Abstract
Background
Ependymoma is a rare adult tumor that originates from ependymal cells of the ventricles and the spinal cord. The diagnosis and management can be very challenging. This study aims to bring out the hypothalamus as an atypical location of ependymoma and to underline the consequences of treatment delay in anaplastic ependymomas through a concrete case.
Case presentation
We present a case of 20 years with no previous medical history, suffering from progressive intracranial hypertension syndrome, tetraparesis, cerebellar ataxia, and a weight loss of 3 kg in 4 months. Cerebral and medullar Magnetic Resonance Imaging showed multiple processes including the temporal region, the cerebellum, the 4th ventricular floor, the interpeduncular cistern, the hypothalamus, and almost the entire spinal cord, in addition to a posterior intradural lesion. The hypothalamic localization was very suggestive of neurohistiocytosis. A stereotactic biopsy and immunohistochemical study confirmed the diagnosis of anaplastic ependymoma. Total resection was impossible and radiotherapy was delayed by inconclusive dosimetric scans. The patient's outcome was unfortunately quickly fatal.
Conclusions
Ependymomas should be evoked whatever the location in the central nervous system. Radiotherapy must be quickly discussed and started when the gross total resection is impossible. The locations, as well as the doses of irradiation, are now well-established and any delay in initiating radiotherapy only darkens the prognosis.
Similar content being viewed by others
Background
Ependymoma is a glial neoplasm that originates from the ependymal cells lining the brain ventricles and the central canal of the spinal cord. There is a clear predominance in infants compared to the adult population. It also remains clinically silent for a long time and appears very heterogeneous on imaging [1, 2].
Many atypical origins and extensive locations of this tumor have been described in the literature and many theories have been proposed to explain them.
This paper aims to point out the atypia, and extensibility and the consequences of treatment delay of anaplastic ependymomas through a concrete case and a review of the literature.
Case presentation
Authors present 20 years, right handed man, a university student with no personal or familiar medical past. He complained of neurological symptoms, which appeared 4 months before the admission and included intracranial hypertension syndrome (headache, vomiting, and blurred vision), progressive tetraparesis, and balance deficit, all in addition to polydipsia, polyuria, and a weight loss of three kilograms in 4 months. We noticed an underweight patient (BMI = 16.5) with asymmetric tetraparesis scored between 3 and 4/5, cerebellar ataxia, and no papillary edema.
Encephalic and spinal cord Magnetic Resonance Imaging MRI (SIEMENS Healthineers, MAGNETOM Aera 1.5 T, Germany) showed multiple intra and extra-axial, supra and infratentorial processes of different sizes and heterogeneous compounds.
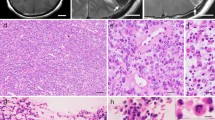
The most voluminous supratentorial lesion was located in the temporal lobe. It was spherical, measuring 11 mm, and with no enhancement after Gadolinium injection (Fig. 1).
Infratentorial lesions involved the cerebellum (Fig. 2), the 4th ventricular floor, and the interpeduncular cistern (Fig. 3). These lesions appeared as T1-hyposignal, T2-hypersignal and included tissue and liquid signals.
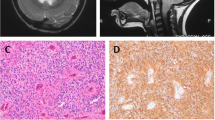
The hypothalamic region was the seat of a voluminous lesion of 21 × 22 mm, T1-isosignal, T2-hyperintense with an obvious enhancement after Gadolinium. It comes into contact with the optic chiasm and internal carotid arteries and compresses the pituitary stem, pushed back (Fig. 4).
T1 sagittal MRI of the brain showing a voluminous lesion of the hypothalamus, measuring 21 × 22 mm, T1-isosignal and T2-hyperintense, with an obvious enhancement after injection of Gadolinium, close to the optic chiasm and the internal carotid arteries and compresses the pituitary stem pushed back (arrows)
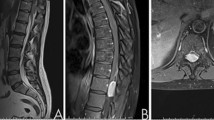
The spinal MRI showed an almost total infiltration of the spinal cord by cystic and tissue lesions, in addition to a posterior intradural lesion of 11 mm, at T4–T5 disc level, compressing the spinal cord, reduced to 4 mm of transversal diameter (Fig. 5).
Clinical and radiological correlation, in this case, was very suggestive of neurohistiocytosis and neurosarcoidosis particularly due to hypothalamic localization. The rapid evolution and the extensive appearance on the MRI were not very suggestive of a tumor, an ependymoma, for example.
The case was discussed in a multidisciplinary meeting and an agreement was found about a biopsy of the dorsal intradural lesion (Fig. 6). The anatomopathological and immunohistochemical evaluation of the tumor samples concluded with anaplastic ependymoma WHO grade III (Fig. 7).
Because of the rapid deterioration of the neurological state of our patient, dosimetry and, therefore, radiotherapy were delayed. The patient's outcome was unfortunately quickly fatal.
Ependymomas are very rare and especially affect children [1, 2, 3]. Different statistics have been published. The variability of reported epidemiologic results can be attributed to the heterogeneity of the patients (age) and tumors’ characteristics (location) [3, 4, 5]. Ependymoma originates from the ependymal cells of the central nervous system. Therefore, it is typically located in the ventricles and the spinal cord.
In regards to intracranial ependymoma, the average age of onset is very heterogeneous. It is 16 ± 1 years in the Maghreb series [6, 7], 46.1 ± 16 years in a French series of 121 cases [8], and 42.2 years in an American meta-analysis of 183 cases (based on 56 studies) [9]. The neoplasm is compressive rather than infiltrative. It remains clinically silent for months or even years [9]. These two data argue for the onset of this neoplasm at a very early age in our patient, with progressive volume gain and extensibility. Headache and vomiting are the most common presenting signs followed by cerebellar syndrome [6–9]. Schwartz and colleagues, in a study of supratentorial ependymomas in adult patients, noted that only one-quarter of ependymomas involve the brain in adults, in contrast to 90% in children [10]. It fits with the results of Sayegh and colleagues’ meta-analysis. Indeed, Sayegh reported that about 61% of ependymomas were supratentorial. However, the French and Tunisian series found a predominance of infratentorial localization, which represented, respectively, 60.1% and 77.4% of intracranial ependymomas. In addition, it has been noted that an extraventricular location is more common than an intraventricular one in the subgroup of supratentorial ependymomas [9].
Intraspinal ependymomas are the most prevalent primary intramedullary spinal tumor in adults [11]. The reported mean age at diagnosis was around 32.4 (Australia), 35.2 ± 17.8 (China), 41.8 (France), and 43.5 (USA) years [5, 12–14]. Pain, neurological deficit, and paresthesia are the main revealing symptoms [13, 15]. Cervical localization is not only recognized as the most common but also as a good prognostic factor of survival. In addition, intramedullary ependymoma often occurs centrally in the spinal cord [16]. Contrariwise, intraspinal extramedullary ependymoma is very unusual. Lars Gardner established a summary table of 18 cases of intraspinal extramedullary ependymomas published from 1951 to 2013 [17]. We noted that this subgroup of intraspinal ependymoma preferentially affects young women (13/18) and the thoracic floor (12/18) and that resection was complete in all cases.
Through the literature review, we noticed that many atypical localizations have been reported (Table 1) [18–22]. The extra-axial cerebral localization, the cerebellopontine angle, the pineal region, and the sella turcica are the most recent. The hypothalamus is the one never described as far as we know. This exceptional localization was very suggestive of neurohistiocytosis and neurosarcoidosis. Larsen and colleagues, trying to resolve the enigma of the pituitary fossa ependymomas, suggested that it is the consequence of neoplastic transformation of heterotopic ependymal cells or embryological remnants of the ependymal cells lining in the infundibular process [23]. Our patient presented a very extensive ependymoma at the time of diagnosis, with supratentorial cerebral localization (extra-axial and hypothalamic), subtentorial and intraspinal; intra and extramedullary development.
Extra-cranial and extra-spinal ependymomas are extremely rare [24]. They mainly occur in children and young adults. Sacrum, mediastinum and ovarium are the most frequent. Over a 25-year period, Yust Katz and colleagues [24] published a paper of 8 cases of which five were sacral. The proximity of the sacrum to the spinal cord may explain why it is, indeed, the most frequent location of ependymomas arising outside the central nervous system. Since 1988, only 10 cases of mediastinal ependymoma have been reported. All patients were women with a median age of 44 years. They suffered from back pain and/or dyspnea and had atypical imaging aspects [25]. Ovary, board ligament, and mediastinum are the most reported extra-neurological ependymomas in women. These localizations support the germinal cell theory.
If the tumor location is very variable in ependymomas, the imaging is even more heterogeneous. In CT (Computed Tomography), ependymoma appears iso, hypo, or hyperdense, with heterogeneous enhancement after contrast product injection [7]. CT scan is superior to MRI in highlighting intralesional calcification. However, MRI remains the gold standard in ependymoma imaging [26]. Ependymomas demonstrate low T1, high T2, and intermediate to high Flair signal, with Cystic formation, calcifications, hemorrhage, and necrosis [16, 26]. Diffusion-weighted imaging, Perfusion MRI, and MRI spectroscopy are increasingly used sequences that orient the tumor nature of a suspicious MRI lesion [26]. The diagnosis of ependymoma is based on pathology and immunohistochemistry. The diagnosis of an intracranial ependymoma requires a complement by medullary MRI ± a study of the CSF (cerebrospinal fluid) in search of medullary dissemination, to guide the therapeutic approach [7].
The management of ependymoma is based on surgery and radiotherapy [27, 28]. Maximal safe resection and relief of obstructive hydrocephalus are the targets of surgery [29]. Indeed, whatever the intracranial or medullary seat of the tumor, the surgical resection must be total whenever feasible [27]. A second-look surgery is only recommended for intracranial ependymoma when postoperative MRI shows an unsatisfactory resection [28]. Postoperative conformal radiotherapy is recommended for patients with WHO grade III (anaplastic) ependymomas (Table 2), regardless of the extent of resection, and for patients with WHO grade II ependymomas following incomplete resection [28]. The use of chemotherapy in newly diagnosed ependymomas remains very controversial [16]. However, chemotherapy might be warranted in patients with recurrent ependymomas, who are no longer eligible for local treatments [28].
Conclusions
The diagnosis of ependymoma is very challenging because of its atypical origins, its extensive sites and its heterogeneous MRI aspects. Consequently, ependymoma should be evoked whatever the location; in the central nervous system and even outside of it, especially the sacrum. Indeed, it should be considered a differential diagnosis of tumors in pineal, intrasellar and hypothalamus regions. Only biopsy and immunohistochemistry study can confirm the tumor nature. The optimal management is gross total resection and radiotherapy. If maximal safe resection if impossible, as the case of CSF or spinal dissemination, craniospinal irradiation is recommended and must be carried out quickly.
Availability of data and materials
The data used during the study are available on request from the corresponding author.
Abbreviations
- BMI:
-
Body Mass Index
- MRI:
-
Magnetic Resonance Imaging
- WHO:
-
World Health Organization
- GFAP:
-
Glial Fibrillary Acidic Protein
- EMA:
-
Epithelial Membrane Antigen
- WI:
-
Weighted imaging
- CT:
-
Computed Tomography
- CSF:
-
Cerebrospinal fluid
- Gy:
-
Gray
References
Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18(Suppl. 1):i1-50.
Desandes E, Guissou S, Chastagner P, Lacour B. Incidence and survival of children with central nervous system primitive tumors in the French National Registry of Childhood Solid Tumors. Neuro Oncol. 2014;16:975–83.
Frappaz D, Vasiljevic A, Beuriat PA, Alapetite C, Grill J, Szathmari A, et al. Pediatric ependymomas: Current diagnosis and therapy. Bull Cancer. 2016;103:869–79.
Campello C, Parker F, Slimani S, Le Floch A, Herbrecht A, Aghakhani N, et al. Tumeurs gliales intramédullaires de l’adulte: la série du rapport [Adult intramedullary gliomas]. Neurochirurgie. 2017;63(5):381–90.
Aghakhani N, Messerer M, David P, Herbrecht A, Parker F. Intramedullary ependymomas: a French retrospective multicenter study of 221 cases. Neurochirurgie. 2017;63:391–7.
Ben Ammar CN, Kochbati L, Frikha H, Gargouri W, Benna F, Besbes M, et al. Primitive intracranial ependymomas, Salah-Azaîz institute experience. Cancer Radiother. 2004;8(2):75–80.
Houjami M, Sahraoui S, Benchakroun N, Jouhadi H, Tawfiq N, Benider A. Intracranial ependymomas: Retrospective study of 16 cases. Cancer Radiother. 2011;15:136–9.
Metellus P, Barrie M, Figarella-Branger D, Chinot O, Giorgi R, Jouvet A, et al. Intracranial ependymomas in adult patients. Retrospective analysis of 121 cases from the Multicentric French Study. Neurochirurgie. 2007;53:66–75.
Sayegh ET, Aranda D, Kim JM, Oh T, Parsa AT, Oh MC. Prognosis by tumor location in adults with intracranial ependymomas. J Clin Neurosci. 2014;21(12):2096–101.
Schwartz TH, Kim S, Glick RS, Bagiella E, Balmaceda C, Fetell MR, et al. Supratentorial ependymomas in adult patients. Neurosurgery. 1999;44(4):721–31.
Harrop JS, Ganju A, Groff M, Bilsky M. Primary intramedullary tumors of the spinal cord. Spine. 2009;34(22 Suppl):S69-77.
Hamilton KR, Lee SS, Urquhart JC, Jonker BP. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J Clin Neurosci. 2019;63:168–75.
Wang Y, Cai R, Wang R, Wang C, Chen C. Outcome predictors in the management of intramedullary classic ependymoma: An integrative survival analysis. Medicine (Baltimore). 2018;97(23): e10870.
Lin Y, Smith ZA, Wong AP, Melkonian S, Harris DA, Lam S. Predictors of survival in patients with spinal ependymoma. Neurol Res. 2015;37(7):650–5.
Borges LF. Spinal intramedullary ependymoma: surgical approaches and outcome. J Neurosurg Sci. 2018;62(1):51–62.
Gerstner ER, Pajtler KW. Ependymoma. Semin Neurol. 2018;38(1):104–11.
Gardner L, Kasliwal MK, Hempeck N, Utset M, Gandhi YN. Ependymoma: Unusual differential for a totally extramedullary intraspinal tumor. Neurol India. 2013;61(6):687–90.
Lan Z, Richard SA, Zhang Y. Cerebellopontine angle ependymoma in a young adult: A case report. Medicine (Baltimore). 2019;98(14): e15019.
Khatami D, Kasper EM, Bhadelia R, Rojas R. Radiologic characteristics of ependymomas: a case-based approach. J Neurosurg Sci. 2018;62(1):38–45.
Berhili S, Aissa A, Kadiri S, Cherradi N, El Majjaoui S, El Kacemi H, et al. Extra-axial ependymoma of the cerebral convexity: A very rare intracranial adult tumor. Neuroradiol J. 2017;30(3):281–5.
Wang S, Zong W, Li Y, Wang B, Ke C, Guo D. Pituitary ependymoma: a case report and review of the literature. World Neurosurg. 2018;110:43–54.
Parish JM, Bonnin JM, Goodman JM, Cohen-Gadol AA. Intrasellar ependymoma: clinical, imaging, pathological, and surgical findings. J Clin Neurosci. 2015;22(4):638–41.
Larsen WJ, Sherman LS, Potter SS, William J. Human embryology. 3rd ed. New York: Churchill Livingstone; 2001.
Yust Katz S, Cachia D, Kamiya-Matsuoka C, Olar A, Theeler B, Penas Prado M, et al. Ependymomas arising outside of the central nervous system: A case series and literature review. J Clin Neurosci. 2018;51:105.
Ye WB, Zhou JP, Xu YQ, Lu BY, Li ZJ. Primary mediastinal ependymoma: a case report and literature review. Medicine (Baltimore). 2019;98(44): e17686.
Dorfer C, Tonn J, Rutka JT. Ependymoma: a heterogeneous tumor of uncertain origin and limited therapeutic options. Handb Clin Neurol. 2016;134:417–31.
Thorp N, Gandola L. Management of ependymoma in children, adolescents and young adults. Clin Oncol (R Coll Radiol). 2019;31(3):162–70.
Rudà R, Reifenberger G, Frappaz D, Pfister SM, Laprie A, Santarius T, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20(4):445–56.
Chan MD, McMullen KP. Multidisciplinary management of intracranial ependymoma. Curr Probl Cancer. 2012;36(1):6–19.
Acknowledgements
The authors would like to thank the multidisciplinary staff who contributed to the diagnosis and care of the patient (radiologists, neurosurgeons, pathologists, and radiotherapists).
Funding
No funding was obtained for the present study.
Author information
Authors and Affiliations
Contributions
All the authors took part in the patient's care and support for his family throughout the hospitalization. They all contributed to the drafting of the manuscript. CM, NL, and KN approved the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
It is a non-interventional study. It is also a retrospective report and the authors respected the anonymity of the patient and his archived medical file. Consequently, only the agreement of the head of the department (KN) was required and obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aballa, L., Chraa, M., Louhab, N. et al. Extensive anaplastic multi-centric ependymoma in a young adult: case report and literature review. Egypt J Neurol Psychiatry Neurosurg 59, 67 (2023). https://doi.org/10.1186/s41983-023-00663-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00663-1