Abstract
Background
Chitosan and Ca+ are natural signal molecules that can be used in agriculture as biostimulants and elicitors. They enhance different physiological responses and mitigate the negative effects of salinity. So, this investigation was done to study the effect of soaking wheat grains in chitosan and CaCO3 (20 and 40 mg/L) on alleviating the adverse effect of salinity stress (0.0 and 5000 mg/L) on growth, some biochemical and physiological and yields of wheat plant.
Results
Shoot length (cm), leaves no/tiller, shoot dry weight (g), root fresh weight (g) and root dry weight (g) were significantly decreased as a result of salt stress. Soaking wheat grains in Chitosan or CaCO3 significantly promoted plant growth under normal and stressed conditions. Irrigation of wheat plants with saline water significantly decreased photosynthetic pigments (Chlo-a, Chlo-b, carotenoids and total pigments) in addition to Chlo-a/Chlo-b ratio, indole acetic acid content in the plant leaves. Meanwhile, saline water significantly increased phenolics, total soluble sugars (TSS) and proline content. H2O2 and lipid peroxidation expressed by malondialdehyde (MDA) content clearly showed significant increases under salinity stress compared with untreated control. Soaking wheat grains in chitosan or CaCO3 before sawing significantly increased the accumulation of H2O2 and MDA in the leaves of wheat plants. Treatment of wheat grains with chitosan or CaCO3 significantly promoted the activity of various antioxidant enzymes (SOD and POX) as compared to the control. CAT activity was significantly decreased as a result of chitosan or CaCO3 treatments. The highest CAT activity was recorded in plants irrigated with 5000 mg/L saline water followed by control plants which recoded 36.40 and 24.82 U/min/g FW, respectively. On the other hand, irrigation of wheat plants with 5000 mg/L saline water significantly decreased spike length (cm), spikelets no/spike, grains wt/plant (g), 1000-grains wt (g), yield and biomass/plant (g) as well as, carbohydrate % and protein % compared with the control. However, treating wheat plants either with Chitosan or calcium carbonate resulted in obvious significant increases in carbohydrates and protein contents, especially in plants treated with 40 mg/L chitosan followed by 40 mg/L calcium carbonate. Soaking wheat grains in chitosan, especially at 40 mg/L, exhibited the strongest scavenging potential (2,2-diphenyl-1-picryl-hydrazyl-hydrate assay (DPPH%) followed by treatment with 40 mg/L CaCO3.
Conclusion
In conclusion, the used treatment enhanced the protective parameters such as antioxidant enzymes, total phenols and free radical scavengers and consequently helped the plants to decrease lipid peroxidation, increased their tolerance and improved yield and spike quality. Application of 40 mg/L chitosan recorded the highest increment in the scavenging ability of the natural antioxidants of the plant extract toward the stable free radical DPPH.
Similar content being viewed by others
Background
Wheat plant (Triticum aestivum L.) is considered one of the most important crops. Salinity stress is a major limiting factor which negatively affects the growth and production of several crops all over the world. Salinity stress limits plant growth by adversely affecting various physiological and biochemical processes, such as photosynthesis, antioxidant phenomena, nitrogen metabolism, ion homeostasis and osmolyte accumulation (Ashraf 2004). Thus, salinity exerts its undesirable effects through osmotic inhibition and ionic toxicity and by disturbing the uptake and translocation of nutritional ions (Misra and Dwivedi 2004).
Chitosan is a natural biopolymer derived from chitin, a polysaccharide found in exoskeleton of crustaceans, insects as well as cell wall of fungi and some algae (Boonlertnirum et al. 2010). It is low toxic and inexpensive compound that is biodegradable and environmentally friendly with various applications in agriculture. Chitosan has been widely used in agricultural applications mainly for stimulation of plant immunity, to protect plants and food products against microorganisms (bacteria and fungi) (Hadwiger et al. 2002; ChunYan et al. 2003; Devlieghere et al. 2004; Patkowska et al. 2006; No et al. 2007). Also, many efforts were done to study the effect of chitosan on plant growth, development and productivity. A positive effect of chitosan was observed on the growth of roots, shoots and leaves of various plant species. Foliar application of chitosan increased growth and yield in sweet pepper, radish and sunflower (Ghoname et al. 2010; Farouk et al. 2011; Bakhoum et al. 2020). Similar results were also observed in grapevine and strawberry (Gornik et al. 2008; Abdel-Mawgoud et al. 2010). Recently, Sheikha and Al-Malki (2011) indicated that application of different concentrations of chitosan enhanced bean shoot and root length, fresh and dry weights of shoots, root and leaf area. In addition, foliar applications with chitosan resulted in higher vegetative growth and improvement in fruit quality of cucumber (Farouk et al. 2008). For other cultivated plants, Bittelli et al. (2001) reported that foliar application of chitosan decreased transpiration in pepper plants, and reduced water use by 26–43% while maintaining biomass production and yield.
Calcium is a universal signaling molecule and the calcium-sensing (CaS) receptor is of fundamental importance for extracellular calcium signaling and calcium homeostasis (Bouschet et al. 2008). Calcium is an important second messenger in signal transduction pathways (Achary et al. 2013), mediating various defense responses to the action under environmental stresses (Pan et al. 2012). Calcium (Ca2+) is micro and multifunctional element in plants used in different biochemical and physiological processes (Barker and Pilbeam 2007). Calcium is an essential nutrient for growth and development of plants which involved in various important functions as in stability of plant membrane and stabilization of cell wall as well as, increases a huge number of key enzymes activities and interacting with phytohormones (White and Broadley 2003). Moreover, it serves in the signaling network pathways as a secondary messenger under different abiotic stress (Sadak 2016). In addition, calcium appears to play a central role in many defense mechanisms which are induced by stress and calcium signaling is required for the acquisition of stress tolerance or resistance (Cousson 2009).
The aim of this study was to study the effect of chitosan and Ca2+ on growth, some physiological and biochemical aspects and yield of wheat plant irrigated with saline water.
Methods
Experimental procedures
Two pot experiments were conducted at the greenhouse of National Research Centre, Dokki, Cairo, Egypt, at the winter seasons of 2018/2019 and 2019/2020. Grains of wheat Sakha 94 were obtained from Agricultural Research Centre, Giza, Egypt. Chitosan and calcium carbonate used in the present work were supplied from Sigma-Aldrich. Wheat grains were soaked in different concentrations of Chitosan and CaCO3 (0.0, 20 and 40 mg/L) for 12 h before sowing. The experimental design was complete randomized block design (CRBD) with 4 replicates. Grains of wheat were sown at November 25th; grains were selected for uniformity by choosing those of equal size and with the same color. Ten uniform air-dried wheat grains were sown along a central row in each pot at 30-mm depth in plastic pots, each filled with about 7 kg clay soil from Giza, consisting of the upper 10 cm of soil collected from an area of undisturbed native vegetation. To reduce compaction and improve drainage, the soil was mixed with yellow sand in a proportion of 3:1(v/v). Ten days after sowing, the seedlings were thinned to 5 seedlings per pot. Granular ammonium sulfate 20.5% N at a rate of 40 kg N ha−1, and single superphosphate (15% P2O5) at a rate of 60 kg P2O5 ha−1 were added to each pot. The N and P fertilizers were mixed thoroughly into soil of each pot immediately before sowing. Soil field capacity in the pots was estimated by saturating the soil in the pots with water and weighing them after they had drained for 48 h. Field water capacity was 0.36. The two saline water levels used were 0.0, 5000 mg/L, respectively. The preparation of salt mixture according to Stroganov (1962) equation is shown in Table 1. The pots were irrigated with equal volumes of the various salinity levels.
The component of specific anions and cations in chloride mixture is expressed as percentage of total mill equivalents.
Plant samples were taken after 75 days from sowing for measurements of growth characters as shoot length (cm), leaves no/tiller, shoot, root fresh and dry weight (g/plant). Plant samples were taken for chemical analysis as photosynthetic pigments, indole acetic acid (IAA µg/100 g fresh weight), hydrogen peroxide (H2O2, µg/100 g fresh weight), lipid peroxidation (MDA, µg/100 g fresh weight) contents and some antioxidant enzymes as super oxide dismutase (SOD), peroxidase (POX) and catalase (CAT) (U/min/g fresh wt). After drying of plant samples, total soluble sugars (TSS, mg/g dry weight) proline (mg/100 g dry wt) were determined. At harvest: spike length (cm), spike weight (g), spikelet's number/spike, grains number/spike, grains weight/plant (g), 1000-grains weight (g) and biomass yield/plant (g). Some nutritional values of the yielded grains were determined as carbohydrate%, protein%, phenolics (mg/100 g dry weight), flavonoids (mg/100 g dry weight) and DPPH%.
Measurements
Photosynthetic pigments: Chlorophyll a, chlorophyll b and carotenoids were determined using spectrophotometric method described by Lichtenthaler and Buschmann (2001). Indole acetic acid content was extracted and analyzed by the method of Larsen et al. (1962). Total soluble sugars (TSS) were extracted by the method of (Homme et al. 1992). TSS were analyzed according to (Yemm and Willis 1954). Proline was extracted and assayed according to the method described by Bates et al. (1973). The level of lipid peroxidation was measured by determining the levels of malondialdehyde (MDA) content using the method of Hodges et al. (1999). Hydrogen peroxide content was determined using the method of Velikova et al. (2000). Enzyme extracts were collected following the method described by Chen and Wang (2006). Superoxide dismutase (SOD, EC 1.12.1.1) activity was spectrophotometrically assayed at 560 nm by nitro-blue-tetrazolium (NBT) reduction method (Chen and Wang 2006). Peroxidase (POX, EC 1.11.1.7) activity assayed according to the method of Bergmeyer (1974). Catalase (CAT) (EC 1.11.1.6) activity was determined spectrophotometrically by following the decrease in absorbance at 240 nm (Chen and Wang 2006). The enzyme activities were calculated by Kong et al. (1999). Total carbohydrate determination was carried out according to Herbert et al. (1971). The protein content was determined by micro-Kjeldahl method according to A.O.A.C. (1990). Total phenol content was measured as described by Danil and George (1972). Flavonoid content of crude extract was determined by the aluminum chloride colorimetric method (Chang et al. 2002). The free radical scavenging activity by grain extracts was done according to the method reported by Gyamfi et al. (2002).
Statistical analysis
The data were statistically analyzed on complete randomized design system (CRBD) with four replicates. Combined analysis of the two growing seasons was carried out. Means were compared by using least significant difference (LSD) at 5% levels of probability (Snedecor and Cochran 1980).
Results
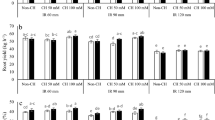
Data presented in Table 2 indicated that irrigation of wheat plants with 5000 mg/L saline water markedly decreased plant growth parameters (shoot length, leaves number per tiller, shoot fresh and dry weights, and root fresh and dry weights). Meanwhile, soaking wheat grains in chitosan or calcium carbonate (either at 20 or 40 mg/L) before sowing significantly promoted all the above-mentioned growth criteria, especially in plants treated with 40 mg/L chitosan, followed by plants treated with CaCO3.
Data presented in Table 3 indicated that irrigation of wheat plants with 5000 mg/L saline water significantly decreased yield and yield components under study (i.e., spike length (cm), spike weight (g), spikelet's number/spike, grains number/spike, grains weight/plant, 1000 grains weight, yield and biomass/ plant (g)). On the other hand, exogenous treatment of chitosan and CaCO3 increased significantly yield and its components of wheat plants grown either under normal irrigation water or salinity stress conditions as compared with their corresponding untreated controls. Meanwhile, treating plants with CaCO3 resulted in similar results, but to a lesser degree (Table 3). 40 mg/L chitosan and 40 mg/L CaCO3 treatments were more effective than 20 mg/L of either chitosan or CaCO3 treatments.
It is obvious that irrigation of wheat plants with saline water significantly decreased photosynthetic pigments (chlorophyll-a, chlorophyll-b, carotenoids and total pigments) in addition to chlorophyll-a/chlorophyll-b ratio and endogenous indole acetic acid contents in the plant leaves (Table 4). On the other hand, soaking wheat grains in chitosan at 40 mg/L significantly increased chlorophyll-a content followed by treating grains with 40 mg/L CaCO3 (Table 4). Chlorophyll-b and carotenoids contents as well as IAA contents followed the same trend. On the other hand, the ratio of chlorophyll-a/chlorophyll-b (a/b) was stable in all treatments irrigated with 5000 mg/L saline water.
Data presented in Table 5 indicated that irrigation of wheat plants with saline water significantly increased total soluble sugars (TSS) (mg/g fresh weight), proline content (mg/100 g dry weight) and phenolics (mg/100 g dry wt). Moreover, treating wheat plants either with chitosan or calcium carbonate resulted in obvious significant increases in the above-mentioned parameters, especially in plants treated with 40 mg/L chitosan followed by 40 mg/L calcium carbonate (Table 5).
Data presented in Table 5 also indicated that soaking wheat grains in 20 mg/L chitosan or CaCO3 caused significant increases in phenolics content. Furthermore, soaking wheat grains in 40 mg/L chitosan or CaCO3 resulted in significant increases in total phenolics.
Lipid peroxidation expressed by malondialdehyde (MDA) content and H2O2 content clearly showed significant increases under salinity stress compared with control plants. Meanwhile, soaking wheat grains in chitosan or CaCO3 before sowing significantly decreased the accumulation of H2O2 and MDA in the leaves of wheat plants as compared with their corresponding untreated controls either at normal irrigated plants or saline treated plants as shown in Table 6.
Regarding to antioxidant enzymes, Table 6 clearly shows that irrigation of wheat plants with 5000 mg/l saline water caused significant increases in different enzymes superoxide dismutase SOD, peroxidase POX and catalase CAT as compared to those plants irrigated with tap water. Treatment of wheat grains with chitosan or CaCO3 significantly promoted the activity of various antioxidant enzymes (SOD and POX) as compared to the control (Table 6). On the other hand, CAT activity was significantly decreased as a result of chitosan or CaCO3 treatments. The highest CAT activity (U/min/g FW) was recorded in plants irrigated with 5000 mg/l saline water followed by control plants which recoded 36.40 and 24.82 U/min/g FW, respectively.
Data presented in Table 7 indicated that irrigation of wheat plants with saline water significantly decreased carbohydrate % and protein % compared with the control plants. Meanwhile, it increased significantly flavonoids content and DPPH activities of the yielded grains of wheat plant as compared with control plants. Treating wheat plants either with chitosan or calcium carbonate with different concentrations resulted in obvious significant increases in carbohydrates, protein content, flavonoids contents and DPPH activities especially in plants treated with 40 mg/l chitosan followed by 40 mg/l calcium carbonate (Table 6). Data in Table 7 showed that salinity stress caused significant increases in the antioxidant activity (as DPPH radical scavenging capacity) of wheat grain. Also, foliar treatment of wheat plant with chitosan and CaCO3 with different concentrations (20 and 40 mMl) caused significant increases in the antioxidant activity as compared with control plants.
Discussion
Data presented in Tables 2 and 3 indicated that irrigation of wheat plants with 5000 mg/l saline water significantly decreased plant growth parameters and yield attributes. The obtained results of salinity stress are in agreement with those recorded by Abdel-Mawgoud et al. (2010), Sheikha and Al-Malki (2011) and Zehra et al. (2012), Rady et al. (2015) and Sadak (2016) on different plant species. In this concern, salinity stress limits plant growth and yield by adversely affecting various physiological and biochemical processes, such as photosynthesis, antioxidant phenomena, nitrogen metabolism, ion homeostasis and osmolyte accumulation (Ashraf 2004). Thus, salinity exerts its undesirable effects through osmotic inhibition and ionic toxicity and by disturbing the uptake and translocation of nutritional ions (Misra and Dwivedi 2004). Moreover, the reductions in yield and yield components of wheat plant under salinity stress might be through reductions in growth (Table 2) and photosynthetic pigments (Table 4) contents, thus reducing the output of photosynthesis (Anjum et al. 2003) and diminishing activities of Calvin cycle enzymes (Ashraf et al. 2013).
A positive effect of chitosan was observed on the growth and yield of various plant species. Foliar application of chitosan increased growth and yield in chilli and maize plants (Chookhongkha et al. 2012; Mondal et al. (2013). Similar results were also observed in grapevine and strawberry (Gornik et al. 2008; Abdel-Mawgoud et al. 2010). Sheikha and Al-Malki (2011) and Khan et al. (2018) indicated that application of different concentrations of chitosan enhanced bean and pea growth and yield through increasing plant immunity to different kinds of stress. Chitosan is considered as one of growth regulators and as a signal molecules in addition to its role as a high effective biomolecule (Górnik et al. 2008). Moreover, this promotive role resulted by enhancing activities of enzymes of nitrogen metabolism as well as improving the translocation of nitrogen in the leaves thus increased growth and development (Sultana et al. 2017). Chitosan treatment induces overexpression of genes involved in photosynthesis, changes in programming of protein metabolism with an enhancement of various storage proteins and hormone metabolism (Landi et al. 2017).
Similar findings were reported earlier concurrent with our obtained positive results of Ca+ on different plant under abiotic stresses as Shoresh et al. (2011), Zehra et al. (2012), Xu et al. (2013), Ibrahim et al. (2016) and Haleema et al. (2018) and Sadak et al. (2020). The positive role of Ca2+ might be attributed to that Ca2+ was suggested to be a crucial secondary messenger that used in signaling-related processes to many defense mechanisms which are induced by salinity stress (Tuteja 2009). Ca2+ can increase membrane stability and protect them from lipid peroxidation and oxidative stress induced by salinity stress, thus improving water status of plant (Nayek et al. 1983 and Shao et al. 2008) as calcium is an important constituent of plant cell wall and plays an important role in cell division and enlargement.
It is obvious that irrigation of wheat plants with saline water significantly decreased photosynthetic pigments in addition to chlorophyll-a/chlorophyll-b ratio and endogenous indole acetic acid contents in the plant leaves (Table 4). These reductions in different photosynthetic pigments components in response to salt stress were confirmed on various plants Sakhonwasee and Phingkasan (2017) on tomato plant. These decreases might be referred to the increased levels of reactive oxygen species (ROS) production and oxidative stress that happened with salinity stress, thus damaging chloroplast membranes and the antagonistic effects of sodium ion on magnesium ion absorption (Smirnoff 1993). As well as, these decreases could be considered as an important regulatory step to avoid the absorbance of high light and the over reduction of photosynthetic electron transport chain, thus increasing the production of ROS (Munne-Bosch and Alegre 2000; Mazars et al. 2010).
The obtained results showed that chitosan treatment increased photosynthetic pigments. These increases could be due to improving cytokinins contents that stimulated chlorophylls synthesis or to the increased availability of amino compounds released from chitosan (Chibu and Shibayama 2001). Farouk and Amany (2012) stated that chlorophylls and carbohydrates of cowpea plant were reduced under water stress, whereas foliar application of chitosan significantly increased these parameters. Moreover, Khan et al. (2002) who reported that chitosan significantly increased photosynthetic pigments in maize and soybean plants. Pereira et al. (2017) found that chitosan treatment increased photosynthetic pigments of Phaseolus vulgaris. In another report, Behboudi et al. (2018) foliar treatment of chitosan increased photosynthetic pigments of barley plant. These results might be due to the increases of nitrogen and magnesium contents in the leaves because nitrogen and magnesium are the most important elements in the chemical composition of chlorophylls (Dzung et al. (2011).
The enhancement of photosynthetic pigment obtained in wheat leaves by foliar treatments of CaCO3 had been proved previously on different species as pepper (Yang et al. 2016) and tung tree (Li et al. 2017). This promotive effect might be attributed to the effect of Ca2+ in preventing dehydration damage of cellular structures via maintaining the osmotic strength of cytoplasm in plants (Arshi et al. 2006).
Salinity stress decreased significantly IAA contents of wheat leaves (Table 4). In agreement with the obtained results, Khater et al. (2018) stated that subjecting cowpea plant to stress decreased IAA contents. Generally, the reduction of different phytohormones among them IAA caused by different abiotic stresses might be attributed the decrease in enzyme activity which participates in phytohormone synthesis and/or increases in enzymes participate in its degradation (Vaseva-Gemisheva et al. 2005). Moreover, different treatments could increase IAA contents of wheat plants. Muthukrishnan et al. (2019) confirmed the promoting role of chitosan on IAA contents of chickpea plant. These increases might be due to the induced effect of Chito on auxin-related gene expression, accelerated IAA biosynthesis and transport and reduced IAA oxidase activity increases (Li et al. 2019).
Data presented in Table 5 indicated that irrigation of wheat plants with saline water significantly increased total soluble sugars (TSS), proline content and phenolics. Moreover, treating wheat plants either with chitosan or calcium carbonate resulted in obvious significant increases in the above-mentioned parameters. The obtained results on proline and TSS of wheat plants are confirmed in various plant species under stress Ku et al. (2012) on Nicotiana benthamiana plant, El-Bassiouny and Sadak (2015) on flax plant, Ibrahim et al. (2016) on sunflower plant. These increases in the two major organic osmolytes contents (proline and TSS) might help plants to regulate osmotic potential of cells which led to improve water absorbance and translocation under salinity stress (Oraki et al. 2012). As well as, proline is involved in protection of cellular structures, different enzymes from oxidative damages and acts as scavenger of free radical (Rady et al. 2015). With respect to phenol contents, phenolic compounds are antioxidants which trigger a series of secondary metabolites formed via shikimic acid or malonic acid cycles, as well as it has a cellular signaling functions (Michalak 2006).
It is well known that free radical-induced peroxidation of membrane lipids is an indicator of damage induced by stress at the cellular level (Jain et al. 2001). Thus, MDA level produced during lipids membrane peroxidation is usually used as an indicator of oxidative damage. Guimaraes et al. (2011) showed that increased MDA content might result in electrolyte leakage, indicating a loss of membrane integrity. As well as, salinity stress increased effect on MDA and H2O2 contents could be attributed to the inadequate induction of antioxidant system (Hossain et al. 2013). The H2O2 may play a role as a secondary messenger in response to abiotic stress, leading to a tolerance increase toward these unfavorable conditions, by maintaining cellular homeostasis through the antioxidant enzymes SOD, POX and CAT. The role of Chitosan on decreasing MDA and H2O2 contents might be proposed that CHT receptors are present on the plasma membrane; however, through a signaling cascade, the chloroplast is the primary CHT action organelle (Hadwiger 2013). Charge–charge interactions between positively charged CHT amine groups and negatively charged phospholipids promote a signal that will lead to the octadecanoid pathway activation; this metabolic pathway is directly related to the decreased H2O2 formation (Pichyangkura and Chadchawan 2015; Almeida et al. 2020).
Meanwhile, 20 and 40 mg/L CaCO3 decreased MDA contents. These results are similar to those obtained by Issam et al. 2012, Xu et al. (2013) and Kaur et al. (2017) on different plant species. Hydrogen peroxide is used in different biochemical processes and signaling cascades required for plant growth and development. Generally, H2O2 contents increased in various plants subjected to stress (Dawood et al. 2014; Sadak, 2016; Kaur et al. 2017). In our investigation, H2O2 content increased in wheat plant subjected to salinity stress, whereas calcium chloride treatment decreased H2O2 contents. These obtained results are in harmony with those obtained by Amor et al. (2010) and Sadak et al. (2020).
Regarding to antioxidant enzymes, Table 6 clearly showed that irrigation of wheat plants with 5000 mg/L saline water caused significant increases in different enzymes superoxide dismutase SOD, peroxidase POX and catalase CAT as compared to those plants irrigated with tap water. Treatment of wheat grains with chitosan or CaCO3 significantly promoted the activity of various antioxidant enzymes (SOD and POX) as compared to the control (Table 6). On the other hand, CAT activity was significantly decreased as a result of chitosan or CaCO3 treatments. The highest CAT activity (U/min/g FW) was recorded in plants irrigated with 5000 mg/l saline water followed by control plants which recoded 36.40 and 24.82 U/min/g FW, respectively. Plants normally cope with oxidative damage by increasing the activity of antioxidant enzymes in which it indicates stress resistance in plants (Weng et al. 2015). The enhancing effect of salinity stress is confirmed earlier on different plant species Ibrahim et al. (2016) on sunflower and Kaur et al. (2017). Oxidative stress is an important sign of abiotic stress and the increased SOD, POX and CAT activities were correlated with increasing protection from destructive resulted from oxidative stress (Miller et al. 2010). Superoxide is converted by the SOD enzyme into H2O2 which is still toxic and must be removed via transformation to H2O in the next step. H2O2 is then eliminated by conversion to H2O by POX enzyme (Parida et al. 2004). Peroxidases are important enzymes in various biochemical processes in plant, either in response to biotic or abiotic stresses. Also in the scavenging of ROS and plant cells protection from H2O2 injury (Bowler et al. 1992). The present study demonstrated that chitosan pretreatment induces the activities of SOD, POX and CAT. It was also reported that chitosan alleviates the adverse effect of water stress by enhanced production of antioxidant enzymes (Hidangmayum et al. 2019). The result also corresponded to the previous studies, which also revealed that chitosan application increases SOD, POX and CAT activities in wheat under salinity stress in wheat and maize seedlings under salt stress condition (Peykani and Sepehr 2018).
Generally, the effect of calcium carbonate on antioxidant enzymes of plant under salinity stress is in accordance with Amor et al. (2010), Xu et al. (2013) and Ibrahim et al. (2016). Eventually, the above-mentioned relationship confirmed that CaCl2 has an important role in alleviation water stress in wheat plant by adjusting membrane integrity, cell wall structure and improving water status of plant or by its direct role on osmolytes and antioxidants. As well as, Ca is involved in the biosynthesis of cell wall and interacts with phytohormones (Barker and Pilbeam 2007). Therefore, calcium acts as a site-specific cofactor on peroxidase enzyme (Mac-laughein and Wimmer 1999).
Data presented in Table 7 indicated that irrigation of wheat plants with saline water significantly decreased carbohydrate % and protein % compared with the control plants. Meanwhile, increased significantly flavonoids content and DPPH activities of the yielded grains of wheat plant as compared with control plants. Treating wheat plants either with chitosan or calcium carbonate with different concentrations resulted in obvious significant increases in carbohydrates, protein content, flavonoids contents and DPPH activities especially in plants treated with 40 mg/L chitosan followed by 40 mg/L calcium carbonate (Table 7). These decreases on carbohydrates contents are mainly due to the reduction in growth parameters (Table 2) and photosynthetic pigments (Table 4). Carbohydrate and protein changes of the yielded grains are of particular importance because of their direct relationship with such physiological processes as photosynthesis, translocation and respiration (Dubey and Pessarakli 2001). Salinity stress decreased chlorophyll contents in leaves, thus causing reduction of photosynthetic activity. So, decreased carbohydrates accumulation in mature leaves might reduce carbohydrate transport from leaves to the developing grains (Neslihan-Ozturk et al. 2002; Liu et al. 2004). On the other hand, the stimulating effect of chitosan and Ca+ treatments on carbohydrate contents of yielded grains might be due to the increases in growth parameters and photosynthetic pigments (Table 3). As well as, these increases in carbohydrate and proteins contents might be due to the increased photosynthetic output so decreased carbohydrates formation in leaves increased the translocation of carbohydrate from leaves to developing grains.
Flavonoids are plant secondary metabolites, the antioxidant activity (DPPH) of which depends on the presence of free OH groups, especially 3-OH. Plant flavonoids have antioxidant activity in vitro and also act as antioxidants in vivo (Geetha et al. 2003). As this is the first report on the antioxidant activity of grains of wheat thorough phytochemical analyses should be done to identify the active phenolic and flavonoid components. The increased contents of flavonoid under saline conditions may reflect some kind of defense against stress conditions (i.e., oxidative burden) since salt stress was accompanied by increased production of reactive oxygen species (Rezazadeh et al. 2012). Exogenous treatment of wheat plants with chitosan or CaCO3 resulted in a significant increase in flavonoids contents compared to untreated plants. These observations reveal that the bioactive molecule of treatments may be an inducer for the biosynthesis of secondary metabolites (flavonoids) which act as oxygen scavengers to reduce oxidative stress and, hence, increase the growth and yield wheat plant (Table 7).
Data in Table 7 showed that salinity stress caused significant increases the antioxidant activity (as DPPH- radical scavenging capacity) of wheat grain. Also, foliar treatment of wheat plant with chitosan and CaCO3 with different concentrations (20 and 40 mMl) caused significant increases in the antioxidant activity as compared with control plants. Yu et al. (2002) suggested that significant levels of antioxidant activities and phenolic components have been detected in wheat and wheat-based food products and indicated that wheat may serve as an excellent dietary source of natural antioxidants for disease prevention and health promotion. The increase in the scavenging activity can be considered an advantage of treatment used. This could be attributed to the increases in total phenols and total flavonoids (Abd Allah et al. 2020; Orabi et al. 2015).
Conclusion
From the obtained data, it could be concluded that salinity stress adversely affected growth and biochemical parameters as compared with control plants. Meanwhile, soaking wheat grains in chitosan or calcium carbonate before sowing, especially in the higher dose (i.e., 40 mg/L) have a significant positive effect on plant growth, photosynthetic pigments, yield, yield components and bio-yield. It is obvious that application of chitosan or CaCO3 significantly increased growth, yield quantity and quality via increasing photosynthetic pigments, indole acetic acid, total soluble sugars, proline, phenolics and the activity of some antioxidant enzymes. Moreover, wheat plants treated with chitosan or CaCO3 gave higher technological characteristics of grain like carbohydrate%, protein%, flavonoids and antioxidant activities.
Availability of data and materials
The datasets generated and/or analyzed during the current study are included in this published manuscript.
Abbreviations
- Ca+ :
-
Calcium
- CaCO3 :
-
Calcium carbonate
- Chlo-a:
-
Chlorophyll a
- Chlo-b:
-
Chlorophyll b
- IAA:
-
Indole acetic acid
- TSS:
-
Total soluble sugars
- MDA:
-
Malondialdehyde
- H2O2 :
-
Hydrogen peroxide
- SOD:
-
Superoxide dismutase
- POX:
-
Peroxidase
- CAT:
-
Catalase
- DPPH:
-
2,2-Diphenyl-1-picryl-hydrazyl-hydrate assay
References
Abdallah MMS, Ramadan AE, El-Bassiouny HMS, Bakry BA (2020) Regulation of antioxidant system in wheat cultivars by using chitosan or salicylic acid to improve growth and yield under salinity stress. Asian J Plant Sci 19(2):114–126
Abdel-Mawgoud AMR, Tantawy AS, El-Nemr MA, Sassine TN (2010) Growth and yield response of strawberry plants to chitosan application. Eur J Sci Res 39:170–177
Achary VMM, Parinandi NL, Panda BB (2013) Calcium channel blockers protect against aluminium-induced DNA damage and block adaptive response to genotoxic stress in plant cells. Toxicol Environ Mutagen 751:130–138
Almeida LG, Magalhães PC et al (2020) Chitosan application in the induction of water deficit tolerance in maize plants. Acta Sci Agron 42:e42463. https://doi.org/10.4025/actasciagron.v42i1.42463
Amor NB, Megdiche W, Jimenez A, Sevilla F, Abdelly C (2010) The effect of calcium on the antioxidant systems in the halophyte Cakile maritima under salt stress. Acta Physiol Plants 32:453–461. https://doi.org/10.1007/s11738-009-0420-2
Anjum F, Yaseen M, Rasul E, Wahid A, Anjum S (2003) Water stress in barley. I. Effect on chemical composition and chlorophyll content. Pak J Agric Sci 40:45–49
AOAC (1990) Official methods of analysis, 20th edn. Association of Official Analytical Chemists, Arlington, Virginia, USA (No 984.13)
Arshi A, Abdin MZ, Iqbal M (2006) Effect of CaCl2 on growth performance, photosynthetic efficiency and nitrogen as simulation of Cichorium intybus L. grown under NaCl stress. Acta Physiol Plant 28:137–147
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants flora - morphology distribution functional. Ecol Plants 199(5):361–376. https://doi.org/10.1078/0367-2530-00165
Ashraf M, Shahbaz M, Ali Q (2013) Drought-induced modulation in growth and mineral nutrients in canola (Brassica napus L.). Pak J Bot 45:93–98
Bakhoum GS, Sadak MS, Badr EA (2020) Mitigation of adverse effects of salinity stress on sunflower plant (Helianthus annuus L.) by exogenous application of chitosan. Bull Natl Res Centre 44:79. https://doi.org/10.1186/s42269-020-00343-7
Barker AV, Pilbeam DJ (2007) Handbook of plant nutrition. Taylor and Francis Group, New York, pp 121–144
Bates LS, Waldan RP, Teare LD (1973) Rapid determination of free proline under water stress studies. Plant Soil 39:205–207
Behboudi F, TahmasebiSarvestani Z, ZamanKassaee M, ModaresSanavi SAM, Sorooshzadeh A, Ahmadi SB (2018) Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J Water Environ Nanotechnol 3(1):22–39. https://doi.org/10.22090/jwent.2018.01.003
Bergmeyer HU (1974) Methods of enzymatic analysis, 2nd edn. Academic Press, New York
Bittelli M, Flury M, Campbell GS, Nichols EJ (2001) Reduction of transpiration through foliar application of chitosan. Agric Forest Meteorol 107:167–175
Boonlertnirum S, Meechoul S, Sarobol E (2010) Physiological and morphological responses of field corn seedlings to chitosan under hypoxic conditions. Sci Asia 36:89–93
Bouschet T, Martin S, Henley JM (2008) Regulation of calcium-sensing-receptor trafficking and cell-surface expression by GPCRs and RAMPs. Trends Pharmacol Sci 29:633–639
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(2002):178–182
Chen JX, Wang XF (2006) Plant physiology experimental guide. Higher Education Press, Beijing, pp 24–25
Chibu H, Shibayama H (2001) Effects of chitosan applications on the growth of several crops. In: Uragami T, Kurita K, Fukamizo T (eds) Chitin and chitosan in life science
Chookhongkha YN, Miyagawa S, Jirakiattikul Y, Photchanachai S (2012) Chili growth and seed productivity as affected by chitosan. In: Proceedings of the international conference on agriculture technology and food sciences, Manila, Philippines
ChunYan L, GuoRui M, WenYing H (2003) Induction effect of chitosan on suppression of tomato early blight and its physiological mechanism. J Zhejiang Univ Agric Life Sci 29:280–286
Cousson A (2009) Involvement of phospholipase C-independent calcium-mediated abscisic acid signaling during Arabidopsis response to drought. Biol Planta 53:53–62
Danil AD, George CM (1972) Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. J Am Soc Hort Sci 17:621–624
Dawood MG, Sadak MS (2014) Physiological role of glycinebetaine in alleviating the deleterious effects of drought stress on canola plants (Brassica napus L.). Middle East J Agric Res 3(3):943–954
Devlieghere F, Vermeulen A, Debevere J (2004) Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microb 27:703–714
Dubey RS, Pessarakli M (2001) Physiological mechanisms of nitrogen absorption and assimilation. In plants under stressful conditions. In: Passarakli M (ed) Handbook of plant and crop physiology, 2nd edn, Marcel Dekker Inc., New York, pp 636–655
Dzung NA, Phuong VT, Dzung TT (2011) Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr Polym 84:751–755
El-Bassiouny HM, Sh M, Sadak, (2015) Impact of foliar application of ascorbic acid and α-tocopherol on antioxidant activity of flax cultivars under salinity stress. Acta Biol Colomb 20(2):209–222
Farouk S, Amany AR (2012) Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egypt J Biol 14(1):14–16
Farouk S, Ghoneem KM, Ali Abeer A (2008) Induction and expression of systematic resistance to downy mildew disease in cucumber plant by elicitors. Egypt J Phytopath 1–2:95–111
Farouk S, Mosa AA, Taha AA, Heba MI, ELGahmery AM (2011) Protective effect of humic acid and chitosan on radish (Raphanus sativus L. var. sativus) plants subjected to cadmium stress. J Stress Physiol Biochem 7:99–116
Geetha M, Sai-Ram SS, Mongia V, Singh G-G et al (2003) Evaluation of antioxidant activity of leaf extract of sea buckthorn (Hippophae rhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J Ethnopharmacol 87:247–251
Ghoname AA, El-Nemr MA, Abdel-Mawgoud AMR, El-Tohamy WA (2010) Enhancement of sweet pepper crop growth and production by application of biological, organic and nutritional solutions. Res J Agric Bio Sci 6:349–355
Gornik K, Grzesik M, Duda BR (2008) The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. J Fruit Ornament Plant Res 16:333–343
Guimarães FVA, de Lacerda CF, Marques EC, de Miranda MRA, de Abreu CEB et al (2011) Calcium can moderate changes on membrane structure and lipid composition in cowpea plants under salt stress. Plant Growth Regul 65:55–63. https://doi.org/10.1007/s10725-011-9574-1
Gyamfi MA, Yonamine M, Aniya Y (2002) Free radical scavenging action of medicinal herbs from Ghana Thonningia sanguine on experimentally induced liver injuries. Gen Pharmacol 2002(32):661–667
Hadwiger LA (2013) Multiple effects of chitosan on plant systems: solid science or hype. Plant Sci 208:42–49. https://doi.org/10.1016/J.PLANTSCI.2013.03.007
Hadwiger LA, Klosterman SJ, Choi JJ (2002) The mode of action of chitosan and its oligomers in inducing plant promoters and developing disease resistance in plants. In: Suchiva K, Chandrkrachang S, Methacanon P, Peter MG (eds) Advances in chitin science, vol 5, Bangkok, pp 452–457. ISBN 974-229-412-7
Haleema B, Rab A, Hussain SA (2018) Effect of calcium, boron and zinc Foliar application on growth and fruit production of tomato. Sarhad J Agric 34(1):19–30
Herbert DP, Phipps J, Strange RE (1971) Chemical analysis of microbial cells. Methods Microbiol 5:209–344. https://doi.org/10.1016/S0580-9517(08)70641-X
Hidangmayum A, Dwivedi P, Katiyar D, Hemantaranjan A (2019) Application of chitosan on plant responses with special reference to abiotic stress. Physiol Mol Biol Plants 25:313–326. https://doi.org/10.1007/s12298-018-0633-1
Hodges DM, De Long JM, Forney C, Prange PK (1999) Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Homme PM, Gonzalez B, Billard J (1992) Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by sources/link modification after cutting. J Plant Physiol 140:282–291
Hossain MA, Mostofa MG, Fujita M (2013) Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed 4:50–70
Ibrahim MFM, Faisal A, Shehata SA (2016) Calcium chloride alleviates water stress in sunflower plants through modifying some physio-biochemical parameters. Am Euras J Agric Environ Sci 16(4):677–693
Issam N, Kawther M, Haythem M, Moez J (2012) Effects of CaCl2 pretreatment on antioxidant enzyme and leaf lipid content of faba bean (Vicia faba L.) seedlings under cadmium stress. Plant Growth Regul 68:37–47. https://doi.org/10.1007/s10725-012-9691-5
Jain M, Mathur G, Koul S, Sarin NB (2001) Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep 20:463–468
Kaur H, Bhardwaj RD, Grewal SK (2017) Mitigation of salinity-induced oxidative damage in wheat (Triticum aestivum L.) seedlings by exogenous application of phenolic acids. Acta Physiol Plant 39:221
Khan WM, Brithiviraj B, Smith DL (2002) Effect of foliar application of chitin and chitosan oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 40:621–624
Khan R, Manzoor N, Zia A, Ahmad I, Ullah A, Shah SM, Naeem M, Ali S, Khan IH, Zia D, Malik S (2018) Exogenous application of chitosan and humic acid effects on plant growth and yield of pea (Pisum sativum). Inter J Biosci 12(5):43–50
Khater MA, Dawood MG, Sadak MS, Shalaby MAF, El-Awadi ME, El-Din KG (2018) Enhancement the performance of cowpea plants grown under drought conditions via trehalose application. Middle East J Agric Res 7(3):782–800
Kong FX, Hu W, Chao WL, Sang WL, Wang LS (1999) Physiological responses of Mexicana to oxidative stress of SO2. Environ Exp Bot 42:201–209
Ku HM, Tan CW, Su YS, Chiu CY, Chen CT, Jan FJ (2012) The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol Plant 56:337–343
Landi L, De Miccolis Angelini RM, Pollastro S, Feliziani E, Faretra F, Romanazzi G (2017) Global transcriptome analysis and identification of differentially expressed genes in strawberry after preharvest application of Benzothiadiazole and chitosan. Front Plant Sci 2017(8):235
Larsen P, Harbo A, Klungron S, Ashein TA (1962) On the biosynthesis of some indole compounds in Acetobacter Xylinum. Physiol Plant 15:552–565
Li Z, Tan XF, Lu K, Liu ZM, Ul L (2017) The effect of CaCl2 on calcium content, photosynthesis, and chlorophyll fluorescence of tung tree seedlings under drought conditions. Photosynthetica 55(3):553–560
Li R, He J, Xie H, Wang W, Bose SK, Sun Y, Hu J, Yin H (2019) Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int J Biol Macromol 126:91–100
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry (CPFA). Wiley, New York, pp F4.3.1–F4.3.8
Liu F, Jensen CR, Andersen MN (2004) Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: Its implication in altering pod set. Field Crops Res 86:1–13
Mac-laughein SB, R. wimmer, (1999) Calcium physiology and terrestrial ecosystem processes, tansley Review n. 104. New Physiol 142:373–417
Mazars C, Thuleaua P, Lamotte O, Bourque S (2010) Cross-talk between ROS and calcium in regulation of nuclear activities. Mol Plant 3(4):706–718
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Plant Cell 15:523–530
Miller G, Suzuki N, Ciftci-yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Misra N, Dwivedi UN (2004) Genotypic difference in salinity tolerance of green gram cultivars. Plant Sci 166(5):1135–1142
Mondal M, Malek M, Puteh A, Ismail M (2013) Foliar application of chitosan on growth and yield attributes of mungbean (Vignaradiata (L.) Wilczek). Bangladesh J Bot 42:179–183
Munné-Bosch S, Alegre L (2000) The xanthophyll cycle is induced by light irrespective of water status in field-grown lavender (Lavandula stoechas) plants. Physiol Plant 108:147–151
Muthukrishnan S, Murugan I, Selvaraj M (2019) Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohyd Polym. https://doi.org/10.1016/j.carbpol.2019.02.037
Nayek B, Biswas AK, Choudhuri MA (1983) Effect of calcium on water-stress-induced biochemical changes and yield of field-grown rice. Biol Plant 25:117–123
Neslihan-Ozturk Z, Talam V, Deyholos M, Michalowski CB, Galbraith DM, Gozukirmizi N, Tuberosa R, Bohnert HJ (2002) Monitoring large-scale changes in transcript abundance in drought- and salt stressed barley. Plant Mol Biol 48:551–573
No HK, Lee KS, Kim ID, Park MJ, S.D. Kim and S.P. Meyers, (2003) Chitosan treatment affects yield, ascorbic acid content and hardness of soybean sprouts. J Food Sci 68:680–685
Orabi SA, Dawood MG, Salman SA (2015) Comparative study between the physiological role of hydrogen peroxide and salicylic acid in alleviating the harmful effect of low temperature on tomato plants grown under sand. Sci Agric 9:49–59
Oraki H, Khanjani FP, Aghaalikhna M (2012) Effect of water deficit stress on proline contents, soluble sugars, chlorophyll and grain yield of sunflower (Helianthus annuus L.) hybrids. Afr J Biotechnol 11:164–168
Pan Z, Zhao Y, Zheng Y, Liu J, Jiang X, Guo Y (2012) Ahigh-throughput method for screening Arabidopsis mutants with disordered abiotic stress-induced calcium signal. J Genet Genom 39:225–235
Parida AK, Das AB, Mohanty P (2004) Investigations on the antioxidative defence responses to NaCl stress in a mangrove, Bruguiera parviflora: differential regulations of isoforms of some antioxidative enzymes. Plant Growth Regul 42:213–226
Patkowska E, Pieta D, Pastucha H (2006) The effect of biochikol 020 pc on microorganisms’ communities in the rhizosphere of Faba bean plants. Polish Chitin Soc Monog 11:171–178
Pereira AS, Silva PM, Olivera JL, Olivera HC, Fraceto LF (2017) Chitosan nanoparticles as carrier systems for the plant growth hormone gibbereillic acid. Collodis Surf B Bioenterfaces 150:141–152
Peykani LS, Sepehr MF (2018) Effect of chitosan on antioxidant enzyme activity, proline, and malondialdehyde content in Triticum aestivum L. and Zea may L. under salt stress condition. Iran J Plant Physiol 9:2661–2670
Pichyangkura R, Chadchawan S (2015) Biostimulant activity of chitosan in horticulture. Sci Hortic 196(30):49–65. https://doi.org/10.1016/j.scienta.2015.09.031
Rady MM, Sadak MS, El-Lethy SR, Abd El-Hamid EM, Abdelhamid MT (2015) Exogenous α-tocopherol has a beneficial effect on Glycine max (L.) plants irrigated with diluted sea water. J Hortic Sci Biotechnol 90(2):195–202
Rezazadeh A, Ghasemnezhad A, Barani M, Telmadarrehe T (2012) Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L.) leaves. Res J Med Plant 6:245–252
Sadak MS (2016) Physiological role of signal molecules in improving plant tolerance under abiotic stress. Int J Chem Technol Res 9:46–60
Sadak MSh, El-Enany MAM, Bakry BA, Abdallah MMS, El-Bassiouny HMS (2020) Signal molecules improving growth, yield and biochemical aspects of wheat cultivars under water stress. Asian J Plant Sci 19:35–53. https://doi.org/10.3923/ajps.2020.35.53
Sakhonwasee S, Phingkasan W (2017) Effects of the foliar application of calcium on photosynthesis, reactive oxygen species production, and changes in water relations in tomato seedlings under heat stress. Hortic Environ Biotechnol 58(2):119–126
Shao H, Song W, Chu L (2008) Advances of calcium signals involved in plant anti-drought. CR Biol 331:587–596
Sheikha SA, Al-Malki FM (2011) Growth and chlorophyll responses of bean plants to chitosan applications. Eur J Sci Res 50:124–134
Shoresh M, Spivak M, Bernstein N (2011) Involvement of calcium mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Radic Biol Med 51:1221–1234. https://doi.org/10.1016/j.freeradbiomed.2011.03.036
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State Univ. Press, Iowa
Stroganov BP (1962) Physiological basis of salt tolerance of plants as affected by various types of salinity. Izadatel ‘Stvo Akademii Nauk, USSR, pp 279
Sultana S, Islam M, Khatun MA, Hassain MdA, Huque R (2017) Effect of foliar application of oligo-chitosan on growth, yield and quality of tomato and eggplant. Asian J Agric Res 11(2):36–42. https://doi.org/10.3923/ajar.2017.36.42
Tuteja N (2009) Integrated calcium signaling in plants. In: Baluška F, Mancuso S (eds) Signaling and communication in plants. Springer, Berlin, pp 29–49
Vaseva-Gemisheva I, Lee D, Karanov E (2005) Response of Pisum sativum cytokinin oxidase /dehydrogenase expression and specific activity to drought stress and herbicide treatments. Plant Growth Regul 46:199–208
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 5:59–66
Weng M, Cui L, Liu F, Zhang M, Shan L, Yang S, Deng XP (2015) Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak J Bot 47(1):49–56
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Xu C, Li X, Zhang L (2013) The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE 8(7):e68214. https://doi.org/10.1371/journal.pone.0068214
Yang BZ, Liu ZB, Zhou SD et al (2016) Exogenous Ca2+ alleviates waterlogging-caused damages to pepper. Photosynthetica 54:620–629
Yemm EW, Willis AJ (1954) The respiration of barley plants. IX. The metabolism of roots during assimilation of nitrogen. New Phytotol 55:229–234
Yu L, Haley S, Perret J, Harris M (2002) Antioxidant properties of hard winter wheat extracts. Food Chem 78:457–461
Zehra A, Gul B, Ansari R, MA, Khan. (2012) Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S Afr J Bot 78:122–128
Funding
There are currently no funding sources in the design of the study and collection, analysis and interpretation of data, and in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
MShS designed and performed the experiment, responsible of all the physiological and biochemical analysis and also wrote and reviewed the manuscript. IMT designed and performed the experiment, statistical analysis, responsible of all the physiological and biochemical analysis and also wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadak, M.S., Talaat, I.M. Attenuation of negative effects of saline stress in wheat plant by chitosan and calcium carbonate. Bull Natl Res Cent 45, 136 (2021). https://doi.org/10.1186/s42269-021-00596-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00596-w