Abstract
Background
Meningiomas are one of the most common tumors of the brain and central nervous system. The key role of endocan in predicting tumor growth and prognosis has been shown for several types of cancers; however, this role in meningiomas has not been evaluated. In the current study, we investigated the relationship between endocan serum levels with low- and high-grade meningiomas.
Results
The serum level of endocan in the group with meningiomas was 283.34 (242.09-358.70) pg/ml and in the control group was 250.29 (207.56-329.71) pg/ml respectively (P = 0.172). Afterwards, patients were divided into three different groups (grades I, II, and III) and compared to the control. The level of endocan in the group with grade I of meningioma showed no significant difference compared to control individuals (P = 0.86). When patients with grade II and grade III compared with the control group, endocan serum levels were statistically significant (P = 0.002, P < 0.001 respectively). Moreover, our findings showed that the different grades of meningiomas were statistically significant compared to each other (P < 0.001) regarding endocan serum levels, meaning that the higher the grade, the higher the endocan serum levels.
Conclusion
Our findings revealed that higher grades of meningioma had higher endocan serum levels, however, the role of endocan in pathogenesis or progression of this type of tumor requiring further exclusively assessment.
Similar content being viewed by others
Background
Brain tumors are one of the most important health problems worldwide. They are responsible for 2% of total mortality in all cancers and include the most common type of severe tumors in young adults. After gliomas, meningiomas are the second most common primary neoplasm of the central nervous system. The origin of the tumor is usually from the arachnoid cap cells of the arachnoid villi in the meninges [1, 2]. The prevalence of pathologically confirmed meningiomas are calculated to be approximately 97.5/100,000 in the USA with over 170,000 patients currently diagnosed with meningiomas [3]. It is difficult to estimate the mortality rate and the prevalence of meningiomas, as some patients become aware of their disease when a CT or MRI is done for other reasons and as an incidental finding meningioma is detected. Data from the Central Brain Tumor Registry of the United States (CBTRUS) demonstrates meningiomas are more than twice among females [3]; however, this ratio may be reversed in the rare pre-pubertal meningiomas [2, 4]. According to the World Health Organization (WHO), meningiomas, considering histopathological features such as cell type, mitotic activity, cellularity, necrosis, and brain invasion, are categorized into 3 different grades. Grade I (benign) meningioma is the most common type of meningiomas with approximately 90% prevalence, grade II (atypical meningioma) represents approximately 5-7% of all meningiomas and grade III or anaplastic meningioma accounts for 3% of all meningiomas [5]. Currently, there is no established serum-based marker which can be used for diagnostic and prognostic purposes for meningiomas. Therefore, identification of non-invasive circulating tumor markers would not only improve early detection of meningiomas but also improve survival rate of meningioma patients [6].
Endocan, previously known as endothelial cell-specific molecule-1(ESM-1), is soluble dermatan sulfate proteoglycan, which is primarily produced by endothelial cells in different organs such as kidney, liver, lung, and gastrointestinal system [7,8,9]. It contains a core protein with 165 amino acids that are enveloped by a mucopolysaccharide chain that is connected to a serine residue of the protein [10]. The gene encoding human endocan is located on chromosome 5 containing two introns and three exons [11]. The expression of endocan is regulated by some inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) as well as some angiogenic factors such as fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor-A (VEGF-A) [12, 13]. Over-expression of endocan is frequently accompanied by hypersecretion of intercellular adhesion molecule-1,2 (ICAM-1 and ICAM-2) leading to increase in leukocyte migration, adhesion, and activation [14, 15]. Due to the potential role of both inflammatory cytokines and intercellular adhesion molecules in an inflammatory response and ischemia/reperfusion injuries, it is now hypothesized that the over-expression of endocan can activate both inflammatory and tumoral processing through activation of inflammatory mediators and adhesion molecules. Recent studies have focused on the central role of endocan over-expression in angiogenesis and tumorigenesis and thus this biomarker seems to be a selective target for cancer therapy [16]. This hypothesis has been examined and even demonstrated in subgroups of patients suffering malignancies of liver, kidney, lungs, breast, pancreas, prostate, ovary, and brain glioblastoma [17,18,19,20,21,22]. However, the key role of endocan in other tumors such as meningiomas has not been evaluated so far. Because of the central role of activating inflammatory pathways and also angiogenesis in the progression of meningiomas, in this study, the relationship between endocan serum levels and meningiomas (low and high grade) were investigated.
Methods
This study consisted of 60 consecutive patients who were suffering from meningiomas as the case group from two university hospitals, as well as 30 age-sex-matched healthy individuals as a control group without any history or evidence of clinical problems especially brain disorders and also with no history of any medication. Patients with any kind of chronic or acute infection, immunological and metabolic diseases, other neoplastic diseases, cardiovascular diseases, and recent major surgical procedures, in which, the endocan level could be affected by such diseases were excluded. Approval for the study was obtained from the Ethical Committee of University of Medical Sciences, and all participants gave written informed consent before participation in the study. All patients and controls were evaluated in terms of detailed medical history and complete physical examinations. Also, routine laboratory tests were considered in both groups on admission. The definitive diagnosis of meningiomas was based on histopathological tissue analysis and specific imaging methods including computed tomography (CT) and or magnetic resonance imaging (MRI) in all patients. In our studied hospitals, Philips brilliance 16 CT scan printed in Netherland and GE light speed 16 slice CT scan manufactured in the USA were used. Furthermore, for MRI imaging GE SIGNA HDX 1.5T, USA, and GE T SIGNA EXCITE HD 1.5, USA, and SIMENS MAGNETOM symphony manufactured in Germany were used. The grade of the tumor was determined based on the WHO classification [5].
Measurement of endocan serum levels in both case and control groups were performed by commercially reliable ELISA kit according to manufacturer’s protocols (MyBioSource, CA, USA), and ELISA reader machine (Jencons Anthos 2020, type: 22 550, Austria), and using the standard samples with known levels of endocan, provided by the manufacturer and presented as pg/ml. The assay range for this ELISA kit was 31.2 pg/ml up to 2000 pg/ml, and its sensitivity was ≤ 10 pg/ml.
Statistical Package for Social Sciences (SPSS, version 22; Chicago, IL, USA) was used for data analysis. The normality of the data was analyzed using Kolmogorov-Smirnoff and the Shapiro-Wilk tests. Variables are presented as median and first and third interquartile ranges (IQR), otherwise, as mean ± standard deviation (SD). Frequencies are presented as percentages. Mann–Whitney U and Kruskal-Wallis tests were used to analyze the differences among groups. To assess the value of endocan to discrimination of meningiomas from the normal conditions, the area under the ROC curve (AUC) was determined and the best cut-off point for this biomarker as well as its sensitivity and specificity were calculated. In our study, P values of 0.05 or less were considered statistically significant.
Results
Of 60 patients with meningiomas, 42 (70%) cases were female and 18 (30%) were male. The age range was between 30 and 84 years in patients with a mean age of 53.93 ± 13.32. The control group included 30 healthy individuals and the majority of them were female (N = 20, 66.6%), and the age range was between 38 and 86 years with a mean age of 54.11 ± 13.72.
In patients’ groups, 39 cases (65.0%) suffered from grade I of meningioma, 15 cases (25%) from grade II of meningioma, and 6 cases (10%) from grade III of the tumor.
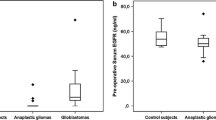
The endocan serum level in the group with meningiomas was 283.34 (242.09-358.70) pg/ml, and in the control group was 250.29 (207.56-329.71) pg/ml respectively (p = 0.172). Based on the grade, patients were divided into three different groups (grade I, II, and III) and the level of endocan was evaluated separately in each group and compared to the control. The serum level of endocan in the group with grade I of meningioma was 259.39 (225.67-287.17) pg/ml and in the control group was 250.29 (207.56-329.71) pg/ml, indicating no significant differences (P = 0.86). While in patients with grade II, serum level was 355.24 (325.14-431.74) and in the control group was 250.29 (207.56-329.71) pg/ml which showed statistically significant differences (P = 0.002). Furthermore, patients with grade III of meningioma had statistically higher serum levels of endocan in comparison with control group [493.93 (406.92-710.97) versus 250.29 (207.56-329.71) pg/ml respectively, P < 0.001] (Fig. 1). In the meningioma and control groups, the endocan serum levels were independent of baseline parameters including gender and age (Table 1).
Regarding different meningioma grades, patients with grade II had significantly higher endocan serum levels compared with grade I (P < 0.001). This is also true for comparison between grade III versus grade I and grade III versus grade II (P < 0.001, P = 0.02 respectively) (Table 2).
According to the ROC curve analysis (Fig. 2), the measuring endocan serum levels could be a probable index for differentiating meningioma from normal condition (AUC = 0.624, 95% CI, 0.515-0.725). The best cut-off value of endocan for this differentiation was estimated to be 215.15 pg/ml yielding a sensitivity of 65% and a specificity of 60%.
Discussion
The majority of meningiomas are benign and can be cured by surgical procedures, but, approximately 20% of meningioma patients suffer from an aggressive clinical course with tumor recurrence or progressive form, leading to significant morbidity and increased mortality of patients [18]. Hence, novel and specific biomarkers that can help to early diagnosis or prognosis to improve patient outcomes are important [23].
Endocan is a proteoglycan that is primarily produced by kidney, liver, lung, and gastrointestinal system endothelial cells. Endocan is also known as an indicator of endothelial dysfunction. It has been demonstrated that it plays an important role in tumor growth and angiogenesis and its plasma levels have been shown to increase in some cancers [24, 25]. It has shown a positive correlation with tumor recurrence and progression. In the present study, a significant endocan serum levels were seen in patients with grades II and III of meningioma compared to healthy individuals. There are several previous studies that have investigated endocan serum levels or expressions in different kinds of cancers, for example, Atukeren and colleagues, studied levels of endocan in patients with meningioma and glioma. They found that the mean level of endocan has a significant incline from the control group to the malignant tumor groups although the meningioma (benign) had the lowest mean level, and the high-grade glioma (most malignant) group had the highest mean level. They also showed that incline expression was significantly correlated with the degree of malignancy, which means that with increasing the degree of malignancy, the endocan expression also increased [26]. In another study, Maurage and colleagues investigated endocan expression in brain tumors. In their study, endocan immune reactivity was detected in hyperplastic endothelial cells in high-grade gliomas, mostly at the tumor margins; endothelial cells were mostly endocan negative in low-grade gliomas, and it was never detected in the cerebral cortex distant from the tumors. No endocan immune reactivity was observed in pure anaplastic oligodendrogliomas, whereas there was endocan immune reactivity in both endothelial and tumor cells in anaplastic oligoastrocytomas and anaplastic astrocytomas. The intensity of immunolabeling in these tumor cells varied considerably from cell to cell, and not all tumor cells expressed endocan in their cytoplasm. In general, as shown in their study, low-grade glioma cases were almost all negative for endocan expression as were cases with grade III oligodendrogliomas [27]. Lassalle and colleagues studied endocan expression in different organs. They found out that endocan may participate in specialized endothelial functions, particularly in the lung vascular spaces. Also, endocan mRNA was detected with less intensity in the kidney, thereby indicating that vascular endothelium from lung and kidney may have common functional entities mediated by endocan. In their study, other endothelial cell-rich tissues, such as heart or placenta, have shown poor expression of endocan mRNA and the reason was unclear. One can suggest that the constitutive expression of endocan mRNA in the vascular endothelial cells may vary considerably, depending on either an organ-specific differentiation state of endothelial cells or specific factors present in the local microenvironment [7]. In another study, Leroy and colleague investigated endocan expression in clear renal cell carcinoma and papillary carcinoma and they illustrated that endocan in papillary carcinomas is weakly or not expressed. This finding is of interest because there are unique molecular mechanisms for clear cell renal carcinoma and papillary carcinomas [28]. As mentioned in our findings, the different grades of meningioma had statistically significant differences compared to each other and control group regarding serum levels of endocan meaning that the higher the grade, the higher the serum endocan level. Previous studies have shown that not only in meningiomas but also in gliomas, endocan is never expressed in the cerebral cortex and endothelial cells in lower grades (grade I and grade II) and endocan expression in glioblastoma is continuously related to abnormal vasculature reflecting neoangiogenesis [27, 29]. Another study was conducted to investigate the endothelial expression of endocan and its relation to tumor progression in pituitary adenoma by Cornelius and colleagues. As a result, they found out that in normal pituitary, endocan is never expressed in the endothelial cells but is observed in few endocrine cells whereas in a subset of pituitary tumors endocan is expressed in endothelial and endocrine cells [30].
Our findings showed the association between endocan serum levels and different grades of meningioma that may emphasize the critical role of endocan as a predicting or prognostic factor for the disease. Additionally, according to the results of the ROC curve analysis, the endocan appears to be a probable marker for meningiomas; however, more investigations with larger sample sizes are warranted.
This study has some limitations. First, due to retrospective nature of the study, there may be risk of patients’ bias. Second, certain proportion of patients without complete data was excluded so study had a relatively small number of patients. Our conclusion needs to be verified in future by a multi-center investigation.
Conclusion
In total, although meningiomas are considered as the heterogeneous brain tumors with aggressive potential, our findings suggest that higher grades of meningioma had higher endocan serum levels, however, the role of endocan in pathogenesis or progression of this type of tumor requiring further exclusively assessment.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author.
Abbreviations
- ELISA:
-
Enzyme-linked immunosorbent assay
- CBTRUS:
-
Central Brain Tumor Registry of the United States
- WHO:
-
World Health Organization
- ESM-1:
-
Endothelial cell-specific molecule-1
- TNF-α:
-
Tumor necrosis factor-α
- ICAM-1 and ICAM-2:
-
Intercellular adhesion molecule-1,2
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
References
Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535–43. https://doi.org/10.1016/S0140-6736(04)16153-9.
Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neuro-Oncol. 2010;99(3):307–14. https://doi.org/10.1007/s11060-010-0386-3.
Li X, Zhao J. Intracranial meningiomas of childhood and adolescence: report of 34 cases with follow-up. Childs Nerv Syst. 2009;25(11):1411–7. https://doi.org/10.1007/s00381-009-0949-9.
Menon G, Nair S, Sudhir J, Rao B, Mathew A, Bahuleyan B. Childhood and adolescent meningiomas: a report of 38 cases and review of literature. Acta Neurochir. 2009;151(3):239–44. https://doi.org/10.1007/s00701-009-0206-8.
Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Saydam O, Erkan EP, Strobel T, Dorfer C, Sontagbauer M, Weinhaeusel A, et al. Circulating tumor biomarkers in meningiomas reveal a signature of equilibrium between tumor growth and immune modulation. Front Oncol. 2019;9:1031.
Lassalle P, Molet S, Janin A, Van der Heyden J, Tavernier J, Fiers W, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271(34):20458–64. https://doi.org/10.1074/jbc.271.34.20458.
Palmiere C, Augsburger M. Endocan measurement for the postmortem diagnosis of sepsis. Leg Med (Tokyo). 2014;16(1):1–7. https://doi.org/10.1016/j.legalmed.2013.09.007.
Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta Rev Cancer. 2006;1765(1):25–37. https://doi.org/10.1016/j.bbcan.2005.08.004.
Yang J, Yang Q, Yu S, Zhang X. Endocan: a new marker for cancer and a target for cancer therapy. Biomed Rep. 2015;3(3):279–83. https://doi.org/10.3892/br.2015.438.
Scherpereel A, Gentina T, Grigoriu B, Sénéchal S, Janin A, Tsicopoulos A, et al. Overexpression of endocan induces tumor formation. Cancer Res. 2003;63(18):6084–9.
Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34(2):532–7. https://doi.org/10.1097/01.CCM.0000198525.82124.74.
Caires NDF, Gaudet A, Portier L, Tsicopoulos A, Mathieu D, Lassalle P. Endocan, sepsis, pneumonia, and acute respiratory distress syndrome. Crit Care. 2018;22(1):280. https://doi.org/10.1186/s13054-018-2222-7.
Béchard D, Scherpereel A, Hammad H, Gentina T, Tsicopoulos A, Aumercier M, et al. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167(6):3099–106. https://doi.org/10.4049/jimmunol.167.6.3099.
Ustyol A, Aycan Ustyol E, Gurdol F, Kokali F, Bekpınar S. P-selectin, endocan, and some adhesion molecules in obese children and adolescents with non-alcoholic fatty liver disease. Scand J Clin Lab Invest. 2017;77(3):205–9. https://doi.org/10.1080/00365513.2017.1292363.
Li C, Geng H, Ji L, Ma X, Yin Q, Xiong H. ESM-1: a novel tumor biomaker and its research advances. Anti Cancer Agents Med Chem. 2019;19(14):1687–94. https://doi.org/10.2174/1871520619666190705151542.
Tanriverdi T, Kemerdere R, Inal BB, Yuksel O, Emre HO, Ahmedov M, et al. Serum endocan levels before and after surgery on low-grade gliomas. Surg Neurol Int. 2017;8(1):32. https://doi.org/10.4103/sni.sni_405_16.
Laloglu E, Kumtepe Y, Aksoy H, Topdagi Yilmaz EP. Serum endocan levels in endometrial and ovarian cancers. J Clin Lab Anal. 2017;31(5):e22079. https://doi.org/10.1002/jcla.22079.
Rebollo J, Geliebter J, Reyes N. ESM-1 siRNA knockdown decreased migration and expression of CXCL3 in prostate cancer cells. Int J Biomed Sci. 2017;13(1):35.
Lin L-Y, Yeh Y-C, Chu C-H, Won JG, Shyr Y-M, Chao Y, et al. Endocan expression is correlated with poor progression-free survival in patients with pancreatic neuroendocrine tumors. Medicine (Baltimore). 2017;96(41).
Sagara A, Igarashi K, Otsuka M, Kodama A, Yamashita M, Sugiura R, et al. Endocan as a prognostic biomarker of triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):269–78. https://doi.org/10.1007/s10549-016-4057-8.
Huang X, Chen C, Wang X, Zhang J-Y, Ren B-H, Ma D-W, et al. Prognostic value of endocan expression in cancers: evidence from meta-analysis. Onco Targets Ther. 2016;9:6297.
Zhi F, Shao N, Li B, Xue L, Deng D, Xu Y, et al. A serum 6-miRNA panel as a novel non-invasive biomarker for meningioma. Sci Rep. 2016;6(1):32067. https://doi.org/10.1038/srep32067.
Lv Z, Fan Y, Chen H, Zhao D. Endothelial cell-specific molecule-1: a potential serum marker for gastric cancer. Tumor Biol. 2014;35(10):10497–502. https://doi.org/10.1007/s13277-014-2319-9.
El Behery MM, Seksaka MA, Ibrahiem MA, Saleh HS, El Alfy Y. Clinicopathological correlation of endocan expression and survival in epithelial ovarian cancer. Arch Gynecol Obstet. 2013;288(6):1371–6. https://doi.org/10.1007/s00404-013-2863-3.
Atukeren P, Kunbaz A, Turk O, Kemerdere R, Ulu MO, Turkmen Inanir N, et al. Expressions of endocan in patients with meningiomas and gliomas. Dis Markers. 2016;2016:1–5. https://doi.org/10.1155/2016/7157039.
Maurage C-A, Adam E, Minéo J-F, Sarrazin S, Debunne M, Siminski R-M, et al. Endocan expression and localization in human glioblastomas. J Neuropathol Exp Neurol. 2009;68(6):633–41. https://doi.org/10.1097/NEN.0b013e3181a52a7f.
Leroy X, Aubert S, Zini L, Franquet H, Kervoaze G, Villers A, et al. Vascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology. 2010;56(2):180–7. https://doi.org/10.1111/j.1365-2559.2009.03458.x.
Dieterich LC, Mellberg S, Langenkamp E, Zhang L, Zieba A, Salomäki H, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J Pathol. 2012;228(3):378–90. https://doi.org/10.1002/path.4072.
Cornelius A, Cortet-Rudelli C, Assaker R, Kerdraon O, Gevaert MH, Prévot V, et al. Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 2012;22(6):757–64. https://doi.org/10.1111/j.1750-3639.2012.00578.x.
Acknowledgements
Not applicable
Funding
This work was supported by a grant from Shiraz University of Medical Sciences (grant no. 15233-01-01-1396).
Author information
Authors and Affiliations
Contributions
MJF: Design of the work, interpretation of data, revision of manuscript, FS: The acquisition, analysis, interpretation of data, drafting the manuscript. MM: Interpretation of data, drafting the manuscript. AAN: interpretation of data, drafting the manuscript. MP: The acquisition and analysis of data. YS: The acquisition and analysis of data. MT: Design of the work, revision of manuscript. AG: Design of the work, revision of manuscript. Note: The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for the study was obtained from the Ethical Committee of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1399.106), and all participants gave written informed consent before participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fattahi, M.J., Sedaghat, F., Malekzadeh, M. et al. Endocan serum levels in patients with low- and high-grade meningiomas: does this biomarker have an indicative role?. Egypt J Neurol Psychiatry Neurosurg 57, 92 (2021). https://doi.org/10.1186/s41983-021-00346-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-021-00346-9