Abstract
Background
MiRNAs play critical roles in the regulation of cellular function, life span, and the aging process. They can affect longevity positively and negatively through different aging pathways.
Main text
MiRNAs are a group of short non-coding RNAs that regulate gene expressions at post-transcriptional levels. The different types of alterations in miRNAs biogenesis, mRNA expressions, and activities of miRNA-protein complexes can affect the regulation of normal post-transcriptional gene process, which may lead to aging, age-related diseases, and an earlier death. It seems that the influence of deregulation of miRNAs on senescence and age-related diseases occurring by targeting aging molecular pathways can be used for diagnosis and prognosis of them. Therefore, the expression and function of miRNAs should be studied more accurately with new applicable and validated experimental tools. However, the current review wishes to highlight simply a connection among miRNAs, senescence and some age-related diseases.
Conclusion
Despite several research indicating the key roles of miRNAs in aging and longevity, further investigations are still needed to elucidate the essential roles of miRNAs in controlling mRNA regulation, cell proliferation, death and/or protection during stress and health problems. Besides, more research on miRNAs will help to identify new targets for alternative strategies regarding effectively screen, treat, and prevent diseases as well as make slow the aging process.
Similar content being viewed by others
MicroRNAs (miRNAs)
MicroRNAs (miRNAs) are a large class of small non-coding RNAs functioning as the important regulators of a wide range of cellular processes [1] that have been identified firstly in C. elegans in 1993 [2,3,4]. They act as mediators that can regulate post-transcriptional gene expressions [5,6,7]; therefore, they are able to control cellular behavior, balance in biological processes, development, and diseases [7, 8] by modulating gene expression [1]. The post-transcriptional gene effect of miRNAs happens by base-pair binding on their related mRNAs targeting untranslated regions (UTRs) of genes and multiple sites within a single UTR [9]. Each miRNA can target multiple mRNAs, and one mRNA can be regulated by multiple miRNAs [10, 11]. MiRNAs have been found in plants, animals, bacteria and some viruses [12] by their gene expression profiling. In animal models, miRNAs contribute into genetic networks and metabolic pathways. It seems that miRNAs play critical roles in the occurrence of pathological conditions like neurodegeneration and cancer following the alterations in the expression of specific miRNAs in the brain and/or homeostasis in the body [6, 9].
Besides, some miRNAs are related to the regulation of senescence in a variety of human cells [8]. As it is shown in Table 1, cancer, cognition, and senescence can be associated with miRNAs [2, 6, 13,14,15,16,17]. Until now, more than 950 miRNAs in humans have been found [18]. It has been indicated that some of miRNAs are tissue- or cell-specific and some of them are house-keeping molecules. However, a relatively small number of miRNAs have probably key functions in order to regulate the human genome and affect post-transcriptional physiological process. It seems that they can influence differentiation and tumor suppression in cells, which may relate to some pathological processes such as carcinogenesis or senescence [10]. Thus, miRNAs have been suggested to be used as biomarkers to evaluate many diseases and aging [9], even though it is difficult to estimate miRNAs quantification due to their small size, low copy number, interference from other small RNAs, and contamination by degradation products of mRNAs or other RNA species [2].
Despite the presence of miRNAs in tissue cells, they can also be found in the body fluids and extracellular environments such as plasma, serum, urine, saliva, seminal fluid, ascites, pleural effusions, and cerebrospinal fluid [4, 19]. They are injected to the circulation in different ways. It can happen through a passive leakage following apoptosis, necrosis, inflammation, or an active secretion by exosomes/microvesicles, lipoproteins, and RNA–protein complex. Circulating miRNAs following packing into exosomes have been found specific to a tissue or a disease, which can indicate degree of tumor progression and stage of cancer. Several studies have also shown that the abnormality of specific circulating miRNAs can be associated with the manifestation, development, invasion, and metastasis of cancer [19]. However, miRNAs-related studies will increase our understanding regarding age-related gene regulation and improve miRNA-based biomarker development for an advance in RNA-based diagnosis and therapies [9]. Thus, the aging process and age-related problems and conditions can be better monitored. In addition, further studies will help to better identify specific miRNAs and their changes related to caloric restriction, aging and age-related diseases pathways.
Biogenesis of miRNA in animals
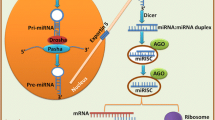
MiRNAs are a group of small non-coding RNA [8, 14, 20, 21] with approximately 21–24 nucleotide (nt) in length [3] that are widespread and probably regulate > 50% of the human genome [22]. They are produced from precursor molecules (pri-miRNAs), which are made through transcription by RNA polymerase II from independent genes or derived from introns after splicing. Pri-miRNAs are subsequently converted to pre-miRNAs by Drosha enzyme and exported to the cytoplasm. Dicer enzyme cleaves them to the mature approximately 20-bp miRNA 5p/3p pairs. One strand of this duplex will incorporate into the miRNA-inducing silencing complex (miRISC) [3, 23,24,25]. The process is summarized in Fig. 1.
The transcription of DNA coding for miRNAs and the related protein-coding genes occurs in a similar way. The mature miRNA directly interacts with a member of the Argonaute protein family, which results in the formation of the RNA-induced silencing complex (RISC). As a component of RISC, miRNAs direct the post-transcriptional repression. Thus, miRNAs show regulatory functions regarding gene expression and can play a central role in several cellular processes including cell growth, differentiation, proliferation, and apoptosis [18].
MiRNAs are potent negative regulators of gene expressions [8]. They regulate their target genes through either translational repression or mRNA degradation. Such functions happen via binding to the complementary regions of messenger transcripts [14] and targeting specific messenger RNAs (mRNAs) [21]. Each miRNA can target up to hundreds of mRNAs [8, 18, 26] and they are able to regulate the expression of more than 60% of protein-coding genes of the human genome [27]. The effect of miRNAs on the regulation of many human genes [28,29,30] indicates their critical roles in a variety of biological processes [8, 21].
MiRNAs are important epigenetic regulators [14] and many of them are also self-regulated epigenetically [8]. For instance, epigenetically transcriptional repression by miRNAs can be through DNA methylation and histone modifications in which to affect subsequently mostly CpG islands located in the promoter regions of genes and result in silencing of genes [31]. Moreover, miRNAs can also down-regulate mRNAs [26] by declining target mRNAs or the levels of translation into proteins [32]. The silence of target mRNAs is based on base-pairing recognition sites [8] and the interaction of miRNAs with the 3’ UTR of target mRNAs. Thus, mRNA degradation and/or translational repression result in gene silencing [4, 29, 33, 34].
However, miRNAs are known as major elements that can contribute into complex functional pathways, control cellular processes [35], and the regulation of biological functions [36] such as differentiation, proliferation, apoptosis, replicative senescence [21], development, and stress responses [35] in plants and animals (Table 2).
MiRNAs are in both intracellular and extracellular regions of the body [61]. Extracellular miRNAs can be used as biomarkers in which to evaluate a variety of diseases such as liver fibrosis and hepatocellular carcinoma. Besides, miRNAs play important roles in intercellular communication. Such miRNAs can be delivered to target cells, where they have hormone-like activities and may act as autocrine, paracrine, and/or endocrine regulators. It can modulate cellular activities [4] by changing the expression of proteins following the specific inhibition of mRNA targets in cell-free miRNAs, which may originate from one cell type and acquired by another cells or other cell types [11]. In addition, miRNAs, gene expression patterns, physiology, and homeostasis in cells are related to the effects of different factors such as stress [135].
Stress
Stress is caused by different stressors and is divided into acute, chronic and several forms. It can play an important role in daily life in which to threat an individual’s homeostasis, well-being, health, and/or survival. Stress is with a systemic physiological response such as inflammatory and cellular reactions, metabolic processes, and epigenetic regulation [18]. The occurrence of stress in cells may happen due to sudden or frequent changes in environmental factors. Stress can damage existing macromolecules in living cells such as proteins, mRNAs, DNA, and lipids, which in turn can increase the risk of death, metabolic imbalances [136], and chronic diseases [20] such as cognitive decline and cancer with advancing age [2].
The severity and duration of stress can affect cellular homeostasis in which to return to stable state or modify to a new state. However, responses to stress in the body can occur through several mechanisms [136] including induction of molecular chaperones [137, 138], rapid clearance of damaged macromolecules [139], activation of specific gene expressions, growth arrest [140], and cell death [20].
MiRNAs play critical roles in main cellular processes. Their identifying helps to understand the cellular stress responses and the occurrence of senescence associated with environmental changes [20]. Nowadays, there is a better understanding regarding the miRNAs involving in physiological reactions and reaction pathways as well as their regulatory roles. For instance, the alteration of miRNAs concentration such as miR-21 has been detected in some stress-related pathological conditions and diseases such as psychiatric diseases [18]. MiRNAs have shown regulatory role in the control of specific mRNA translation. It leads to a gene-specific control over protein translation, which reversibly and rapidly can regulate cellular landscape and also enables survival during periods of extreme stress with efficient utilization of ATP turnover [12]. Responses of cells to stresses are in the form of restoring or reprogramming of gene expression patterns mediated by miRNA functions, the amounts of miRNAs, the amount of mRNA targets, and the activity of miRNA-protein complexes. The levels of cells reactions can determine specificity, time, and concentration of genes related to products expressed at each stress situation [20].
Several studies indicate that miRNAs can have a major regulatory influence over a number of cellular processes in which to play essential roles in prolonged environmental stress survival [12]. For instance, several miRNAs are involved in the regulation of psychological stress effects on the genesis and maintenance of many diseases [18]. In addition, stress regulates both transcription and the biogenesis of miRNAs, which results in the accumulation of pre-miRNAs, the reduction of mature miRNAs, or facilitating the processing of some miRNAs. Such regulatory effect happens by mediating of some important factors such as SMADs, p53 and breast cancer 1 (BRCA1) protein [24]. Besides, psychological stress results in oxidative stress by induction of the sympathetic-adrenal-medullary (SAM) and later the hypothalamic–pituitary–adrenal (HPA) axis, which in turn can cause protein damages and induce specific cellular stress response pathways [18].
Such connections between several miRNAs and pathways in the body can probably explain the effect of stress on aging and age-related changes. For example, there is an association between the expression of some miRNAs (miR-217, miR-100, miR-34a, miR-199a, and miR-132) and different factors of sirtuin 1 (SIRT1) protein, chemokine production, and hypoxia-inducible factor-1 alpha (HIF-1α). It seems that such relation can affect hemostasis, normal growth, and maintenance of cells in the body [2]. The effect of miRNAs happens through recognizing partly complementary sequences in their own mRNA targets and inhibition of their expression by translational repression or degradation of the target mRNAs. As follows, it results in controlling protein synthesis in cells, which in turn plays an important role in regulating cell proliferation, development, and aging [135].
Aging
Aging is due to the accumulation of genetically and environmentally damages [8, 141, 142] accompanied with the unregulated repair systems of DNA [8]. The accumulations of cellular and molecular damages can cause aging and the functional decline of organs, which results in the increased risk of susceptibility to diseases and mortality [21, 143].
The nine hallmarks indicating aging are genomic instability, telomere reduction, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Besides, further studies show the presence of other hallmarks such as dysregulation of the extracellular matrix in aging, for example aging lung [144].
The senescent cell phenotype is under the combination effects of cell changes in morphology, behavior, structure, and functions. These changes occur due to alterations probably happening in gene expressions [145], protein secretions [146], and inducibility of apoptosis, which may increase in senescent fibroblasts [147] and decrease in endothelial cells [148].
The harmful altered senescent cells functions may accelerate senescence and/or loss of cells within tissues, resulting in the age-associated decline of body function and the increased rate of age-associated diseases [8]. It has been indicated that tissue micro-environment changes happening due to the age-related accumulation of senescent cells can promote age-related phenotypes, cancer [149,150,151], and neurological disorders [8]. However, changes in patterns of gene expression in cells following the effects of non-coding RNAs, particularly miRNAs can lead to different functions in senescent cells [8].
As it has been noted, miRNAs play key roles in the regulation of development, apoptosis and metabolism in the body; therefore, they regulate aging and processes responsible for life span determination in vertebrates [152]. Studies on aging in animal models have supported the major roles of miRNAs in modulating life span and the aging process [2]. Age‐related diseases can also be associated with changes in the expression of circulating miRNAs in the body fluids including serum and plasma. Such miRNAs are released during tissue injury or shed from the plasma membranes of various cell types. They are remarkably stable as well as resistant to heat, pH changes, long time storage, and repeated freeze and thaw cycles. Despite these findings, exact molecular pathways underlying aging are not yet well understood [8] and also little is known about the role of circulating miRNAs related to aging in humans [21].
Some of factors and pathways involved in the aging process are insulin/insulin-like growth factor (IGF)-1, phosphoinositide 3-kinase (PI3K), target of rapamycin (TOR), mitogen-activated protein kinase (MAPK), AMP-dependent protein kinase (AMPK), protein kinase C signaling pathway (PKC), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸb), transforming growth factor beta (TGF-β), WNT signaling pathway (wingless-related integration site), Notch signaling pathway, receptor tyrosine kinase (c-Kit), and H2A histone family member X (H2AX). It seems that miRNAs affect the aging process by targeting these mentioned pathways including insulin/IGF-1 pathway [2, 153]. For example, miR-100, miR-30a, and miR-34a contribute into TOR pathway (miR-100, miR-30a) and aging signaling pathway (miR-34a) as the common pathways affecting life span and aging. It has also been found that changes in miRNAs expression with age are opposite to mRNAs expression [2]. However, miRNAs have roles in the regulating aging and age-related specific phenotypes of tissue and cell type with up-regulation, down-regulation and targeting the genes involved in aging pathways during cellular senescence. Various miRNAs expressions involved in aging process can be associated with specificity of tissue and the tissue-specific functions of aging signaling pathways. Despite the up-regulation of some of miRNAs, such as miR-34 and miR-71 with aging, the vast majority of C. elegans miRNAs expression is down-regulated with aging. Such differences in their expression can be due to the globality or specificity of those miRNAs related to a tissue or differences found among plants, animals and viruses. Thus, it is important to find various factors that can actively affect up- or down-regulation of miRNAs with aging [153].
Recent studies have shown miRNAs effects on aging and age-related diseases. For instance, miRNAs can regulate all related aspects of cutaneous biogenesis, functionality, and aging. It has been found that some miRNAs, such as let-7, miR-17, and miR-34, were down-regulated in long-lived individuals. Such conserved miRNAs in humans, known as longevity-related miRNAs, presumably promote life span prolongation. Conversely, miRNA let-7, miR-17, and miR-34 are up-regulated in some age-related diseases such as cancers [48] and cardiovascular diseases [154]. Therefore, further investigations are needed to elucidate the relation between miRNAs and healthy aging.
Longevity
The solution of longevity and how to have a healthy aging is one of the principal challenges in biology and medicine. The improvement of lifestyle and reduction of environmental hazards can prevent diseases and increase health in general population. Besides, the genetic assessment of exceptional individuals can provide important biological insights regarding the basis of healthy aging and human longevity [155]. However, genetics in aging is investigated in which to evaluate life span, longevity, exceptional longevity, and healthy aging. Longevity is defined as a specific advanced age or older, which is often considered 100 years or above. Besides, healthy aging states a combination of old age and health. It indicates the absence of certain diseases, disabilities, and health problems such as cognitive impairment and mobility disorders in older people. It is probably because such people live much healthier [155]. Several studies indicated that life span can be associated with miRNAs expression changes [21]; therefore, the identification of miRNAs roles in the induction and maintenance of senescence [20] will clarify the mechanisms associated with age-related diseases [8]. Thus, miRNAs can be good biomarkers used in order to facilitate predicting individuals’ longevity [21] or treat premature aging and age-related diseases such as cognitive function in the future [156].
Different investigations have shown that changes in the expression of some miRNAs such as let-7, miR-17, and miR-34 have been involved in life span prolongation among long-lived individuals as well as age-related diseases such as cancers [48] and cardiovascular diseases [154]. Further studies suggested the expression profiles of miRNAs miR-211‐5p, miR-374a‐5p, miR-340‐3p, miR-376c‐3p, miR-5095, miR-1225‐3p [129], miR-146a, miR-21, and miR-483 can be used as useful biomarkers for predicting and evaluating aging and age-related diseases. It has been suggested that miRNAs with the decreased production of melatonin, the increased levels of inflammatory and ROS markers [51], and their association with mRNA and epigenetic factors can affect aging and age-related diseases [154] like neurodegenerative and cognitive disorders [157].
Cognition
Neurodegenerative diseases are progressive disorders of the nervous system [6]. There are many cognitive disorders such as Alzheimer, Parkinson, Schizophrenia, Huntington’s diseases, and Autism spectrum disorders [157]. Their pathogenesis is complex and involves the alterations of multiple basic cellular pathways and non-coding RNAs [6]. Non-coding RNAs like miRNAs [6] play key roles in a wide range of physiological processes in the body [158, 159]. For example, miRNAs can regulate the neuronal activity, which contributes into neurogenesis, neurodegeneration, and cognition [6, 160, 161]. Several studies showed that the genetic deletion of dicer and the disruption of miRNAs expressions can affect development, differentiation, morphogenesis, and signaling in neurons [158, 159].
Alzheimer disease is one of neurodegenerative diseases that is the most common cause of dementia. It is mostly related to the aging process [162]. Many cases of dementia have been progressed from mild cognitive impairment (MCI), which is a transitional phase between the cognitive changes of normal aging and senile dementia. It presents with memory or cognitive impairment without significant effect on daily living. The conversion rate of MCI to dementia is approximately 10–15% each year. As many people suffer from dementia; therefore, early and effective diagnosis and intervention of MCI can have a great effect to reduce or delay the progression of dementia [163, 164].
MiRNAs are one of the main regulators of homeostasis in neurons. Their dysregulation can result in pathological conditions in the brain; therefore, their regulatory functions may have great impact on neurodegenerative diseases [128]. Thus, using miRNAs especially multiple miRNAs and serum-based miRNA assays suggested as a method with a high sensitivity and specificity can be used in which to diagnose cognitive impairment [163]. For instance, miR-206 and miR-567 (hsa-mir-567) have been introduced as good biomarkers that can be used to evaluate MCI and the earliest stages of dementia due to their effects on the involved genes in the biological processes in neuronal cells and their crucial roles in the neuronal differentiation and brain development [128]. Many of miRNAs are specific for cells and tissues [165,166,167,168]. For example, axons, dendrites and synapses have their own miRNAs and various expressions of miRNAs in neurons in neurodegenerative diseases such as Alzheimer disease (AD) [14] are probably associated with dendritogenesis and axonal path [169].
AD damages are started in the hippocampus and spread progressively throughout the brain. Although pathological hallmarks of AD are intracellular neurofibrillary tangles, the abnormal deposition of tau protein, and the accumulation of extracellular plaques of β-Amyloid (Aβ) peptides [6], miRNAs network (Fig. 2), and changes such as alterations in miRNAs targeting APP (miR-29a, miR-29b) can also affect the process of this disease [54].
Deregulation of miRNAs can lead to the Aβ-accumulation, which occurs probably due to the activation of several pathogenic cascades. MiRNAs play important roles in homeostasis and pathogenesis of diseases in the brain by targeting 3'-UTRs mRNA that are related to several important proteins such as amyloid-beta precursor protein (APP), transforming growth factor-beta-induced protein (TGFBI), Tripartite Motif Containing protein 2 (TRIM2), SIRT1, and BTB domain containing protein 3 (BTBD3) [6, 170]. For instance, miRNAs let-7 and miR-320 can affect cognition through DAF-12 signaling and insulin/IGF signaling (IIS) pathways, respectively [2].
Further studies showed that several miRNAs such as miR-34, miR-9, miR-124 [171], miR-137 [157], miR-132, and miR-212 play important roles in cognition and memory function. Besides, miRNAs reactions to the higher expression of SIRT1 (miR-132 and miR-212) [76], NF-kB protein complex (mir-125b and mir-146a), cell cycle proteins (mir-26b, mir-107, mir-125b, mir-107, and mir-34a) [53], mitochondrial fission, synaptic function, ATP generation, presynaptic calcium level (miR-484) [120], and the increased production of ROS (mir-210 and mir-146a) can lead to a possible explanation regarding the induction of aging and aging-related diseases. Such correlations may result in releasing factors including IL6, altered genes function such as p53 and/or altered Wnt signaling, which in turn may affect cell cycle control, apoptosis, DNA, and cellular senescence [53]. It is now necessary to obtain consistent knowledge about the role of miRNAs in the brain for the maintenance of cognitive function or the appearance of cognitive deficits. A variety of miRNAs and their combinatorial effects may mediate their roles in pathological disorders in the brain. Several studies suggest that miRNAs-based therapy can be an alternative in the future for diagnosis and treatment of diseases such as neurodegenerative diseases [157, 172] and cancers [19].
Cancer
MiRNAs are key molecular components of cells and play important roles in both normal and pathologic states in the body [173]; hence, changes in their expression can lead to human diseases such as cancers [61, 174, 175]. They act as regulators for controlling a wide range of biological functions including apoptosis, tumor cell proliferation, differentiation, cell cycle progression, invasion, and metastasis [61, 175, 176].
Cancers are a group of age-related diseases. The initiation and progression of cancers can be related to miRNAs deregulation in a cause-effect manner [8] as it has been indicated in Table 3. Thus, miRNAs can be good biomarkers [8] for diagnosis, prognosis, and prediction of tumors. Several investigations have identified specific miRNAs related to solid tumors and hematological malignancies [176, 178] and their actions and effects vary among cancers [61]. For instance, let-7 miRNA and miR-146 act in cancers through their corresponding pathways, namely RAS and KIT, respectively [82]. The effects of miRNAs on proliferation, invasion and cell survival in prostate and pancreatic cancers occur by targeting cyclin D1 (CCND1), Wnt family member 3A (WNT3A), and B-cell lymphoma 2 (BCL2). MiRNAs can regulate metabolism and energy production in oral cancers by targeting NAD-dependent deacetylase SIRT3 (miR-31). Tumor formation and metastasis in germ cell tumors and gastric cancer can be promoted by miRNAs, which happens through targeting tumor suppressor gene TOB1 (transducer of ERBB2. 1) (miR-371-3) [61]. More than half of miRNAs-related genes in cancers are located in either cancer-associated genomic regions leading to amplification, loss of heterozygosity, and breakpoints, or fragile sites [176, 178]. As shown in Fig. 3, miRNAs play important roles in cancers as oncogenes or tumor suppressors [35, 61]. The expressions of tissue-specific miRNAs may lead to malignant transformation changes as tumor formation and growth by repressing tumor suppressor genes or increasing oncogene expressions. Thus, miRNAs can be good biomarkers in order to evaluate cancers and their classifications [61]. Changes of miRNAs function as tumor suppressors may mediate suppression of normal cells functions, which may result in initiation of malignant transformation in cells. Alterations such as mutations, genomic deletions, epigenetic silencing, and miRNA processing alterations can change the function of miRNAs in the regulation of normal cell proliferations. The oncogenic role of miRNAs can also happen by triggering mRNAs, which may encode tumor suppressor proteins. Moreover, miRNAs affect the progression of tumor by influencing the inflammation system, the components of the innate immune system [175, 176], and the modulation of apoptosis [5].
MiRNAs abnormal expression and their effects on inflammation and cell proliferation [179, 180] in cancers [35, 181, 182] can be due to various factors such as defect in miRNA biogenesis machinery, activity changes in drosha, dicer [183], and transcription factors [184], as well as epigenetic changes such as DNA methylation, and histone modifications (histone methylation, and histone acetylation) [35]; therefore, the controlling mechanism of miRNAs expression is almost same to protein-coding genes [182]. As miRNAs play important roles in the regulation of many cancer-related genes, identification of specific ones of them may act as powerful tools to aid in diagnosis and treatment of tumors [82].
One of miRNAs is MiR-34a that can be used as an important diagnostic and therapeutic miRNA in many cancers due to its general expression. MiRNAs can also be used in order to evaluate chemotherapy resistance in cancers such as lung cancer and breast cancer. Despite this, miRNAs expression among different cancer types varies in a wide range, as it has been shown for miR-155, miR-221, and miR-222 expression [61].
It has been noted that miRNAs can be associated with the risk of recurrence and the chance of relapse‐free survival in cancers. For example, the overexpression of miR‐210 in breast cancer is with a higher risk of relapsing and lower chance of survival [22]. On the other hand, some miRNAs such as miR-34 and miR-1 contribute into several biological processes of tumor cells, including differentiation, proliferation, and apoptosis through their down-regulation [185]. Besides, miRNAs can affect drug resistance of tumor cells, which may happen by targeting drug resistance-related genes and/or influencing genes that are related to cell proliferation, cell cycle, and apoptosis [103]. Thus, further research are required in order to understand the exact roles of miRNAs in the development [82], diagnosis, prognosis, treatment [5], and survival [13] of cancers.
Accordingly, gene therapy using miRNAs might be used in the future in which to block the progression of cancers [175] by inhibiting the overexpression of oncogenic miRNAs or replacing those that are effective on tumor suppressive genes [176]. As present cancer-related biomarkers are not specific for tissues and have poor early diagnosis and prognostic value; therefore, they cannot be used as targeted therapy for cancers. Thus, miRNAs sound to be good non-invasive cellular and molecular biomarkers that can be replaced in order to early diagnosis, prognosis, and treatment of cancers [19]. However, further studies are needed for increasing the related knowledge in order to cope with challenges such as miRNAs stability, delivery drug systems, and the control of target effects [176] in which to use the miRNA-based therapeutic approaches in cancers [19]. Despite several therapeutic miRNA delivery systems such as virus-based delivery, non-viral delivery (lipid-based, polymer-based, or chemical structures), and the emerged extracellular vesicle (EV)-based delivery, miRNA-based therapeutic approach is still one of the great challenges. However, it seems that further improvements in techniques such as the targeted-therapeutic miRNA delivery in miRNAs-therapy for the treatment of different cancers will happen in the future [61].
Limitations
However, there are some limitations that can restrict miRNA investigations. Identification of specific miRNAs and their own targets in different diseases such as cancers and dementia is difficult and it limits the use of the miRNA-based therapeutic approaches. MiRNA and the related targeted mRNA must express at the same time in which to change gene expression, protein and biological function. It would be even more complex, when a single miRNA can target hundreds of mRNAs and vice versa. Therefore, it is crucial to identify and validate miRNA/mRNA target pairs and verify their interactions. In addition, technical limitations and delivery problems should be considered as extra factors that can confine such related studies.
Conclusion
This review highlights some relationships among miRNAs, senescence, cancer, and cognitive decline. Different investigations have shown that the actions and biological roles of miRNAs vary in various biological conditions. As noted in the review, further studies are needed to understand better the roles of miRNAs in the development and death of cells in aging and age-related diseases. Such new investigations can help to identify new targets for alternative strategies in order to treat or prevent diseases such as cancers and cognitive decline. The discovery of specific miRNAs will lead to find new biomarkers for screening and diagnosis of diseases as well as exploring of new therapeutic applications in numerous diseases in the future.
Availability of data and materials
Not applicable.
Abbreviations
- miRNAs:
-
MicroRNAs
- UTRs:
-
MRNAs targeting untranslated regions
- miRISC:
-
MiRNA-inducing silencing complex
- RISC:
-
RNA-induced silencing complex
- mRNAs:
-
Messenger RNAs
- ATP:
-
Adenosine Triphosphate
- ROS:
-
Reactive oxygen species
- SAM:
-
Sympathetic-adrenal-medullary
- HPA:
-
Hypothalamic–pituitary–adrenal
- HIF-1α:
-
Hypoxia-inducible factor-1 alpha
- IGF-1:
-
Insulin/insulin-like growth factor
- PI3K:
-
Phosphoinositide 3-kinase
- TOR:
-
Target of rapamycin
- MAPK:
-
Mitogen-activated protein kinase
- AMPK:
-
AMP-dependent protein kinase
- PKC:
-
Protein kinase C signaling pathway
- NF-ĸb:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- TGF-β:
-
Transforming growth factor beta
- wingless-related integration site:
-
WNT signaling pathway
- c-Kit:
-
Notch signaling pathway, receptor tyrosine kinase
- H2AX:
-
H2A histone family member X
- MCI:
-
Mild cognitive impairment
- Aβ peptides:
-
β-Amyloid
- APP:
-
Amyloid-beta precursor protein
- TGFBI:
-
Transforming growth factor-beta-induced protein
- TRIM2:
-
Tripartite Motif Containing protein 2
- BTBD3:
-
BTB domain containing protein 3
- CCND1:
-
Cyclin D1
- WNT3A:
-
Wnt family member 3A
- BCL2:
-
B-cell lymphoma 2
References
Van Rooij E (2011) The art of microRNA research. Circ Res 108(2):219–234
Jung HJ, Suh Y (2012) MicroRNA in aging: from discovery to biology. Curr Genomics 13(7):548
Lou S, Sun T, Li H, Hu Z (2018) Mechanisms of microRNA-mediated gene regulation in unicellular model alga Chlamydomonas reinhardtii. Biotechnol Biofuels 11(1):1–12
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402
Ahmad J, Hasnain SE, Siddiqui MA, Ahamed M, Musarrat J, Al-Khedhairy AA (2013) MicroRNA in carcinogenesis & cancer diagnostics: A new paradigm. Indian J Med Res 137(4):680
Bicchi I, Morena F, Montesano S, Polidoro M, Martino S (2013) MicroRNAs and molecular mechanisms of neurodegeneration. Genes 4(2):244–263
Alberti C, Cochella L (2017) A framework for understanding the roles of miRNAs in animal development. Development 144(14):2548–2559
Schraml E, Grillari J (2012) From cellular senescence to age-associated diseases: the miRNA connection. Longevity Healthspan 1(10):256
Fehlmann T, Lehallier B, Schaum N, Hahn O, Kahraman M, Li Y et al (2020) Common diseases alter the physiological age-related blood microRNA profile. Nat Commun 11(1):1–14
ElSharawy A, Keller A, Flachsbart F, Wendschlag A, Jacobs G, Kefer N et al (2012) Genome-wide miRNA signatures of human longevity. Aging Cell 11(4):607–616
Umansky S (2018) Aging and aging-associated diseases: a microRNA-based endocrine regulation hypothesis. Aging (Albany NY) 10(10):2557
Biggar KK, Storey KB (2018) Functional impact of microRNA regulation in models of extreme stress adaptation. J Mol Cell Biol 10(2):93–101
Almeida MI, Reis RM, Calin GA (2011) MicroRNA history: discovery, recent applications, and next frontiers. Mutat Res Fundam Mol Mech Mutagen 717(1):1–8
Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR (2012) Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging (Albany NY) 4(9):590
Ai J, Sun L-H, Che H, Zhang R, Zhang T-Z, Wu W-C et al (2013) MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J Neurosci 33(9):3989–4001
Holohan KN, Lahiri DK, Schneider BP, Foroud TM, Saykin AJ (2013) Functional microRNAs in Alzheimer’s disease and cancer: differential regulation of common mechanisms and pathways. Front Genet 3:323
Nidadavolu LS, Niedernhofer LJ, Khan SA (2013) Identification of microRNAs dysregulated in cellular senescence driven by endogenous genotoxic stress. Aging (Albany NY) 5(6):460
Wiegand C, Savelsbergh A, Heusser P (2017) MicroRNAs in psychological stress reactions and their use as stress-associated biomarkers, especially in human saliva. Biomed Hub 2(3):1–15
Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R et al (2019) Circulating microRNAs in cancer: potential and challenge. Front Genet 10:626
Leung AK, Sharp PA (2010) MicroRNA functions in stress responses. Mol Cell 40(2):205–215
Hooten NN, Fitzpatrick M, Wood WH 3rd, De S, Ejiogu N, Zhang Y et al (2013) Age-related changes in microRNA levels in serum. Aging (Albany NY) 5(10):725
Tan W, Liu B, Qu S, Liang G, Luo W, Gong C (2018) MicroRNAs and cancer: Key paradigms in molecular therapy. Oncol Lett 15(3):2735–2742
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11(9):597–610
Olejniczak M, Kotowska-Zimmer A, Krzyzosiak W (2018) Stress-induced changes in miRNA biogenesis and functioning. Cell Mol Life Sci 75(2):177–191
Allen L, Dwivedi Y (2020) MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol Psychiatry 25(2):308–320
Faraonio R, Salerno P, Passaro F, Sedia C, Iaccio A, Bellelli R et al (2011) A set of miRNAs participates in the cellular senescence program in human diploid fibroblasts. Cell Death Differ 19(4):713–721
Catalanotto C, Cogoni C, Zardo G (2016) MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci 17(10):1712
Kato M, Slack FJ (2008) microRNAs: small molecules with big roles-C. Elegans to human cancer. Biol Cell 100(2):71–81
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10(2):126–139
Morales S, Monzo M, Navarro A (2017) Epigenetic regulation mechanisms of microRNA expression. Biomol Concepts 8(5–6):203–212
Shin K-H, Pucar A, Kim RH, Bae SD, Chen W, Kang MK et al (2011) Identification of senescence-inducing microRNAs in normal human keratinocytes. Int J Oncol 39(5):1205
Eulalio A, Huntzinger E, Izaurralde E (2008) Getting to the root of miRNA-mediated gene silencing. Cell 132(1):9–14
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136(4):642–655
Wang J, Sen S (2011) MicroRNA functional network in pancreatic cancer: from biology to biomarkers of disease. J Biosci 36(3):481–491
Wang Z, Yao H, Lin S, Zhu X, Shen Z, Lu G et al (2013) Transcriptional and epigenetic regulation of human microRNAs. Cancer Lett 331(1):1–10
Mitchelson KR, Qin W-Y (2015) Roles of the canonical myomiRs miR-1,-133 and-206 in cell development and disease. World J Biol Chem 6(3):162
Liu C, Zhang S, Wang Q, Zhang X (2017) Tumor suppressor miR-1 inhibits tumor growth and metastasis by simultaneously targeting multiple genes. Oncotarget 8(26):42043
Ma J-C, Duan M-J, Sun L-L, Yan M-L, Liu T, Wang Q et al (2015) Cardiac over-expression of microRNA-1 induces impairment of cognition in mice. Neuroscience 299:66–78
Lin X, Zhan J-K, Wang Y-J, Tan P, Chen Y-Y, Deng H-Q et al (2016) Function, role, and clinical application of microRNAs in vascular aging. BioMed Res Int 2016:145
Jia B, Dao J, Han J, Huang Z, Sun X, Zheng X et al (2021) LINC00958 promotes the proliferation of TSCC via miR-211-5p/CENPK axis and activating the JAK/STAT3 signaling pathway. Cancer Cell Int 21(1):1–16
Chirshev E, Oberg KC, Ioffe YJ, Unternaehrer JJ (2019) Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin Transl Med 8(1):1–14
Derkow K, Rössling R, Schipke C, Krüger C, Bauer J, Fähling M et al (2018) Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’s disease. PLoS ONE 13(7):e0200602
Suh N (2018) MicroRNA controls of cellular senescence. BMB Rep 51(10):493
Chen X, Yang F, Zhang T, Wang W, Xi W, Li Y et al (2019) MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J Exp Clin Cancer Res 38(1):1–16
Basavaraju M, De Lencastre A (2016) Alzheimer’s disease: presence and role of microRNAs. Biomol Concepts 7(4):241–252
An X, Sarmiento C, Tan T, Zhu H (2017) Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm Sin B 7(1):38–51
Gerasymchuk M, Cherkasova V, Kovalchuk O, Kovalchuk I (2020) The role of microRNAs in organismal and skin aging. Int J Mol Sci 21(15):5281
Kou X, Chen D, Chen N (2020) The regulation of microRNAs in Alzheimer’s disease. Front Neurol 11:154
Gong B, Liu W-W, Nie W-J, Li D-F, Xie Z-J, Liu C et al (2015) MiR-21/RASA1 axis affects malignancy of colon cancer cells via RAS pathways. World J Gastroenterol WJG 21(5):1488
Rusanova I, Diaz-Casado ME, Fernández-Ortiz M, Aranda-Martínez P, Guerra-Librero A, García-García FJ et al (2018) Analysis of plasma MicroRNAs as predictors and biomarkers of aging and frailty in humans. Oxid Med Cell Long 2018:1547
Amakiri N, Kubosumi A, Tran J, Reddy PH (2019) Amyloid beta and microRNAs in Alzheimer’s disease. Front Neurosci 13:430
Swarbrick S, Wragg N, Ghosh S, Stolzing A (2019) Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol Neurobiol 56(9):6156–6167
Hébert SS, Horré K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN et al (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci 105(17):6415–6420
Wang Y, Chang W, Chang W, Chang X, Zhai S, Pan G et al (2018) MicroRNA-376c-3p facilitates human hepatocellular carcinoma progression via repressing AT-rich interaction domain 2. J Cancer 9(22):4187
Zhao W, Cheng L, Quek C, Bellingham SA, Hill AF (2019) Novel miR-29b target regulation patterns are revealed in two different cell lines. Sci Rep 9(1):1–17
Xia T, Dong S, Tian J (2020) miR-29b promotes the osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue via the PTEN/AKT/β-catenin signaling pathway. Int J Mol Med 46(2):709–717
Tabak S, Schreiber-Avissar S, Beit-Yannai E (2021) Crosstalk between MicroRNA and oxidative stress in primary open-angle glaucoma. Int J Mol Sci 22(5):2421
Jiang L-H, Zhang H-D, Tang J-H (2018) MiR-30a: a novel biomarker and potential therapeutic target for cancer. J Oncol 2018:894
Rege SD, Geetha T, Pondugula SR, Zizza CA, Wernette CM, Ramesh Babu J (2013) Noncoding RNAs in neurodegenerative diseases. Int Scholarly Res Not 2013:256
Forterre A, Komuro H, Aminova S, Harada M (2020) A comprehensive review of cancer MicroRNA therapeutic delivery strategies. Cancers 12(7):1852
Yu T, Ma P, Wu D, Shu Y, Gao W (2018) Functions and mechanisms of microRNA-31 in human cancers. Biomed Pharmacother 108:1162–1169
Barros-Viegas AT, Carmona V, Ferreiro E, Guedes J, Cardoso AM, Cunha P et al (2020) miRNA-31 improves cognition and abolishes amyloid-β pathology by targeting APP and BACE1 in an animal model of Alzheimer’s disease. Mol Therapy Nucleic Acids 19:1219–1236
Cho J-H, Dimri M, Dimri GP (2015) MicroRNA-31 is a transcriptional target of histone deacetylase inhibitors and a regulator of cellular senescence. J Biol Chem 290(16):10555–10567
Rokavec M, Li H, Jiang L, Hermeking H (2014) The p53/miR-34 axis in development and disease. J Mol Cell Biol 6(3):214–230
Qiang J, Zhu X-W, He J, Tao Y-F, Bao J-W, Zhu J-H et al (2020) miR-34a regulates the activity of HIF-1a and P53 signaling pathways by promoting GLUT1 in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) under hypoxia stress. Front Physiol 11:1245
Ruediger C, Shapira M (2019) mir-71 mediates age-dependent opposing contributions of the stress activated kinase KGB-1 in Caenorhabditis elegans. bioRxiv. 835355
Zhang X, Zabinsky R, Teng Y, Cui M, Han M (2011) microRNAs play critical roles in the survival and recovery of Caenorhabditis elegans from starvation-induced L1 diapause. Proc Natl Acad Sci 108(44):17997–18002
Finger F, Ottens F, Springhorn A, Drexel T, Proksch L, Metz S et al (2019) Olfaction regulates organismal proteostasis and longevity via microRNA-dependent signalling. Nat Metab 1(3):350–359
Blick C, Ramachandran A, Wigfield S, McCormick R, Jubb A, Buffa F et al (2013) Hypoxia regulates FGFR3 expression via HIF-1α and miR-100 and contributes to cell survival in non-muscle invasive bladder cancer. Br J Cancer 109(1):50–59
Kumar S, Reddy PH (2016) Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim Biophys Acta BBA Mol Basis Dis 1862(9):1617–1627
Zhang Z-C, Liu J-X, Shao Z-W, Pu F-F, Wang B-C, Wu Q et al (2017) In vitro effect of microRNA-107 targeting Dkk-1 by regulation of Wnt/β-catenin signaling pathway in osteosarcoma. Medicine 96(27):584
Jiao S, Liu Y, Yao Y, Teng J (2017) miR-124 promotes proliferation and differentiation of neuronal stem cells through inactivating Notch pathway. Cell Biosci 7(1):1–12
Sun H-Y, Huang Y-H, Yu Z-H, Qin Q-W (2020) MiR-124 involvement of apoptosis, immunity and regulator of diseases. Afr J Biotech 19(3):142–147
Wang Y, Zeng G, Jiang Y (2020) The emerging roles of miR-125b in cancers. Cancer Manag Res 12:1079
Hadar A, Milanesi E, Walczak M, Puzianowska-Kuźnicka M, Kuźnicki J, Squassina A et al (2018) SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s disease. Sci Rep 8(1):1–10
El Fatimy R, Li S, Chen Z, Mushannen T, Gongala S, Wei Z et al (2018) MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol 136(4):537–555
Mziaut H, Henniger G, Ganss K, Hempel S, Wolk S, McChord J et al (2017) Mir-132 controls beta cell proliferation and survival in mouse model
You J, Li Y, Fang N, Liu B, Zu L, Chang R et al (2014) MiR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS ONE 9(3):e91827
Mahmoudi E, Cairns M (2017) MiR-137: an important player in neural development and neoplastic transformation. Mol Psychiatry 22(1):44–55
Tian R, Wu B, Fu C, Guo K (2020) miR-137 prevents inflammatory response, oxidative stress, neuronal injury and cognitive impairment via blockade of Src-mediated MAPK signaling pathway in ischemic stroke. Aging (Albany NY) 12(11):10873
Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM (2006) MicroRNA expression and function in cancer. Trends Mol Med 12(12):580–587
McGarty TP (2014) Notch pathway, MiR‐146a and melanoma
Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D et al (2013) Micro RNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 5(7):1017–1034
Ye E-A, Steinle JJ (2016) miR-146a attenuates inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediat Inflam 2016:1487
Wani JA, Majid S, Khan A, Arafah A, Ahmad A, Jan BL et al (2021) Clinico-pathological importance of miR-146a in lung cancer. Diagnostics 11(2):274
Czyzyk-Krzeska M, Zhang X (2014) MiR-155 at the heart of oncogenic pathways. Oncogene 33(6):677–678
Mashima R (2015) Physiological roles of miR-155. Immunology 145(3):323–333
Han B, Wang S, Zhao H (2020) MicroRNA-21 and microRNA-155 promote the progression of Burkitt’s lymphoma by the PI3K/AKT signaling pathway. Int J Clin Exp Pathol 13(1):89
Readhead B, Haure-Mirande J-V, Mastroeni D, Audrain M, Fanutza T, Kim SH et al (2020) miR155 regulation of behavior, neuropathology, and cortical transcriptomics in Alzheimer’s disease. Acta Neuropathol 140(3):295–315
Soliman B, Salem A, Ghazy M, Abu-Shahba N, El Hefnawi M (2018) Bioinformatics functional analysis of let-7a, miR-34a, and miR-199a/b reveals novel insights into immune system pathways and cancer hallmarks for hepatocellular carcinoma. Tumor Biol 40(5):1010428318773675
Wu L, Xi Y, Kong Q (2020) Dexmedetomidine protects PC12 cells from oxidative damage through regulation of miR-199a/HIF-1α. Artif Cells Nanomed Biotechnol 48(1):506–514
Wang Q, Ye B, Wang P, Yao F, Zhang C, Yu G (2019) Overview of microRNA-199a regulation in cancer. Cancer Manag Res 11:10327
Yamakuchi M (2012) Endothelial senescence and microRNA. Biomol Concepts 3(3):213–223
Deng M, Qin Y, Chen X, Wang Q, Wang J (2019) MiR-206 inhibits proliferation, migration, and invasion of gastric cancer cells by targeting the MUC1 gene. Onco Targets Ther 12:849
Liu X, Yang Z, Meng Q, Chen Y, Shao L, Li J et al (2020) Downregulation of microRNA-206 alleviates the sublethal oxidative stress-induced premature senescence and dysfunction in mesenchymal stem cells via targeting alpl. Oxid Med Cell Longev 2020:1236
Bavelloni A, Ramazzotti G, Poli A, Piazzi M, Focaccia E, Blalock W et al (2017) MiRNA-210: a current overview. Anticancer Res 37(12):6511–6521
Bu H, Wedel S, Cavinato M, Jansen-Dürr P (2017) MicroRNA regulation of oxidative stress-induced cellular senescence. Oxid Med Cell Longev 2017:748
Fan C, Wu Q, Ye X, Luo H, Yan D, Xiong Y et al (2016) Role of miR-211 in neuronal differentiation and viability: implications to pathogenesis of Alzheimer’s disease. Front Aging Neurosci 8:166
Chen W, Song J, Bian H, Yang X, Xie X, Zhu Q et al (2020) The functions and targets of miR-212 as a potential biomarker of cancer diagnosis and therapy. J Cell Mol Med 24(4):2392–2401
Ramalinga M, Roy A, Srivastava A, Bhattarai A, Harish V, Suy S et al (2015) MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget 6(33):34446
Zhang N, Lu C, Chen L (2016) miR-217 regulates tumor growth and apoptosis by targeting the MAPK signaling pathway in colorectal cancer. Oncol Lett 12(6):4589–4597
Si W, Shen J, Zheng H, Fan W (2019) The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenet 11(1):1–24
Song Q, An Q, Niu B, Lu X, Zhang N, Cao X (2019) Role of miR-221/222 in tumor development and the underlying mechanism. J Oncol 2019:526
Manzine PR, Pelucchi S, Horst MA, Vale FA, Pavarini SC, Audano M et al (2018) microRNA 221 Targets ADAM10 mRNA and is downregulated in Alzheimer’s disease. J Alzheimers Dis 61(1):113–123
Zeng Q, Zou L, Qian L, Zhou F, Nie H, Yu S et al (2017) Expression of microRNA-222 in serum of patients with Alzheimer’s disease. Mol Med Rep 16(4):5575–5579
Wan C, Wen J, Liang X, Xie Q, Wu W, Wu M et al (2021) Identification of miR-320 family members as potential diagnostic and prognostic biomarkers in myelodysplastic syndromes. Sci Rep 11(1):1–11
Luo L, Yang R, Zhao S, Chen Y, Hong S, Wang K et al (2018) Decreased miR-320 expression is associated with breast cancer progression, cell migration, and invasiveness via targeting Aquaporin 1. Acta Biochim Biophys Sin 50(5):473–480
Hatse S, Brouwers B, Dalmasso B, Laenen A, Kenis C, Schöffski P et al (2014) Circulating MicroRNAs as easy-to-measure aging biomarkers in older breast cancer patients: correlation with chronological age but not with fitness/frailty status. PLoS ONE 9(10):e110644
Martinez-Gutierrez AD, Cantú de León D, Millan-Catalan O, Coronel-Hernandez J, Campos-Parra AD, Porras-Reyes F et al (2020) Identification of miRNA master regulators in breast cancer. Cells 9(7):1610
Huang Z, Xu Y, Wan M, Zeng X, Wu J (2021) miR-340: a multifunctional role in human malignant diseases. Int J Biol Sci 17(1):236
Sahu N, Stephan J-P, Cruz DD, Merchant M, Haley B, Bourgon R et al (2016) Functional screening implicates miR-371-3p and peroxiredoxin 6 in reversible tolerance to cancer drugs. Nat Commun 7(1):1–10
Ji R, Zhang X, Gu H, Ma J, Wen X, Zhou J et al (2019) miR-374a-5p: A new target for diagnosis and drug resistance therapy in gastric cancer. Mol Therapy-Nucleic Acids 18:320–331
Bian H, Zhou Y, Zhou D, Zhang Y, Shang D, Qi J (2019) The latest progress on miR-374 and its functional implications in physiological and pathological processes. J Cell Mol Med 23(5):3063–3076
Unterbruner K, Matthes F, Schilling J, Nalavade R, Weber S, Winter J et al (2018) MicroRNAs miR-19, miR-340, miR-374 and miR-542 regulate MID1 protein expression. PLoS ONE 13(1):e0190437
Ekowati AL, Pramono ZAD, Soeselo DA, Budiyanto A, Astuti I, Mubarika S (2019) hsa-miR-376c-3p in the circulating plasma is upregulated in the elderly javanese male when compared to their younger counterparts. Indonesian Biomed J 11(3):304–313
Cao X, Zhang J, Apaer S, Yao G, Li T (2021) microRNA-19a-3p and microRNA-376c-3p promote hepatocellular carcinoma progression through SOX6-mediated Wnt/β-catenin signaling pathway. Int J Gener Med 14:89
Wang L-L, Min L, Guo Q-D, Zhang J-X, Jiang H-L, Shao S et al (2017) Profiling microRNA from brain by microarray in a transgenic mouse model of Alzheimer’s disease. BioMed Res Int 2017:1254
Zhou W, Yang W, Ma J, Zhang H, Li Z, Zhang L et al (2018) Role of miR-483 in digestive tract cancers: from basic research to clinical value. J Cancer 9(2):407
Wingo TS, Yang J, Fan W, Canon SM, Gerasimov ES, Lori A et al (2020) Brain microRNAs associated with late-life depressive symptoms are also associated with cognitive trajectory and dementia. NPJ Genom Med 5(1):1–8
Holubekova V, Kolkova Z, Grendar M, Brany D, Dvorska D, Stastny I et al (2020) Pathway analysis of selected circulating miRNAs in plasma of breast cancer patients: a preliminary study. Int J Mol Sci 21(19):7288
Lee D, Tang W, Dorsey TH, Ambs S (2020) miR-484 is associated with disease recurrence and promotes migration in prostate cancer. Biosci Rep 40(5):124
Yu C, Tian F, Liu J, Su M, Wu M, Zhu X et al (2019) Circular RNA cMras inhibits lung adenocarcinoma progression via modulating miR-567/PTPRG regulatory pathway. Cell Prolif 52(3):e12610
Bertoli G, Cava C, Diceglie C, Martelli C, Rizzo G, Piccotti F et al (2017) MicroRNA-567 dysregulation contributes to carcinogenesis of breast cancer, targeting tumor cell proliferation, and migration. Breast Cancer Res Treat 161(3):605–616
Han M, Hu J, Lu P, Cao H, Yu C, Li X et al (2020) Exosome-transmitted miR-567 reverses trastuzumab resistance by inhibiting ATG5 in breast cancer. Cell Death Dis 11(1):1–15
Elkady MA, Doghish AS, Elshafei A, Elshafey MM (2021) MicroRNA-567 inhibits cell proliferation and induces cell apoptosis in A549 NSCLC cells by regulating cyclin-dependent kinase 8. Saudi J Biol Sci 28(4):2581–2590
Zhang F, Li K, Yao X, Wang H, Li W, Wu J et al (2019) A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine 44:311–321
De Felice B, Montanino C, Oliva M, Bonavita S, Di Onofrio V, Coppola C (2020) MicroRNA expression signature in mild cognitive impairment due to Alzheimer’s disease. Mol Neurobiol 57(11):4408–4416
Smith-Vikos T, Liu Z, Parsons C, Gorospe M, Ferrucci L, Gill TM et al (2016) A serum miRNA profile of human longevity: findings from the Baltimore Longitudinal Study of Aging (BLSA). Aging (Albany NY) 8(11):2971
Urh K, Žlajpah M, Zidar N, Boštjančič E (2021) Identification and validation of new cancer stem cell-related genes and their regulatory microRNAs in colorectal cancerogenesis. Biomedicines 9(2):179
Gong F, Hou G, Liu H, Zhang M (2015) Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med Oncol 32(2):25
Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan S-J et al (2018) Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via inhibition of miR-5095. Biochem Biophys Res Commun 496(2):712–718
Wu Y, Qian Z (2019) Long non-coding RNAs (lncRNAs) and microRNAs regulatory pathways in the tumorigenesis and pathogenesis of glioma. Discov Med 28(153):129–138
Lan X, Liu X, Sun J, Yuan Q, Li J (2019) CircRAD23B facilitates proliferation and invasion of esophageal cancer cells by sponging miR-5095. Biochem Biophys Res Commun 516(2):357–364
Funikov SY, Zatcepina O (2017) Regulation of microRNA activity in stress. Mol Biol 51(4):496–505
Kültz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
Buchberger A, Bukau B, Sommer T (2010) Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40(2):238–252
Richter K, Haslbeck M, Buchner J (2010) Life on the verge of death: the heat shock response revisited. Mol Cell 40:253–266
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482
Spriggs KA, Bushell M, Willis AE (2010) Translational regulation of gene expression during conditions of cell stress. Mol Cell 40(2):228–237
Eshkoor S, Ismail P, Rahman S, Moin S (2011) Does GSTP1 polymorphism contribute to genetic damage caused by ageing and occupational exposure? Arch Ind Hyg Toxicol 62(4):291–298
Eshkoor SA, Ismail P, Rahman SA, Moin S, Adon MY (2012) The Association of DNA damage level with early age at the occupational exposure in the mechanical workshops workers. Asian J Biotechnol 4(2):83–91
Eshkoor S, Jahanshiri F, Ismail P, Rahman S, Moin S, Adon M (2012) Association between telomere shortening and ageing during occupational exposure. J Med Biochem 31(3):211–216
Ong J, Woldhuis RR, Boudewijn IM, van den Berg A, Kluiver J, Kok K et al (2019) Age-related gene and miRNA expression changes in airways of healthy individuals. Sci Rep 9(1):1–8
Campisi J, di Fagagna FA (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9(2):81–94
Wang E (1997) Regulation of apoptosis resistance and ontogeny of age-dependent diseases. Exp Gerontol 32(4):471–484
Hampel B, Malisan F, Niederegger H, Testi R, Jansen-Dürr P (2004) Differential regulation of apoptotic cell death in senescent human cells. Exp Gerontol 39(11):1713–1721
Krtolica A, Campisi J (2002) Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol 34(11):1401–1414
Rodier F, Campisi J, Bhaumik D (2007) Two faces of p53: aging and tumor suppression. Nucleic Acids Res 35(22):7475–7484
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192(4):547–556
Boehm M, Slack F (2005) A developmental timing microRNA and its target regulate life span in C. elegans. Science 310(5756):1954–1957
Smith-Vikos T, Slack FJ (2012) MicroRNAs and their roles in aging. J Cell Sci 125(1):7–17
Huan T, Chen G, Liu C, Bhattacharya A, Rong J, Chen BH et al (2018) Age-associated micro RNA expression in human peripheral blood is associated with all-cause mortality and age-related traits. Aging Cell 17(1):e12687
Brooks-Wilson AR (2013) Genetics of healthy aging and longevity. Hum Genet 132(12):1323–1338
Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P et al (2011) microRNA-34c is a novel target to treat dementias. EMBO J 30(20):4299–4308
Deraredj Nadim W, Simion V, Bénédetti H, Pichon C, Baril P, Morisset-Lopez S (2017) MicroRNAs in neurocognitive dysfunctions: new molecular targets for pharmacological treatments? Curr Neuropharmacol 15(2):260–275
Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S et al (2005) MicroRNAs regulate brain morphogenesis in zebrafish. Science 308(5723):833–838
Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E et al (2008) Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci 105(14):5614–5619
Im H-I, Hollander JA, Bali P, Kenny PJ (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13(9):1120–1127
Konopka W, Schütz G, Kaczmarek L (2011) The microRNA contribution to learning and memory. Neuroscientist 1073858411411721
Qiu C, Kivipelto M, von Strauss E (2009) Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 11(2):111
Shi D, Han M, Liu W, Tao J, Chen L (2020) Circulating MicroRNAs as diagnostic biomarkers of clinical cognitive impairment: a meta-analysis. Am J Alzheimer’s Dis Other Dement 35:1533317520951686
Eshkoor SA, Hamid TA, Mun CY, Ng CK (2015) Mild cognitive impairment and its management in older people. Clin Interv Aging 10:687
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12(9):735–739
Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM et al (2004) Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci 101(1):360–365
Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH (2006) Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci 29:77–103
Kosik KS (2006) The neuronal microRNA system. Nat Rev Neurosci 7(12):911–920
Hansen K (2012) miRNA-132’s role as a dynamic regulator of memory
Schonrock N, Humphreys DT, Preiss T, Götz J (2012) Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci 46(2):324–335
Malmevik J, Petri R, Knauff P, Brattås PL, Åkerblom M, Jakobsson J (2016) Distinct cognitive effects and underlying transcriptome changes upon inhibition of individual miRNAs in hippocampal neurons. Sci Rep 6(1):1–14
Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D et al (2020) miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells 9(2):276
Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20(8):460–469
McManus MT (2003) MicroRNAs and cancer. Semin Cancer Biol 13(4):253–258
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6(4):259–269
Rothschild SI (2014) microRNA therapies in cancer. Mol Cell Ther 2(1):7
Balacescu O, Visan S, Baldasici O, Balacescu L, Vlad C, Achimas-Cadariu P (2018) MiRNA-based therapeutics in oncology, realities, and challenges. Antisense Therapy
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 101(9):2999–3004
Tili E, Michaille J-J, Cimino A, Costinean S, Dumitru CD, Adair B et al (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179(8):5082–5089
Jiang S, Zhang H-W, Lu M-H, He X-H, Li Y, Gu H et al (2010) MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Can Res 70(8):3119–3127
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M et al (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 102(39):13944–13949
Iorio MV, Piovan C, Croce CM (2010) Interplay between microRNAs and the epigenetic machinery: an intricate network. Biochim Biophys Acta BBA Gene Regul Mech 1799(10):694–701
Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM (2006) Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev 20(16):2202–2207
Chang T-C, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH et al (2007) Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26(5):745–752
Han C, Yu Z, Duan Z, Kan Q (2014) Role of microRNA-1 in human cancer and its therapeutic potentials. BioMed Res Int 2014:875
Funding
There is no funding source for supporting this article.
Author information
Authors and Affiliations
Contributions
SAE involved in contributions to the conception, design of the work, and drafted the work or substantively revising. NG involved in contributions to drafting the article and revising. MA-Z involved in contributions to drafting the article and revising. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There is no report on or involving the use of any animal or human data or tissue in this review paper; therefore, ethics approval is not applicable to our paper. This article is just based on the reviewing of the other published articles with giving references to all texts correctly.
Consent for publication
All authors give consent for the publication of all details in this article including graphs, tables and details within the text for being published in the journal of Egyptian Journal of Medical Human Genetics.
Competing interests
All authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eshkoor, S.A., Ghodsian, N. & Akhtari-Zavare, M. MicroRNAs influence and longevity. Egypt J Med Hum Genet 23, 105 (2022). https://doi.org/10.1186/s43042-022-00316-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00316-7