Abstract
Background
Surgical transepicondylar axis (sTEA) is frequently used for positioning of femoral component rotation in total knee arthroplasty (TKA). Previous studies showed that intraoperative identification of sTEA was not reliable. While surgeons or engineers need to identify sTEA with three-dimensional (3D) computer-aid techniques pre- or intraoperatively, the reproducibility of sTEA identification on preoperative 3D images has not been explored yet. This study aimed to investigate the reproducibility of identifying sTEA in preoperative planning based on computed tomography (CT).
Methods
Fifty-nine consecutive patients (60 knees involved) who received TKA in our center from April 2019 to June 2019 were included in this study. Six experienced TKA surgeons identified sTEA three times on 3D model established on the basis of knee CT data. The projection angle of each sTEA and the posterior condyle axis on the transverse plane were measured and analyzed.
Results
The overall intra-observer reproducibility was moderate. The median intra-observer variation was 1.27°, with a maximum being up to 14.07°. The median inter-observer variation was 1.24°, and the maximum was 11.47°. The overall intra-class correlation coefficient (ICC) for inter-observer was 0.528 (95% CI 0.417, 0.643).
Conclusion
The identification of sTEA on a 3D model established on the basis of knee CT data may not be reliable. Combined with the previous cadaveric and surgical studies, caution should be exercised in determining femoral component rotation by referencing sTEA both preoperatively and intraoperatively.
Level of evidence
III
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Total knee arthroplasty (TKA) is a successful procedure to manage late-stage knee osteoarthritis. Multiple factors may influence the clinical results of TKA. Equal extension and flexion gap is one of the key objectives of TKA to achieve satisfactory soft tissue balance. Femoral component malrotation could be one of the major causes of unbalanced flexion gap. It may cause pain, knee stiffness, patellofemoral mal-tracking and reduced implant longevity after TKA [1,2,3,4,5,6,7]. The references for femoral component rotation include: posterior condyle axis [8], Whiteside's line [9], transepicondylar axis [10], sulcus line [11], and tibia osteotomy platform [12]. Among all the commonly used references for measured resection technique, surgical transepicondylar axis (sTEA) is deemed the best flexion-extension axis of knee joint [10, 13,14,15,16,17], starting from the most prominent point of the lateral femoral epicondyle to the most concave point of the medial femoral epicondyle [10, 14].
However, it is somehow difficult to accurately identify sTEA during operation by palpating bony prominences, due to poor vision, soft tissue coverage, obscure bony landmarks, etc. [18,19,20,21]. The individual differences between operators in locating sTEA are obvious [18,19,20, 22,23,24]. In order to achieve better femoral component rotation and flexion gap, more precise measurement is needed to locate femoral rotation axis based on sTEA methodology. With wider application of navigation, patient-specific instrumentation (PSI), robotics or other computer-assisted surgical techniques [25,26,27,28], sTEA could be identified by surgeons or engineers during preoperative planning with assistance of three-dimensional (3D) images. Intraoperative femoral rotation osteotomy could be guided by robot, PSI or navigation after identification of sTEA preoperatively or intraoperatively [29]. Even without these techniques, the divergence between sTEA and posterior condylar axis could be measured on a preoperative 3D model based on computed tomography (CT) data and serve to orientate sTEA as a reference during surgery [27].
However, identifying sTEA on a 3D model does not go further than defining sTEA by recognizing bony prominences. Although most soft tissue signals were removed from 3D CT images, the prominence, as a landmark, may not be reproducible to draw a unique sTEA. The purpose of this study was to investigate the intra- and inter-observer reproducibility for identification of the sTEA on 3D images. Previous studies on reproducibility of sTEA were carried out on cadeveric specimens or patients' knees [18,19,20, 22,23,24]. To our knowledge, this was the first attempt to explore the accuracy and reproducibility of sTEA measurement on 3D images. We hypothesized that the reproducibility of sTEA identification on 3D image remains too poor to make 3D sTEA a reliable landmark for attaining precise femoral rotation and flexion gap.
Materials and methods
Preoperative femoral CT data were retrospectively collected from a series of consecutive TKAs at our center from April 2019 to June 2019. Patients with any bony deformities that affected recognition of femoral bony prominence and sTEA positioning were excluded, such as previous fractures, severe osteophytes and any extra-articular deformities. Finally, 59 consecutive patients involving 60 knees were included in the study. There were 9 males and 50 females, with 29 left and 31 right knees included. Mean age at the time of surgery was 67.7 years ± 8.2 years (range, 50 to 86). Mean height was 156.9 cm ± 6.2 cm (range, 145 to 172) and mean weight was 62.8 Kg ± 9.9 Kg (range, 40 to 90), with a mean body mass index of 25.5 Kg/m2 ± 3.9 Kg/m2 (range, 16.4 to 35.2).
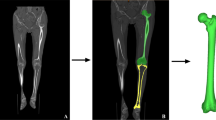
A 3D reconstruction was conducted on the basis of preoperative femoral CT data (thinner scan of 1 mm around knee and thicker scan of 3 mm for the rest parts) by employing Mimics Research 19.0 (Materialise NV, Belgium). The best spherical fitting of femoral head was performed with CATIA 5.20 (Dassault System, France), and the line between the obtained center point and the apex of femoral intercondylar notch was defined as the mechanical axis of femur; the plane perpendicular to the femoral mechanical axis was recorded as the transverse plane; the tangent line to the most posterior part of the femoral condyles was defined as the posterior condyle axis [17, 27]. Six experienced TKA surgeons identified sTEA three times independently on axial, coronal, sagittal, and 3D views [27, 30] (Fig. 1), with an interval of more than 15 days. Each identification was based on the initial model to ensure that there was no interference from other marker information.
A total of 18 sTEA were marked for each 3D model of the knee joint (Fig. 2). Unigraphics NX 12.0 (Siemens PLM Software, USA) was used to measure projection angle of each sTEA and its corresponding posterior condyle axis on the transverse plane, which was denoted as posterior condylar angle (PCA). Since there was only one fixed posterior condyle axis in each model, the variation of PCA was only related to precision errors on the sTEA. Relative to the posterior condyle axis, sTEA external rotation would record PCA as positive and internal negative (Fig. 3).
To evaluate intra-observer reproducibility, intra-class correlation coefficients (ICC) and their 95% confident intervals (CI) were calculated based on a two-way mixed, absolute agreement, single-measure model. For inter-observer reproducibility, two-way random, absolute agreement, single-measure model was utilized [31]. And ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 were indicative of poor, moderate, good and excellent reproducibility, respectively [31]. Variables with normal distribution were expressed as \(\overline{x}\pm s\), variables with high skew were presented as median and interquartile range (IQR). Statistical analyses were performed by using SPSS 25.0 (SPSS Inc, Chicago, IL).
This study has been approved by the local Ethics Committee (KY2020057).
Results
Intra-observer ICCs were 0.720, 0.516, 0.652, 0.717, 0.548, 0.503, respectively. Overall intra-observer reproducibility was moderate, standing somewhere between 0.5 and 0.75. Median (IQR) of overall intra-surgeon variation was 1.27° (0.52°, 2.41°), with a maximum of up to 14.07° (Table 1). Median (IQR) of inter-surgeon variation was 1.24° (0.55°, 2.37°), and the maximum was 11.47°. Overall ICC for inter-surgeon was 0.528 (95%CI 0.417, 0.643) (Table 2). Descriptive statistics of PCA for each knee model are given in the appendix (Appendix 1).
Discussion
The application of computer-assisted techniques in TKA is growing as the importance of bone cut precision in gap-balancing has been increasingly recognized [32]. When a surgeon uses measured resection technique to perform TKA with the assistance of image-based navigation, PSI or robotics, the determination of femoral component rotation plan is relied on the identification of sTEA on the basis of preoperative imaging data (CT or Magnetic Resonance Imaging) [25,26,27,28]. However, previous studies on reproducibility of sTEA were carried out on cadaveric knees or patients’ knees during TKA [18,19,20, 22,23,24]. Stoeckl et al. marked sTEA on cadaveric knees multiple times and acquired a nearly 3 cm2 area on both medial and lateral condyles [23]. Jerosch, Yan and Siston yielded comparable results on cadavers [19, 22, 24]. Jenny et al. identified sTEA on patients' knees during TKA and evaluated the results with navigation. They found that intra-observer deviation was 5° to 6° with a maximum deviation of 15° and the inter-observer deviation was about 9° with a maximum deviation of 15° [18]. Kinzel’s study also arrived at similar conclusion [20]. All these studies came to the same conclusion that identification of sTEA is not reliable on bony prominences. Although the 3D color mapping proposed by Xiang et al. might be a feasible alternative for locating sTEA, it still warrants further clinical verification [33]. Reproducibility of sTEA is poor as a routine reference for TKA regardless of whether sTEA is the true center for femoral rotation.
This study focused, for the first time, on the preoperative reproducibility of sTEA identified on the basis of 3D CT data. It was found that when the same observer identified sTEA at different times, the median variation was 1.27°, with a maximum variation of up to 14.07°. Even if surgeons repeatedly identified sTEA and calculated the average, the median variation among different surgeons was 1.24°, and the maximum was 11.47°. This finding indicated that reproducibility of sTEA based on knee CT data was only moderate in preoperative planning. Many studies showed that PSI could not improve the rotational alignment of femoral implant compared with conventional methods [21, 34,35,36]. And similar conclusions could be drawn for navigation and robotic surgeries [37,38,39,40,41]. The poor reproducibility of sTEA in preoperative or intraoperative 3D planning may be one of the main reasons for the aforementioned phenomenon.
This study also indicated that identifiability of bony anatomical landmarks was positively correlated with the reproducibility of sTEA identification (Appendix 1). Besides, there may exist a possible positive correlation between intra-observers' reproducibility and their experience in TKA, which is worth further study. Only in patients with clear anatomical bony prominence on medial and lateral condyles, techniques, such as image-based navigation, PSI, robotics or personalized 3D preoperative planning, could reproduce the femoral component rotation axis. And the accuracy of sTEA identification relies on a surgeon’s experience. Surgeons should be more cautious when using sTEA before and during TKA. It may be more reliable to refer to multiple reference axes or use gap balancing technique to get the appropriate femoral rotation and flexion gap.
This study has multiple limitations. First, the number of observers and cases for identifying sTEA were limited. Second, this study was a single-center one, and its reproducibility and execution need to be further verified in other centers. Third, further researches are needed to establish the relationship of sTEA reproducibility with bony prominence identifiability and observer experience.
Conclusion
The identification of sTEA on 3D model established on the basis of knee CT data in preoperative planning may not be reliable. Combined with the previous cadaveric and surgical studies, caution should be exercised in determining femoral component rotation by referencing sTEA both preoperatively and intraoperatively.
Availability of data and materials
All data and materials are available on reasonable request.
Abbreviations
- TKA:
-
Total knee arthroplasty
- sTEA:
-
Surgical transepicondylar axis
- PSI:
-
Patient specific instrumentation
- 3D:
-
Three-dimensional
- CT:
-
Computed tomography
- PCA:
-
Posterior condylar angle
- ICC:
-
Intraclass correlation efficient
- CI:
-
Confidence interval
- IQR:
-
Interquartile range
References
Aglietti P, Sensi L, Cuomo P, et al. Rotational position of femoral and tibial components in tka using the femoral transepicondylar axis. Clin Orthop Relat Res. 2008;466(11):2751–5. https://doi.org/10.1007/s11999-008-0452-8.
Barrack RL, Schrader T, Bertot AJ, et al. Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop Relat Res. 2001;392:46–55. https://doi.org/10.1097/00003086-200111000-00006.
Boldt JG, Stiehl JB, Hodler J, et al. Femoral component rotation and arthrofibrosis following mobile-bearing total knee arthroplasty. Int Orthop. 2006;30(5):420–5. https://doi.org/10.1007/s00264-006-0085-z.
Lee JK, Lee S, Chun SH, et al. Rotational alignment of femoral component with different methods in total knee arthroplasty: a randomized, controlled trial. BMC Musculoskelet Disord. 2017;18(1):217. https://doi.org/10.1186/s12891-017-1574-5.
Romero J, Stähelin T, Binkert C, et al. The clinical consequences of flexion gap asymmetry in total knee arthroplasty. J Arthroplast. 2007;22(2):235–40. https://doi.org/10.1016/j.arth.2006.04.024.
Romero J, Stähelin T, Wyss T, et al. Significance of axial rotation alignment of components of knee prostheses. Orthopade. 2003;32(6):461–8. https://doi.org/10.1007/s00132-003-0475-5.
Lee DH, Padhy D, Park JH, et al. The impact of a rectangular or trapezoidal flexion gap on the femoral component rotation in tka. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1141–7. https://doi.org/10.1007/s00167-011-1422-3.
Laskin RS. Flexion space configuration in total knee arthroplasty. J Arthroplast. 1995;10(5):657–60. https://doi.org/10.1016/s0883-5403(05)80211-6.
Whiteside LA, Arima J. The anteroposterior axis for femoral rotational alignment in valgus total knee arthroplasty. Clin Orthop Relat Res. 1995;321:168–72.
Berger RA, Rubash HE, Seel MJ, et al. Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylar axis. Clin Orthop Relat Res. 1993;286:40–7.
Talbot S, Dimitriou P, Mullen M, et al. Referencing the sulcus line of the trochlear groove and removing intraoperative parallax errors improve femoral component rotation in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2743–50. https://doi.org/10.1007/s00167-015-3668-7.
Insall JN, Binazzi R, Soudry M, et al. Total knee arthroplasty. Clin Orthop Relat Res. 1985;192:13–22.
Churchill DL, Incavo SJ, Johnson CC, et al. The transepicondylar axis approximates the optimal flexion axis of the knee. Clin Orthop Relat Res. 1998;356:111–8. https://doi.org/10.1097/00003086-199811000-00016.
Griffin FM, Insall JN, Scuderi GR. The posterior condylar angle in osteoarthritic knees. J Arthroplast. 1998;13(7):812–5. https://doi.org/10.1016/s0883-5403(98)90036-5.
Miller MC, Berger RA, Petrella AJ, et al. Optimizing femoral component rotation in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:38–45. https://doi.org/10.1097/00003086-200111000-00005.
Anouchi YS, Whiteside LA, Kaiser AD, et al. The effects of axial rotational alignment of the femoral component on knee stability and patellar tracking in total knee arthroplasty demonstrated on autopsy specimens. Clin Orthop Relat Res. 1993;287:170–7.
Victor J. Rotational alignment of the distal femur: a literature review. Orthop Traumatol Surg Res. 2009;95(5):365–72. https://doi.org/10.1016/j.otsr.2009.04.011.
Jenny JY, Boeri C. Low reproducibility of the intra-operative measurement of the transepicondylar axis during total knee replacement. Acta Orthop Scand. 2004;75(1):74–7. https://doi.org/10.1080/00016470410001708150.
Jerosch J, Peuker E, Philipps B, et al. Interindividual reproducibility in perioperative rotational alignment of femoral components in knee prosthetic surgery using the transepicondylar axis. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):194–7. https://doi.org/10.1007/s00167-001-0271-x.
Kinzel V, Ledger M, Shakespeare D. Can the epicondylar axis be defined accurately in total knee arthroplasty? Knee. 2005;12(4):293–6. https://doi.org/10.1016/j.knee.2004.09.003.
Parratte S, Blanc G, Boussemart T, et al. Rotation in total knee arthroplasty: no difference between patient-specific and conventional instrumentation. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2213–9. https://doi.org/10.1007/s00167-013-2623-8.
Siston RA, Patel JJ, Goodman SB, et al. The variability of femoral rotational alignment in total knee arthroplasty. J Bone Joint Surg Am. 2005;87(10):2276–80. https://doi.org/10.2106/jbjs.D.02945.
Stoeckl B, Nogler M, Krismer M, et al. Reliability of the transepicondylar axis as an anatomical landmark in total knee arthroplasty. J Arthroplast. 2006;21(6):878–82. https://doi.org/10.1016/j.arth.2005.10.020.
Yan CH, Yau WP, Ng TP, et al. Inter- and intra-observer errors in identifying the transepicondylar axis and whiteside's line. J Orthop Surg (Hong Kong). 2008;16(3):316–20. https://doi.org/10.1177/230949900801600310.
Agarwal N, To K, Mcdonnell S, et al. Clinical and radiological outcomes in robotic-assisted total knee arthroplasty: a systematic review and meta-analysis. J Arthroplast. 2020;35(11):3393–3409.e3392. https://doi.org/10.1016/j.arth.2020.03.005.
Jones CW, Jerabek SA. Current role of computer navigation in total knee arthroplasty. J Arthroplast. 2018;33(7):1989–93. https://doi.org/10.1016/j.arth.2018.01.027.
Lei K, Liu LM, Xiang Y, et al. Clinical value of ct-based patient-specific 3d preoperative design combined with conventional instruments in primary total knee arthroplasty: a propensity score-matched analysis. J Orthop Surg Res. 2020;15(1):591. https://doi.org/10.1186/s13018-020-02123-5.
Lin Y, Cai W, Xu B, et al. Patient-specific or conventional instrumentations: a meta-analysis of randomized controlled trials. Biomed Res Int. 2020;2020:2164371. https://doi.org/10.1155/2020/2164371.
Lei K, Liu L, Chen X, et al. Navigation and robotics improved alignment compared with psi and conventional instrument, while clinical outcomes were similar in tka: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2021. https://doi.org/10.1007/s00167-021-06436-8.
Talbot S, Dimitriou P, Radic R, et al. The sulcus line of the trochlear groove is more accurate than whiteside's line in determining femoral component rotation. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3306–16. https://doi.org/10.1007/s00167-014-3137-8.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63. https://doi.org/10.1016/j.jcm.2016.02.012.
Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet. 2018;392(10158):1672–82. https://doi.org/10.1016/s0140-6736(18)32344-4.
Xiang BY, Wu XD, Zhou N, et al. Three-dimensional color map: a novel tool to locate the surgical transepicondylar axis. Ann Transl Med. 2020;8(21):1401. https://doi.org/10.21037/atm-20-1887.
Kosse NM, Heesterbeek PJC, Schimmel JJP, et al. Stability and alignment do not improve by using patient-specific instrumentation in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2018;26(6):1792–9. https://doi.org/10.1007/s00167-017-4792-3.
Randelli PS, Menon A, Pasqualotto S, et al. Patient-specific instrumentation does not affect rotational alignment of the femoral component and perioperative blood loss in total knee arthroplasty: a prospective, randomized, controlled trial. J Arthroplast. 2019;34(7):1374–1381.e1371. https://doi.org/10.1016/j.arth.2019.03.018.
Thienpont E, Schwab PE, Fennema P. A systematic review and meta-analysis of patient-specific instrumentation for improving alignment of the components in total knee replacement. Bone Joint J. 2014;96-b(8):1052–61. https://doi.org/10.1302/0301-620x.96b8.33747.
Schmitt J, Hauk C, Kienapfel H, et al. Navigation of total knee arthroplasty: rotation of components and clinical results in a prospectively randomized study. BMC Musculoskelet Disord. 2011;12:16. https://doi.org/10.1186/1471-2474-12-16.
Der Linden-Van V, Der Zwaag HM, Bos J, Van Der Heide HJ, et al. A computed tomography based study on rotational alignment accuracy of the femoral component in total knee arthroplasty using computer-assisted orthopaedic surgery. Int Orthop. 2011;35(6):845–50. https://doi.org/10.1007/s00264-010-1082-9.
Oberst M, Bertsch C, Würstlin S, et al. ct analysis of leg alignment after conventional vs. navigated knee prosthesis implantation. Initial results of a controlled, prospective and randomized study. Unfallchirurg. 2003;106(11):941–8. https://doi.org/10.1007/s00113-003-0686-6.
Restrepo C, Hozack WJ, Orozco F, et al. Accuracy of femoral rotational alignment in total knee arthroplasty using computer assisted navigation. Comput Aided Surg. 2008;13(3):167–72. https://doi.org/10.3109/10929080802045640.
Der Linden-Van V, Der Zwaag HM, Valstar ER, Van Der Molen AJ, et al. Transepicondylar axis accuracy in computer assisted knee surgery: a comparison of the ct-based measured axis versus the cas-determined axis. Comput Aided Surg. 2008;13(4):200–6. https://doi.org/10.3109/10929080802240134.
Acknowledgements
We thank Xin Chen, DiShi Yan and XinLiang Zou for their help during this research.
Funding
This study was funded by National Key R&D Program of China (2021YFC2401300). There was no financial conflict of interest with regards to this study.
Author information
Authors and Affiliations
Contributions
K. L., LM. L., JM. L., C. M., L. G. and L. Y. participated in the identification of sTEA. LM. L. and K. L. completed the measurement work. L. G. and K. L. wrote the manuscript. Q. F. completed the statistical analysis. L. G. and L. Y. conceived the idea of the study. All authors contributed to the writing of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the local ethical committee (KY2020057). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
All authors read, approved the final manuscript, and consented to publication. The authors agreed to publish the current work in Arthroplasty and declare that this work is not being concurrently submitted to any other publisher.
Competing interests
L. G. is a member of the Editorial Board of Arthroplasty and other authors declare that they have no competing interests. All authors were not involved in the journal's review of or decisions related to this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Descriptive statistics of PCA for each knee model. The above four dynamic graphics (if dynamic graphics are not moving, please check out videos in Appendix 2, 3, 4 and 5) showed 3D knee model of No. 6, 15, 29 and 56, respectively. It could be found that bony anatomical landmarks of No. 6 and No. 15 were obscurer than those of No. 29 and No. 56, especially in the medial femoral epicondyle, which indicates that identifiability of bony anatomical landmarks is positively correlated with the reproducibility of identifying sTEA.
Additional file 2.
Additional file 3.
Additional file 4.
Additional file 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lei, K., Liu, L.M., Luo, J.M. et al. Could surgical transepicondylar axis be identified accurately in preoperative 3D planning for total knee arthroplasty? A reproducibility study based on 3D-CT. Arthroplasty 4, 46 (2022). https://doi.org/10.1186/s42836-022-00147-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42836-022-00147-2