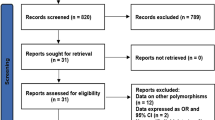
Abstract
Background
Statins are well known for their efficacy to improve lipid profiles. Their efficacy varies between individuals and can be modified by patient factors such as genetic polymorphisms. This study used a cross-sectional retrospective design to assess the effect of selected single nucleotide polymorphisms (SNPs) and other patient-specific clinical variables on statin-related lipid profile changes in a subgroup of Malaysians. The impact of low and moderate intensity of statin doses (10–40 mg/day for at least six weeks), regardless of statin types, was assessed between SNPs of previously identified genes with clinical relation to statin efficacy and lipid profile changes before (baseline) and after statin treatment; two ranges of treatment durations, i.e. ≤ 6 months and 7–12 months. DNA was extracted from patient's venous blood (3 mL), and SNP genotyping was performed using PCR–RFLP method. Using a dominant genetic model, the association between selected SNPs from six genes of interest (ABCG2, ABCC2, APOE, APOA5, GATM and COQ2) and the patients' lipid profiles was investigated.
Results
A total of 229 statin-treated patients were included. The mean age of the patients was 53 ± 7.16 years, and they were mostly females (53.3%), Malay (96.1%), and were taking atorvastatin and simvastatin (90.4%). Seven SNPs genotyped from six genes investigated were related to different lipid profile before and after statin treatment. At baseline, ABCG2 rs2231142 (P = 0.035) and APOA5 rs662799 (P = 0.007) variants had higher HDL-c levels, while ABCC2 rs717620 variants had higher TC (P = 0.040) and LDL-c levels (P = 0.022). Following statin treatment, ABCC2 rs717620 (lower TG, P = 0.009) and APOA5 rs662799 (higher HDL, P = 0.031; lower TG, P = 0.037) were associated with improved lipid profiles, with the association being substantially related to males carrying minor alleles of the SNPs. None of the investigated SNPs were related to significant statin-related LDL-c lowering effects during statin therapy.
Conclusion
To better understand inter-individual heterogeneity in lipid profiles during statin therapy, it would be helpful to take patient genetics and gender into consideration before and after administering statins.
Similar content being viewed by others
Background
Hyperlipidemia (HPL) is one of the risk factors for cardiovascular diseases (CVD), as previously reported in the Framingham Offspring Cohort [1]. A meta-analysis of 32 cohort studies conducted in the Asia–Pacific region found that HPL was related to a significant increase in CVD mortality, while triglycerides (TG) and high density lipoprotein cholesterol (HDL-c) levels predicted CVD risk [2]. Other lipid such as low-density lipoprotein cholesterol (LDL-c) has also been identified as primary targets for lipid reduction in HPL patients. A meta-analysis study conducted by the Cholesterol Treatment Trialists' Collaborators found that lowering LDL-c by 1 mmol/L reduced coronary mortality by 19.0% (risk ratio = 0.81, P < 0.0001) [3]. The National Cholesterol Education Program Adult Treatment Panel III has recognized two treatment options for lowering LDL-c levels: therapeutic lifestyle changes and lipid-lowering drugs [4].
Statins, one of the most commonly used lipid-lowering drugs, have been recognized as the first line of defence against HPL [5, 6]. Statin works in the liver by competitively inhibiting the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. The enzyme involves in the conversion of HMG-CoA to L-mevalonate, decreases cholesterol synthesis in the organ and increases cholesterol uptake from the circulation as a compensatory mechanism [7, 8]. Statins have been reported to reduce LDL-c levels by 24–60% [9], with inter-individual heterogeneity for LDL-c lowering effects that could be attributable to a variety of factors, including genetic polymorphisms [10].
In comparison with Western countries, the Southeast Asian region has less data on statin pharmacogenetics. Several genetic variants have been proposed as the most widely investigated candidate genes determining statin efficacy among Asian populations: genes in transmembrane transporters, cytochrome P450 isoenzymes, and apolipoproteins (APO). Based on previously promising associations with statin efficacy and toxicity, the gene association approach in this study considered the following single nucleotide polymorphisms (SNPs) based on their clinical necessities: APOA5 rs662799, rs429358, and rs7412 in the APOE (regulation in lipid metabolism), ABCG2 rs2231142 and ABCC2 rs717620 (statin transport and disposition) and GATM rs9806699 and COQ2 rs4693075 (associated with statin toxicity) [11,12,13,14,15,16,17,18]. As such, the current study sought to investigate the potential association between the aforementioned SNPs, as well as other patient-specific clinical factors, and lipid profiles in patients treated with low and moderate intensity statin doses (10–40 mg/day) in a subset of outpatient statin users in Malaysia.
Methods
Patient recruitment
Ethical approval for the study was provided by the Human Research Ethics Committee (JePeM), Centre for Research Initiatives Clinical and Health, Universiti Sains Malaysia (USM) Health Campus (approval number: USM/JePeM/19070437). This cross-sectional retrospective study involved 229 hyperlipidemic patients who received low and moderate intensity statin doses (10–40 mg/day) from an outpatient clinic at Hospital USM (HUSM), a university-affiliated teaching hospital on Malaysia’s east coast. Patients were consecutively recruited between February 2018 and September 2020 during their routine lipid monitoring. Following informed written consent, medical records of the included subjects were reviewed, and a face-to-face interview was conducted by a qualified research nurse. Inclusion criteria include: (i) being between the ages of 18 and 75 and (ii) taking statin for at least six weeks. Exclusion criteria include: (i) diagnosed with familial hypercholesterolemia, hepatic, renal, thyroid, or malignant diseases; (ii) taking other drugs that have been demonstrated to interfere with statin efficacy; and (iii) being prescribed with other types of lipid-lowering drugs. Data for overnight fasting lipid levels (TC, HDL-c, LDL-c, and TG) were recorded on the day of patient visit to the clinic in the morning and categorized into two ranges of treatment durations, i.e. ≤ 6 months and 7–12 months. The corresponding baseline lipid levels (when the patients first started statin treatment; therefore, they were considered measurements without statin exposure), statin type and doses, and other clinical parameters were collected from the hospital database.
SNP genotyping
In addition to routine serum biochemical measurements (“Biochemical analysis” section), each patient provided a 3 mL venous blood sample, which was transferred into EDTA tubes and stored at − 80 °C for subsequent genotyping. The DNA was extracted according to the manufacturer’s protocols (GeneAll Biotechnology, Korea) and stored at − 20 °C until further use. SNP genotyping was performed using polymerase chain reaction-restriction fragment length polymorphisms (PCR–RFLP). Table 1 presents information about primer sequences and specific PCR–RFLP settings. The PCR process began with 5 min of pre-denaturation at 95 °C, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at varying temperatures for each SNP (Table 1), and extension at 72 °C for 30 s. Post-extension was conducted at 72 °C for 10 min. To avoid technical errors and maintain the quality in genotyping, 5–10% of all samples were picked at random and sent to the Human Identification Unit DNA at USM for sequencing analysis. Additional file 1 shows the figures for the representative electrophoresis gels and chromatograms that, when possible, capture the wild-type, heterozygous and homozygous recessive genotypes for each SNP studied.
Biochemical analysis
Following an overnight fast (9–12 h), blood samples (2 ml) were collected from each participant for lipid assessment as part of the patients’ usual follow-up. Biochemical parameters such as TC, TG, HDL-c, and LDL-c were determined using an enzymatic colorimetric method on a Hitachi 912 autoanalyzer (RANDOX laboratories, UK) at the HUSM’s department of Chemical Pathology.
Sample size calculation
The sample size for the current study was calculated using an online calculator (https://wnarifin.github.io/sscweb.html) and was based on the variant allele frequencies for a particular SNP (i.e. CETP rs708272) in a representative Asian population, as previously described [19].
Statistical analysis
SPSS software version 26.0 (IBM, USA) was used to perform the statistical analysis. Using East Asian (https://asia.ensembl.org/index.html) as reference population, the observed genotype frequencies were checked for deviations from the Hardy–Weinberg equilibrium (HWE). Continuous data were presented as mean ± standard deviation (SD) tested for normality with histograms and box plots before the Kolmogorov–Smirnov test. A genetic dominant model was applied to evaluate patient genotypes between minor allele carriers (heterozygous + homozygous mutant) and wild-type (homozygous dominant). To compare lipid levels in two groups with normally distributed data, an independent T-test was used; non-normally distributed data were evaluated using the Mann–Whitney U test. Lipid levels before and after statin treatment were compared using one-way repeated measures ANOVA for parametric data and the Friedman test for nonparametric data. A multivariate binary logistic regression analysis was used to evaluate the association between independent factors and patients achieving the LDL-c target of 2.6 mmol/L or below, which was the outcome measured. Statistical significance was defined as P values less than 0.05 (P < 0.05).
Results
Characteristics of the patients
Characteristics of the recruited patients and their clinical data are shown in Table 2. The patients had a mean age of 53 ± 7.16 years, with the majority being females (53.3%), Malays (96.1%), and treated with lipophilic statins, i.e. atorvastatin and simvastatin (90.4%), and followed by pravastatin (7.0%), and lovastatin (2.6%). All statin doses recorded at the time of recruitment ranged from 10 to 40 mg/day (with the majority of patients taking 10–20 mg/day). Drug adherence was verified by patient self-report during interviews; in the case of non-adherence, such as due to mild muscle pain (2 cases), statin re-challenge resolved the issue and was therefore included in the analysis. The patients’ diagnosed comorbidities, concurrent drugs, particular antihypertensive drugs that have been prescribed with statin, as well as baseline lipid levels when patients were initially starting statin treatment, are presented in Table 2. Patients with diagnosed comorbidities included diabetes mellitus (DM) and hypertension (HPT) (39.7%), HPT only (37.1%), HPL only (12.7%), and DM and HPL (10.5%), therefore they were provided drugs such as antihypertensive and diabetic drugs concurrently (Table 2). In particular, 38.4% of the patients were prescribed both diabetic and antihypertensive drugs, 37.1% were on antihypertensive drugs, 15.3% were on statin alone, and 9.2% were on diabetic drugs. Out of 173 patients prescribed with antihypertensive drugs, the majority (57.2%) were prescribed with two or more combination, followed by calcium channel blockers (19.1%), angiotensin-converting enzyme inhibitor (13.9%), angiotensin receptor blocker (5.8%), diuretic drugs (2.3%), and β-blocker (1.7%).
Genotypic and allelic frequencies
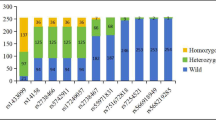
Table 3 compares genotypic and allelic frequencies for the current study to a reference population from the Ensembl Genome Browser website (http://asia.ensembl.org/index.html). The reference population is a healthy cohort of East Asians. The minor allele frequency (MAF) of each SNP in the six genes studied is as follows: ABCG2 rs2231142 = 0.12, ABCC2 rs717620 = 0.58, APOE E4 = 0.35, GATM rs9806699 = 0.63, COQ2 rs4693075 = 0.96, and APOA5 rs662799 = 0.45. All SNPs were not in HWE with the reference population (P < 0.05) except for COQ2 rs4693075 (P = 0.333).
The impact of genetic polymorphisms and other factors on lipid profile
The effects of the studied genetic polymorphisms on lipid levels of statin users are shown in Table 4. Before starting statins, certain SNPs were associated with distinct lipid levels: ABCG2 rs2231142 (P = 0.035) and APOA5 rs662799 (P = 0.007) were associated with higher HDL-c levels, and ABCC2 rs717620 was associated with higher TC (P = 0.040) and LDL-c levels (P = 0.022). During statin treatment, ABCG2 rs2231142 (TC, P = 0.038) and APOA5 rs662799 (TG, P = 0.037) showed a significant association with lipid profiles. With regard to statin-related LDL-c lowering effects, none of the SNPs studied were found to predict significant LDL-c reductions (P < 0.001) with statin treatment.
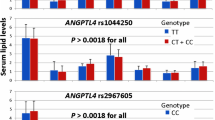
We previously reported that the gender factor resulted in different lipid profiles especially for LDL-c and TG levels in the patient cohort prior to statin treatment, as shown with CETP rs708272 [19]. Females with minor allele A carriers for CETP rs708272 had significantly higher LDL-c (P = 0.007) and TG levels (P = 0.044) [19]. Interestingly, although CETP rs708272 no longer resulted in significant LDL-c level changes in the minor allele A carriers after statin exposure [19], it appears that patient gender determined improvements in HDL and TG profiles, especially in males carrying minor allele G of APOA5 rs662799; higher HDL-c (P = 0.006) and lower TG (P = 0.038) in males (Fig. 1a), but not in females (data not shown). In contrast, male with variants genotypes for ABCC2 rs717620 (-24C > T) had higher TC (P = 0.018) and LDL-c (P = 0.008) levels before statin treatment (Fig. 1b); the SNP was no longer determined for both lipids after statin treatment. In multiple binary logistic regression analysis, only the use of a hydrophilic statin, i.e. pravastatin (P = 0.040), but none of the studied SNPs, age and gender factors independently predicted patient’s achieving LDL-target of < 2.6 mmol/L (Table 5).
Discussion
Statins are the first-line drugs for both primary and secondary CVD prevention in HPL patients [20]. Numerous clinical studies have demonstrated that statin drugs are effective against CVD [3], and their efficacy may be modified by a variety of factors including genetic polymorphisms [21]. The current study expands on previous pharmacogenetic studies of statin efficacy in other populations, and we seek to learn more about how particular genetic polymorphisms, together with other patient or clinical factors, predict statin-related lipid profile changes in a subset of Malaysians with HPL. Indeed, by comparing two geographically distinct populations, such as Malaysian (a proxy for East Asians) and British (a proxy for Europeans), pharmacogenetic data can be utilized to predict different clinical outcomes of pharmacological therapy [22]. The findings in this study highlight a clear association between certain SNPs (e.g., ABCG2 rs2231142, APOA5 rs662799, and ABCC2 rs717620) and lipid profiles of HDL-c or LDL-c in Malays prior to statin treatment, suggesting a different lipid metabolism status among the patients thus the SNPs may exhibit protective effects or risk factors for CVD. Following statin treatment with low and moderate intensity statin doses (10–40 mg/day). Possession of at least one minor allele of the SNPs, i.e. ABCC2 rs717620 and APOA5 rs662799, significantly improved statin-related lipid profile changes, particularly HLD-c and TG, but not LDL-c levels. Statins, but not any variants in genes studied, were significantly beneficial in lowering LDL-c levels (P < 0.001), implying that the LDL-c lowering effects of statins were exclusively pharmacological.
Only two of the seven SNPs investigated, i.e. ABCC2 rs717620 (C > T) and APOA5 rs662799 (A > G), were associated with different lipid profiles in HPL patients both before and after statin treatment. Following longer statin treatment (within 7 to 12 months duration), both SNPs associated with improved TG profile; reduced TG levels were found in minor allele carriers of the SNPs (Table 4). Minor allele T carriers of ABCC2 rs717620 were shown to have a lower TG/HDL index ratio (P = 0.030) in Chilean population (n = 127) treated with a low-dose atorvastatin (10 mg/day) [23], indicating a greater efficacy of atorvastatin-related TG-lowering effects with the SNP. The ABCC2 gene, which encodes the multidrug resistance-associated protein 2 (MRP2) membrane efflux transporter, is necessary for cellular efflux of its substrates, including statin, and controlling its hepatobiliary excretion [24]. The ABCC2 rs717620 variants have been associated with decreased MRP2 expression and function, resulting in higher bioavailability and thus improved the efficacy of atorvastatin and other statins [24, 25]. In this study, minor allele T carriers of the ABCC2 rs717620 SNP also had higher TC (P = 0.040) and LDL-c (P = 0.022) levels at the baseline prior to statin treatment (Table 4), suggesting an increased CVD risk among the SNP variants, and the risk was encountered with significant TC- and LDL-lowering effect with statin treatment. Since our analysis was not corrected by means of body mass index (BMI), one of the most prominent confounding factors in lipid levels [26], we were unable to determine whether the minor allele T carriers had high BMI values, which reflected their high TC and LDL-c levels. However, stratification based on individual genotypes and patient gender in the analysis would eliminate the confounding effects. A large cross-sectional study from the USA (n = 12,383) and Spain (n = 11,765) found that LDL-c levels increased significantly (P < 0.001) by 23.0 mg/dL and 24.1 mg/dL, respectively, per kg/m2 increase in BMI, though the effect was only observed below the BMI inflection points (27.1 kg/m2 and 26.5 kg/m2, respectively) [27]. Similarly, an obese group (BMI ≥ 25 kg/m2) had higher (P < 0.01) LDL-c than the lean group (BMI < 25 kg/m2) in a non-diabetic Chinese population (n = 1538) [28], further suggesting the impact of BMI on LCL-c levels.
In terms of TG profiles, we found an association between APOA5 rs662799 (A > G) and lower TG levels, which were predominantly observed in male patients carrying minor allele G (Fig. 1) suggesting a higher TG metabolism among the minor allele carriers of the SNP. The TG-lowering effects were most likely related to the atorvastatin treatment, regardless of the specified dose (data not shown). The APOA5 gene was identified as a key regulator of plasma TG levels [11]. Despite the fact that most evidence from both animal and human studies indicated that APOA5 rs662799 (found to result in a 50% decrease in the APOA5 gene expression) was associated with higher plasma TG levels [29], minimal inter-ethnic heterogeneity were discovered [30]. A study in Hong Kong (n = 1375) and Guangzhou (n = 1996) populations also found that GG genotypes had 36.1% (P = 2.6 × 10–13) and 30.0% (P = 1.3 × 10–12) higher plasma TG levels, respectively, than homozygous dominant AA genotypes [31], while another Chinese ethnic (Han) population (n = 200) found that GG genotypes were significantly associated with reduced TG levels (P = 0.047), compared with other genotypes, in just three months of atorvastatin (20 mg/day) treatment [32]. Similar findings supporting the former observations were observed in other populations including Pakistani (n = 712) and North Iranian (n = 199) [33, 34]. Our study found no association between APOA5 rs662799 and the statin-related LDL-lowering levels in patients. However, minor allele G carriers of the SNP resulted in a significant LDL-c reduction (P < 0.005) following three months of low dose statin, regardless of the type of statin, in Caucasians (n = 154) [35]. Considering APOA5 rs662799 had a strong association with higher HDL-c levels (Table 4 and Fig. 1) at baseline (P = 0.007) and during statin treatment (P = 0.031), we assumed that this SNP may have a protective effects against CVD risk, as previously demonstrated [36,37,38]. Our findings supported those of the Turkish Cypriot population (n = 100), which indicated that GG genotypes had considerably higher HDL-c levels (P = 0.014) than other genotypes [39].
Gender and, to a lesser extent, ethnicity are the key factors affecting inter-individual variability in lipid levels such as TG and HDL-c [40]. Thus, it is critical to corroborate our findings on the impact of gender on the lipid profiles. It is worth noting that APOA5 rs662799 and ABCC2 rs717620 had gender-specific effects on lipid profiles, thus corresponded with our previous findings with the CETP gene [19]. Before statin treatment, males carrying the minor allele G for APOA5 rs662799 had higher HDL-c levels (P = 0.007) than the wild-type AA genotypes, and HDL-c levels remained significantly higher (P = 0.031) during statin treatment. Also, during statin treatment, TG levels were significantly lower in the APOA5 rs662799 variants but not in the wild-type AA genotypes (Fig. 1a), suggesting that the SNP has a protective effect against CVD risk. The gender-specific effect on TG levels in males in our study, to some extent, explained previous findings in humans and mice [41]. In a large longitudinal study (n = 4329), AA genotypes of the SNP had a higher incidence of dyslipidemia (OR 1.50, 95%CI, P < 0.001) than their AG and GG counterparts [42]. In contrast, prior to statin treatment, the ABCC2 rs717620 variants may have a higher CVD risk, probably due to increased TC and LDL-c levels. The lipid profiles during statin treatment were determined by the pharmacological effect of the drug since the significant statin-related lipid-lowering effects were unaltered with different genotypes (Table 4). Above all, the specific type of statin, i.e. pravastatin, determined the patient’s attainment of the LDL-target of 2.6 mmol/L (Table 5), rather than the effect of other variables such as SNPs or patient’s demographic profiles.
In addition to female gender [43], there is consistent evidence that advanced age and low body mass contribute to statin adverse effects [44, 45]. In this study, gender and age factors did not independently predict patient’s attainment of the LDL-target of 2.6 mmol/L (Table 5). The mean age of patients in this cohort (53 ± 7.16 years old) was not different between males and females, as reported previously [19]. In terms of statin efficacy, there is conflicting evidence among older people (generally defined as more than 65 years old); a meta-analysis of 28 randomized controlled trials found that statin therapy, regardless of patient age, resulted in significant reductions in major vascular events [46], implying a minimal effect of patient age on statin efficacy, but this was not evident for statin-related adverse effects [44]. However, the temporal relation between the study outcomes and the above-mentioned patient factors may be easier to be interpreted in a prospective design, rather than this cross-sectional retrospective approach.
Our study has a few limitations. First, the current study examined the effect of a single SNP on lipid-lowering effects of statins without taking gene–gene interaction into account. The possibility of gene–gene interactions has been demonstrated in relation to statin efficacy and toxicity. Females with the APOE E4 variant allele, for example, reduced the effect of APOA5 rs662799 on TG levels in Caucasian (n = 2500), suggesting a sex-specific interaction between the two genes [47]. Similarly, the inclusion of an important genetic predictor in determining statin efficacy, such as solute carrier organic anion transporter family member 1B1 (SLCO1B1), the most relevant gene underlying statin-related side effects from a genome-wide association study [48], is necessary. In fact, the gene has been replicated in many gene association studies. The SLCO1B1 polymorphism, along with the gender factor, was found to be the only significant gene candidate predicting statin-related muscle toxicity [49]. Next, our findings were most likely restricted to the effect of low and moderate intensity statin doses (approximately 86% of the included patients were on 10 to 20 mg/day) on the lipid profile for all types of statins. Although we were unable to directly determine which statin has the optimum effect on lipid profile, our findings did, in part, explain genetic involvement in lipid profiles changes before and after statin treatment in general. In order to prescribe personalized medicine among statin users, future studies should focus on individual statins because their effect on lipid profile varies, and the consideration of pharmacogenetic-related gender involvement in patient management is necessary. For example, rosuvastatin resulted in significantly higher LDL-c reductions across dose range compared to other statins [50]. Our study also lacked sufficient study power to assess the impact of each type of statin on the measured lipid profiles because of the unequal number of patients among different statin users. Furthermore, the different properties of statins (hydrophilic versus lipophilic statins) were more relevant in explaining statin-related adverse effects [44]. In this study, we also included the two cases of statin-related mild muscle pain since they did not result in statin intolerance; the muscle symptom was resolved with statin re-challenge. Finally, given that the study subjects were Malay ethnicity with HPL, the findings should be regarded with caution when replicated in other ethnic groups in Malaysia or healthy cohorts. In other multi-ethnic nationalities, such as Singaporeans (n = 1589), certain genetic polymorphisms were found to be associated with HDL-c levels in Chinese males alone (P = 0.004), but not in other ethnicities [51], emphasizing the importance of careful interpretation when implementing statin pharmacogenetic data across different ethnicities.
Future investigations should consider the effects variables such as smoking, alcohol intake, and BMI, which were relatively underrecognized contributors to high blood cholesterols and affecting statin response [52, 53]. The inclusion of epigenetic signatures, such as the ABCG1 gene, is particularly attractive owing to its promising signal of statins’ diabetogenic effects in a current epigenome-wide association study [54]. Furthermore, statins have been linked to epigenetic changes, particularly at genes related to lipid metabolism (i.e. ADAL gene, the most significantly differentially methylated with respect to CHD status) in subjects of European ancestry [55], and would be of clinical interest if replicated in the Asian population.
Conclusion
This study found that certain lipid profiles in HPL patients before and during statin treatment are influenced, at least in part, by specific genetic polymorphisms (primarily ABCG2, APOA5 and ABCC2 genes) and patient gender. However, we found no association between statin-related LDL-c lowering effects and the SNPs studied, suggesting a strong pharmacological effect of statins. The findings warrant further investigation and replication in other Asian cohorts of different ethnicities.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Navar-Boggan AM, Peterson ED, D’Agostino RB et al (2015) Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 131(5):451–458
Barzi F (2005) A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann Epidemiol 15(5):405–413
Baigent C, Keech A, Kearney P et al (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 366(9493):1267–1278
Safeer RS, Ugalat PS (2002) Cholesterol treatment guidelines update. Am Fam Physician 65(5):871–880
Feingold KR (2000) Cholesterol lowering drugs. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K (eds) South Dartmouth. MDText.com, Inc
Schachter M (2005) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19(1):117–125
Buhaescu I, Izzedine H (2007) Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 40(9–10):575–584
Stancu C, Sima A (2001) Statins: mechanism of action and effects. J Cell Mol Med 5(4):378–387
Schaiff RAB, Moe RM, Krichbaum DW (2008) An overview of cholesterol management. Am Health Drug Benefits 1(9):39–48
Vladimirova-Kitova LG, Kitov SI (2015) Resistance of statin therapy, and methods for its influence. In: Sekar K, Ashok K (eds) Hypercholesterolemia. IntechOpen, pp 185–202
Forte TM, Ryan RO (2015) Apolipoprotein A5: extracellular and intracellular roles in triglyceride metabolism. Curr Drug Targets 16(12):1274–1280
Husain MA, Laurent B, Plourde M (2001) APOE and Alzheimer’s Disease: from lipid transport to physiopathology and therapeutics. Front Neurosci 15:1–15
Phillips MC (2014) Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life 66(9):616–623
Liu N, Yang G, Hu M et al (2018) Association of ABCC2 polymorphism and gender with high-density lipoprotein cholesterol response to simvastatin. Pharmacogenomics 19(14):1125–1132
Prado Y, Zambrano T, Salazar LA (2018) Transporter genes ABCG2 rs2231142 and ABCB1 rs1128503 polymorphisms and atorvastatin response in Chilean subjects. J Clin Pharm Ther 43(1):87–91
Wan Z, Wang G, Li T et al (2015) Marked alteration of rosuvastatin pharmacokinetics in healthy Chinese with ABCG2 34G.A and 421C.A homozygote or compound heterozygote. J Pharmacol Exp Ther 354(3):310–315
Kitzmiller JP, Mikulik EB, Dauki AM et al (2016) Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers Med 9:97–106
Liu M, Fan F, Zhang Y, Li J (2020) The association of GATM polymorphism with statin-induced myopathy: a systematic review and meta-analysis. Eur J Clin Pharmacol 77(3):349–357
Shamsudin AF, Bakar NS (2023) Gender differences in the association between cholesteryl esters transfer protein polymorphism (rs708272) and plasma lipid levels in hyperlipidaemic participants at hospital Universiti Sains Malaysia. Malays J Med Sci 30(2):96–110
Blais JE, Wei Y, Yap KK et al (2021) Trends in lipid-modifying agent use in 83 countries. Atherosclerosis 328:44–51
Zhao Z, Du S, Shen S et al (2019) Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: a frequentist network meta-analysis. Medicine (Baltimore) 98(6):1–12
Bakar NS (2021) Pharmacogenetics of common SNP affecting drug metabolizing enzymes: comparison of allele frequencies between European and Malaysian/Singaporean. Drug Metab Pers Ther 36(3):173–181
Prado Y, Arencibia A, Zambrano T et al (2018) Gender-specific association between ABCC2 -24C>T SNP and reduction in triglycerides in chilean patients treated with atorvastatin. Basic Clin Pharmacol Toxicol 122(5):517–522
Becker ML, Elens LL, Visser LE et al (2013) Genetic variation in the ABCC2 gene is associated with dose decreases or switches to other cholesterol-lowering drugs during simvastatin and atorvastatin therapy. Pharmacogenomics J 13(3):251–256
Oh ES, Kim CO, Cho SK et al (2013) Impact of ABCC2, ABCG2 and SLCO1B1 polymorphisms on the pharmacokinetics of pitavastatin in humans. Drug Metab Pharmacokinet 28(3):196–202
Li Y, Zheng R, Li J et al (2020) Association between triglyceride glucose-body mass index and non-alcoholic fatty liver disease in the non-obese Chinese population with normal blood lipid levels: a secondary analysis based on a prospective cohort study. Lipids Health Dis 19(1):29
Laclaustra M, Lopez-Garcia E, Civeira F et al (2018) LDL cholesterol rises with BMI only in lean individuals: cross-sectional U.S. And Spanish representative data. Diabetes Care 41(10):2195–2201
Luo Y, Ma X, Shen Y et al (2014) Positive relationship between serum low-density lipoprotein cholesterol levels and visceral fat in a Chinese nondiabetic population. PLoS ONE 9(11):e112715
Schaap FG, Rensen PCN, Voshol PJ et al (2004) ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglycerides (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J Biol Chem 279(27):27941–27947
Hubacek JA (2016) Apolipoprotein A5 fifteen years anniversary: lessons from genetic epidemiology. Gene 592(1):193–199
Jiang CQ, Liu B, Cheung BMY et al (2010) A single nucleotide polymorphism in APOA5 determines triglyceride levels in Hong Kong and Guangzhou Chinese. Eur J Hum Genet 18(11):1255–1260
Yue YH, Bai XD, Zhang HJ et al (2016) Gene polymorphisms affect the effectiveness of atorvastatin in treating ischemic stroke patients. Cell Physiol Biochem 39(2):630–638
Fiaz M, Shaiq PA, Raj GK et al (2019) Association study of Apolipoprotein A5 gene (APOA5 gene) variant with the metabolic syndrome in local Pakistani population. J Pak Med Assoc 69(3):301–305
Halalkhor S, Jalali F, Tilaki KH et al (2014) Association of two common polymorphisms of apolipoprotein A5 gene with metabolic syndrome indicators in a North Iranian population, a cross-sectional study. J Diabetes Metab Disord 13(48):1–7
Hubacek JA, Adamkova V, Prusikova M et al (2009) Impact of apolipoprotein A5 variants on statin treatment efficacy. Pharmacogenomics 10(6):945–950
Bartlett J, Predazzi IM, Williams SM et al (2016) Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? Circ Cardiovasc Qual Outcomes 9(3):206–212
Nelson RH (2013) Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 40(1):195–211
Rosenson RS, Davidson MH, Le NA et al (2015) Underappreciated opportunities for high-density lipoprotein particles in risk stratification and potential targets of therapy. Cardiovasc Drugs Ther 29(1):41–50
Fahrioğlu U, Ergören MÇ (2018) The association between APOA5 gene polymorphisms and plasma lipids in the Turkish Cypriot population: a possible biomarker for preventing cardiovascular diseases. Biochem Genet 56(3):176–187
Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC et al (2013) Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res 54(10):2795–2799
Pennacchio LA, Olivier M, Hubacek JA et al (2001) An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 294(5540):169–173
Lin H, Xuan L, Xiang J et al (2022) Changes in adiposity modulate the APOA5 genetic effect on blood lipids: a longitudinal cohort study. Atherosclerosis 350:1–8
Puccetti L, Ciani F, Auteri A (2010) Genetic involvement in statins induced myopathy: preliminary data from an observational case-control study. Atherosclerosis 211(1):28–29
Schech S, Graham D, Staffa J et al (2007) Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf 16(3):352–358
Feng Q, Wilke RA, Baye TM (2012) Individualized risk for statin-induced myopathy: current knowledge, emerging challenges and potential solutions. Pharmacogenomics 13(5):579–594
Collaboration CTT (2019) Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 393(10170):407–415
Hubacek JA, Lánská V, Škodová Z et al (2008) Sex-specific interaction between APOE and APOA5 variants and determination of plasma lipid levels. Eur J Hum Genet 16(1):135–138
SEARCH Collaborative Group, Link E, Parish S, Armitage J et al (2008) SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med 359(8):789–799
Bakar NS, Neely D, Avery P et al (2018) Genetic and clinical factors are associated with statin-related myotoxicity of moderate severity: a case-control study. Clin Pharmacol Ther 104(1):178–187
Jones PH, Davidson MH, Stein EA, STELLAR Study Group et al (2003) Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 92(2):152–160
Lu Y, Tayebi N, Li H et al (2013) Association of CETP Taq1B and -629C > A polymorphisms with coronary artery disease and lipid levels in the multi-ethnic Singaporean population. Lipids Health Dis 12(1):1–13
Denke MA, Sempos CT, Grundy SM (1993) Excess body weight: an underrecognized contributor to high blood cholesterol levels in white American men. Arch Intern Med 153(9):1093–1103
Zhang L, He S, Li Z et al (2019) Apolipoprotein e polymorphisms contribute to statin response in Chinese ASCVD patients with dyslipidemia. Lipids Health Dis 18(1):129–139
Ochoa-Rosales C, Portilla-Fernandez E, Nano J et al (2020) Epigenetic link between statin therapy and type 2 diabetes. Diabetes Care 43(4):875–884
Dogan MV, Grumbach IM, Michaelson JJ et al (2018) Integrated genetic and epigenetic prediction of coronary heart disease in the Framingham Heart Study. PLoS ONE 13(1):e0190549
Elmadbouh I, Elghobashy Y, Abd-Allah E et al (2013) Relationship of apolipoprotein E polymorphism with lipid profiles in atherosclerotic coronary artery disease. Egypt Heart J 65(2):71–78
Halalkhor S, Jalali F, Tilaki KH, Shojaei S (2014) Association of two common polymorphisms of apolipoprotein A5 gene with metabolic syndrome indicators in a North Iranian population, a cross-sectional study. J Diabetes Metab Disord 13(48):1–7
Acknowledgements
The authors would like to thank everyone at the Family Medicine Clinic, Hospital Universiti Sains Malaysia (USM), for their assistance, and to all the patients who participated in this study. The authors also thank all staffs at the Human Genome Centre, School of Medical Science USM for their technical assistance with the genotyping. The authors also acknowledge statistical advice from Dr Wan Nor Arifin bin Wan Mansor (Department of Biostatistics and Research Methodology, School of Medical Sciences, USM). Finally, heartfelt gratitude is expressed to the Director of Hospital USM for granting permission to publish this medical research work. Special thanks go to Universiti Sains Malaysia for the financial assistance through the Short Term Research Grant (304/PPSK/6315087).
Funding
This work was supported by the USM’s Short Term Grant (304/PPSK/6315087).
Author information
Authors and Affiliations
Contributions
NSB designed the study, obtained funding and interpreted data analysis of the manuscript. AFS drafted the manuscript and contributed to the acquisition, analysis and interpretation of data for the work. AFS also involved in patient recruitment and performed the genotyping. IA and SS co-supervised the student and involved in patient selection and interpreted genotyping results, respectively. All authors revised the manuscript and gave final approval and agreed to be accountable for all aspects of the work, ensuring integrity and accuracy of any part of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee (JEPeM-USM) Centre for Research Initiatives Clinical and Health USM Health Campus (Approval number: USM/JePeM/19070437). An informed written consent has been taken from all subjects while enrolling them for this study.
Consent for publication
The consent to publish has been taken from each subject at the start of this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Appendix 1. Figures for gels and sequence allignments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shamsudin, A.F., Sulong, S., Ahmad, I. et al. Association between genetic polymorphisms and other attributing factors with lipid profiles among statin users: a cross-sectional retrospective study. Egypt J Med Hum Genet 25, 53 (2024). https://doi.org/10.1186/s43042-024-00523-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-024-00523-4