Abstract
Background
Attempts based on increasing the efficacy of Baculovirus and/or reducing the application concentration of synthetic insecticides through integrated lepidopteran management are appreciated role for conserving the environment. Impact of the multiple nucleopolyhedrosis virus (SpliMNPV) with emamectin benzoate (Em) against the cotton leaf worm, Spodoptera littoralis, was examined to identify the effective strategy for applying both agents in the control program successfully.
Main body
The LC50 and LC90 were drastically decreased from 1.9 × 106 and 1.0 × 1010 PIB/ml in SpliMNPV treatment to reach 8.87 × 101 and 1 × 104 PIB/ml, respectively in the SpliMNPV concentrations + Em LC25 treatment. This interaction was considered as potentiation. Larvicidal activity of Em was highly increased by Em concentrations + SpliMNPV LC25 treatment than the separately Em treatment; however, this interaction was considered as additive. Moreover, the mixture treatment (SpliMNPV LC99 + Em LC50) provided almost full protection of viral pathogenicity up to 48 h at natural exposure periods. Furthermore, the mixture treatment had a negative impact on the insect survival and reproduction of treated individuals.
Conclusion
Results indicated that the virus infectivity was increased by a mixture treatment of SpliMNPV + Em in particular facing UV sunlight, which causes virus degradation as well as reduced the effective doses of Em. These findings suggest that this simultaneous treatment maybe an effective technique to be applied in S. littoralis control strategy.
Similar content being viewed by others
Background
The use of insecticides to control the cotton leaf worm, Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), and other lepidopteran pests has an adverse impact on the ecosystem and its fauna, devastation of natural enemies, and development of insect resistance (Ahmad et al. 2009). Research into alternative strategies for limited use and/or reducing the dose application with that type of synthetic pesticide might be beneficial to save the environment as well as successfully suppress the insect population (Lalouette et al. 2016). Additionally, developing the efficacy of bio-pesticides such as natural enemies and pathogens, which are considered environmentally friendly methods to be more effective against insect pests, is appreciated. Baculoviruses is considered one of the bio-control methods against lepidopteran insects, which is commercially produced in some areas with a trade name (Sayed et al. 2020). The cotton leaf worm S. littoralis multiple nucleopolyhedrosis virus (SpliMNPV) belongs to Baculoviruses that may be used as a promising agent for its bio-control (Yang et al. 2012). Admittedly, sunlight UV radiation has adversely affected SpliMNPV persistence in the environment, causing pyrimidine dimers of the viral DNA chain, and rapid degradation of the virus (Yoon et al. 2000). Efforts have been made to protect the pathogenicity of SpliNPV against UV radiation, using various substances. Ignoffo and Batzer (1971) and Rabindra et al. (1989) proposed coax boric acid acted as UV protectants, respectively. Nevertheless, the researches into appropriate techniques to improve Baculovirus infectivity rather than UV protectants were also employed. Consequently, attempts have been conducted to boost NPV infectivity, such as juvenile hormones (Liao et al. 2016) and gamma irradiation (Sayed and El-Helaly 2018). Moreover, synthetic insecticides have been suggested as synergic agents when combined with Baculoviruses such as spinosad with both SfNPV and PgNPV on Spodoptera frugiperda (J.E. Smith) and Pectinophora gossypiella (Saund.), respectively (Méndez et al. 2002; Jackson et al. 2014); methoxyfenozide with SpliMNPV on S. littoralis (Pineda et al. 2009); and azadirachtin with SfMNPV on S. frugiperda (Nathan and Kalaivani 2006). Similarly, emamectin benzoate (Em) may interact with SpliNPV for improving S. littoralis control. Em is isolated from soil actinomycete, Streptomyces avermitilis, that is occurring in nature and is known to be an important natural chemistry insecticide against lepidopteran pests (Jansson et al. 1996). The key action of Em is to cause permanent paralysis on the nerve transmitter (Jansson and Dybas 1998). Given the high toxicity of Em on Lepidoptera, it is less harmful on most beneficial arthropods (e.g., honey bees, parasitoids, predators (Wolterink et al. 2012). However, its extensive use and lethal doses applied may have deleterious effect on biodiversity.
Thus, the aim of the study was to identify the Em-SpliNPV interaction in order to improve S. littoralis control. Besides, the larvicidal activity of the Em + SpliMNPV mixture to elucidate the pathogenicity of the virus and its persistence against UV sunlight was examined.
Materials and methods
Insect colony
A laboratory stock culture of the cotton leaf worm S. littoralis was initiated from eggs samples of infested tomato plant at Giza region, Egypt. Collected eggs were maintained under laboratory conditions of 25 ± 2 °C and 65 ± 5% RH inside a plastic cage until hatching. The newly hatched larvae were transferred to a larval rearing cage (40 × 40 × 10 cm) containing castor plant leaves, Ricinus communis as food for adaptation until pupation. The upcoming larval colony was maintained on a semi synthetic diet (Shorey and Hale 1965).
Bioassay of emamectin benzoate toxicity and SpliMNPV pathogenicity
Local isolate of SpliMNPV was used in the bioassay experiments. The pathogenicity of SpliMNPV was evaluated on the 2nd instar larvae of S. littoralis. Eight different virus concentrations from 1 × 102 to 1 × 109 BIPs/ml (polyhedral inclusion bodies/ml) were prepared from the stock concentration (2.3 × 109 PIB/ml); it was diluted in distilled water to adjust the experimental concentrations. Em (1.9% EC) was provided by Elhelb, Pesticides and Chemicals Company. Eight concentrations, 0.01, 0.1, 0.3, 0.5, 0.7, 1.0. 2.0, and 4.0 ppm, were prepared, using distilled water. The concentration-mortality response of both SpliMNPV and Em was calculated, following the diet surface contamination technique (Cisneros et al. 2002); 2 ml of each treatment was spread on the surface special plate divided into 50 cells containing 50 ml semi-artificial diet. Distilled water was used for untreated control experiments. Each treatment was repeated in 5 replicates with 50 larvae each. Mixture treatments (SpliNPV + Em) were conducted, using different SpliMNPV concentrations + LC25 of Em and vice versa, following the methods mentioned above. Mortality responses of larvae were recorded daily in each treatment. Comparing evaluation of the mixture treatment to the single ones was carried out according to (Mansour et al. 1966) as follows:
Co-toxicity factor (%) = (% Observed mortality − % Expected mortality/% Expected mortality) 100, where the factor (− 20 to + 20) is additive, + 20 or more is potentiation, and − 20 or more is antagonism.
SpliMNPV protection against UV irradiation
An experimental area (500 m2) of tomato was set up in the spring season with the conditions of 24–27 °C, 61–65% RH, and daylight was approximately 14 h. At the time of field application, LC50 concentration of Em and LC99 of SpliMNPV were prepared and kept in the fridge till spraying. The virus and Em were thoroughly mixed (v/v) together, and the measured volume was used into a hand sprayer. Virus suspension and separate treatments were applied on tomato foliage, using hand sprayer (1 l). Leaves were randomly collected from treated and untreated plants at 0, 10, 24, 48, 96, and 168 days post application and kept individually. Every leaf was placed in a glass bottle that allowed 10 neonate larvae to feed for 48 h before being transferred to fresh leaves from the same treatment. Larval mortality was daily recorded as descripted by Shapiro et al. (2008). The experiments were repeated in 5 replicates.
Latent effect of Em and/or SpliMNPV on S. littoralis biology
The impact of sub-lethal concentrations of SpliMNPV and Em either alone or in a mixture (Em 0.05 ppm + SpliMNPV 1 × 104 BIPs) treatment on the biological parameters of S. littoralis 2nd instar larvae was evaluated. Survival rate was estimated through the larvae survived at various treatments in comparison with the control; also, larval duration, pupal period, and adult longevity were included. Moreover, the daily eggs laid by emerged females and their hatchability were recorded. Each treatment was replicated 5 times, and every replicate contained 50 individuals.
Statistical analysis
Mortality data of either SpliMNPV + Em or the separated treatments were analyzed, using Probit analysis, slope. LC50 was calculated according to Finney (1971). Average rates of reduction in virus activity expressed in mortality percentages and the percentages of original activity remaining (% OAR) were conducted according to Muro and Paul (1985) and were calculated as the formula of Sun’s model at each exposure time (Sun et al. 2004). The data of biological studies were analyzed by the analysis of variance (ANOVA) technique, and the means were analyzed, using Duncan’ s multiple range test (P = 0.05) (Steel and Torrie 1960). The potential of Em to prolong the virus persistence was analyzed as described by Muro and Paul (1985).
Results and discussion
Toxicity effect of Em on the 2nd instar larvae of S. littoralis is shown in Fig. 1. The LC50 and LC90 were estimated at 0.13 and 0.68 ppm, respectively. The results indicated that the larvae of S. littoralis was highly sensitive to Em in comparison to those stated by Bengochea et al. (2014) on S. exigua and Ahmad et al. (2003) on Helicoverpa armigera. Furthermore, the data of SpliNPV pathogenicity is illustrated in Fig. 2; the concentrations of 1.9 × 106 and 1.0 × 1010 PIB/ml of SpliNPV were reported in LC50 and LC90, respectively. The SpliNPV pathogenicity was significantly improved by adding 0.1 Em concentration, where the LC50 and LC90 concentrations of the mixture decreased to 8.87 × 101 and 1 × 104 PIB/ml, respectively (Fig. 2). The co-toxicity factors measured in this experiment indicated that they were more than + 20 at all tested concentrations and could be considered as potentiation. This positive interaction resulted from the overall mortality rate of Em and SpliMNPV separately was lower than that for their mixture (Fig. 3a). The synergistic effect of the mixture treatment can be referred to a high toxic effect of Em against insect mid gut cells (Aljabr et al. 2014) that may increase the penetration of viral bodies into the nucleus and/or an immunity degradation in the treated larvae by Em (Birah et al. 2008; Zamora-Avilés et al. 2013) and may support the pathogen infection. Meanwhile, the data of other (SpliMNPV 1 × 104PIB/ml + Em concentrations) treatment revealed that the toxicity of Em increased in the mixture than in separate ones, where LC50 and LC90 decreased to 0.055 and 0.026 ppm, respectively (Fig. 2). The co-toxicity factors of this experiment ranged from − 20 to + 10 at the tested concentrations and could be considered as additive (Fig. 3b). This interaction was based on the overall mortality rate of Em and SpliMNPV separately that was approximately equal with the mortality rate of their mixture. The present findings contradict with those reported an antagonistic effect when chemical pesticides combined with NPV, for instance cartap hydrochloride with S. littura granulovirus SpltGV (Baculovirus) on S. littura (Subramanian et al. 2005) and carbamate methomyl with Autographa californica nucleopolyhedrovirus AcNPV on Heliothis virescens (McCutchen et al. 1997). Recently, Dader et al. (2020) identified a synergy of emamectin with AcMNPV and SpliNPV on S. exigua and S. littoralis when they were sequentially feeding of NPV where the LC50 of Em was followed by the LC50 of NPV.
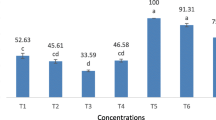
Field test presented higher rates of virus protection against natural sunlight in the (SpliMNPV + Em) treatment than the separate ones (Figs. 4 and 5). Percentages of larval mortality were significantly high at the exposure periods from 10 to 48 h than SpliMNPV separately. The original activity remaining (OAR) revealed that Em could extent the viral persistence since the OAR was similar until 48 h of natural sunlight exposure time as compared to the SpliMNPV separately. The percentages of larval mortality in the mixture treatment were significantly reduced at 69 and 168 h. These results could refer to those reported in a degradation degree of Em under UV light, where its photodegradation was relatively high (Zhu et al. 2011). The median lethal inactivation time (LIT50) showed slightly higher in the mixture treatment than SpliMNPV separately (Fig. 6). It gave in ascending potency 3.5-folds that means a high preservation to the virus. In the obtained results on the interaction of Em + SpliMNPV in mixture, synergistic effect identified may lead to many benefits, enhancing the S. littoralis control in short-term application, delaying the development of insect resistance, and replacing the conventional pesticides with that environmentally friendly product.
The impact of mixture treatment on insect survival is shown in Fig. 7. The data showed that the reduction in larval period in Em was significantly shorter than in SpliMNPV and in mixture treatment; the reduction in the pupal duration was non-significant among various treatments. Additionally, the periods of adult longevity in Em and mixture treatments were significantly shorter than those observed in spliMNPV and control treatments. Such variability in insect survival via Em treatment may be attributed to the direct action of Em on insect cell physiological functions (Rothman and Myers 1996). Moreover, the average numbers of daily eggs laid/female that treated as larvae were significantly lower in Em and mixture tratments than in spliMNPV and control treatments (Fig. 8), while the hatched egg reductions were significantly higher in all tested treatments than the control (Fig. 9). These findings are consistent with Lalouette et al. (2016) who reported hormonal changes in the insect pests when sub lethal concentrations were applied.
Conclusion
Baculovirus and Em are safe promising tools against S. littoralis. The larvicidal activity of Em + SpliMNPV LC25 increased than the single Em treatment. Evidently, the treatment of SpliMNPV LC99 + Em LC50 effectively protected the SpliMNPV against natural sunlight. The synergistic effect of the mixture treatment improved the pathogenicity of SpliMNPV.
Availability of data and materials
The authors declare that they have no objection to the availability of data and materials.
Abbreviations
- SpliMNPV:
-
Spodoptera littoralis multiple nucleopolyhedrosis virus
- PIB:
-
Polyhedral inclusion bodies
- Em:
-
Emamectin benzoate
- LC50 :
-
Median lethal concentration or lethal concentration 50
- LC99 :
-
Lethal concentration 99
- UV:
-
Ultraviolet radiation
References
Ahmad M, Arif MI, Ahmad Z (2003) Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to new chemistries in Pakistan. Crop Prot 22(3):539–544
Ahmad M, Saleem MA, Sayed AH (2009) Efficacy of insecticide mixtures against pyrethroid and organophosphate resistant population of Spodoptera littoralis (Lepidoptera: Noctuidae). Pest Manag Sci 65:266–274
Aljabr AM, Rizwan-ul-Haq M, Hussain A, Al-Mubarak AI, AL-Ayied HY (2014) Establishing midgut cell culture from Rhynchophorus ferrugineus (Olivier) and toxicity assessment against ten different insecticides. In Vitro Cell Dev Biol Anim 50(4):296–303
Bengochea P, Sánchez-Ramos I, Saelices R, Amor F, Del Estal P, Viñuela E, Adán Á, López A, Budia F, Medina P (2014) Is emamectin benzoate effective against the different stages of Spodoptera exigua (Hübner)(Lepidoptera, Noctuidae)? Irish J Agric Food Res 1:37–49
Birah A, Mahapatro G, Gupta G (2008) Toxicity evaluation of emamectin benzoate against tobacco caterpillar (Spodoptera litura) by three different assay techniques. Indian J Entomol 70(3):200–205
Cisneros J, Peterz JA, Penagos DI, Ruiz D, Goulson P, Caballero P, Williams T (2002) Formulation of baculovirus with boric acid for control of Spodoptera frugiperda (Lepidoptera: Noctuidae). Biol Control 23:87–95
Dader B, Aguirre E, Caballero P, Medina P (2020) Synergy of Lepidopteran nucleopolyhedrovirus AcMNPV and SpliNPV with insecticides. Insects 11(5):316
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge Univ, Cambridge
Ignoffo CM, Batzer OP (1971) Microencapsulation and ultraviolet protectants to increase sunlight stability of an insect virus. J Econ Entomol 64:8531–8600
Jackson DM, Shapiro M, Shepard BM (2014) Effects of spinosad and neem on the efficacy of a nucleopolyhedrovirus on pickleworm larvae1. J Agric Urban Entomol 30(1):28–38
Jansson RK, Dybas RA (1998) Avermectins: biochemical mode of action, biological activity and agricultural importance. In: Ishaaya I, Degheele D (eds) Insecticides with novel modes of action—mechanisms and application. Springer, Berlin, Heidelberg, New York, pp 153–170
Jansson RK, Peterson RF, Halliday WR, Mookerjee PK, Dybas RA (1996) Efficacy of solid formulations of emamectin benzoate at controlling lepidopterous pests. Florida Entomol 79:434–449
Lalouette L, Pottier MA, Wycke MA, Boitard C, Bozzolan F, Maria A, Demondion E, Chertemps T, Lucas P, Renault D, Maibeche M (2016) Unexpected effects of sublethal doses of insecticide on the peripheral olfactory response and sexual behavior in a pest insect. Environ Sci Pollut Res 23(4):3073–3085
Liao ZH, Kuo TC, Shih CW, Tuan SJ, Kao YH, Huang RN (2016) Effect of juvenile hormone and pyriproxyfen treatments on the production of Spodoptera litura nuclear polyhedrosis virus. Entomol Exp Appl 161(2):112–120
Mansour NA, Eldefrawi ME, Tappozada A, Zied M (1966) Toxicolo-gical studies on the Egyptian cotton leaf worm Prodenia litura F. VII. Potentiation and antagonism of órgano-phosphorus and carbamates. J Econ Entomol 59:307–311
McCutchen BF, Hoover K, Preisler HK, Betana MD, Herrmann R, Robertson JL, Hammock BD (1997) Interactions of recombinant and wild-type baculoviruses with classical insecticides and pyrethroid-resistant tobacco budworm (Lepidopteran: Noctuidae). J Econ Entomol 90:1170–1180
Méndez WA, Valle J, Ibarra JE, Cisneros J, Penagos DI, Williams T (2002) Spinosad and nucleopolyhedrovirus mixtures for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Biol Control 25(2):195–206
Muro EM, Paul JI (1985) Laboratory evaluation of new ultraviolet absorbers for protection of Douglas-fir tussock moth (Lepidoptera: Lymantriidae) baculovirus. J Econ Entomol 78:951–957
Nathan SS, Kalaivani K (2006) Combined effects of azadirachtin and nucleopolyhedrovirus (SpltNPV) on Spodoptera litura Fabricius (Lepidoptera: Noctuidae) larvae. Biol Control 39:96–104
Pineda S, Martínez AM, Figueroa JI, Schneider MI et al (2009) Influence of azadirachtin and methoxyfenozide on life parameters of Spodoptera littoralis (Lepidoptera: Noctuidae). J Econ Entomol 102(4):1490–1496
Rabindra RJ, Sathiah N, Muthiah C, Jayaraj S (1989) Controlled droplet application of nuclear polyhedrosis virus with adjuvants and UV protectants for the control of Heliothis armigera (Hbn) on chickpea. J Biol Control 3:37–39
Rothman LD, Myers JH (1996) Debilitating effect of viral diseases on host Lepidoptera. J Invertebr Pathol 67:1–10
Sayed WAA, El-Bendary H, El-Helaly AMA (2020) Increasing the efficacy of the cotton leaf worm Spodoptera littoralis nucleopolyhedrosis virus using certain essential oils. Egypt J Biol Pest Control 30(1):1–7
Sayed WAA, El-Helaly AMA (2018) Effect of gamma irradiation on the susceptibility of the cotton leaf worm, Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae) to the infection with nucleopolyhedrosis virus. Egypt J Biol Pest Control 28(1):73
Shapiro M, El-Salamouny S, Shepard BM (2008) Green tea extracts as ultraviolet protectants for the beet armyworm, Spodoptera exigua nucleopolyhedrovirus. Biocontrol Sci Technol 18(6):591–603
Shorey H, Hale RL (1965) Mass rearing of the larvae of nine noctuid species on a simple artificial medium. J Econ Entomol 58:522–524
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill Book Company, New York, p 481
Subramanian S, Rabindra RJ, Palaniswamy S, Sathiah N, Rajasekaran B (2005) Impact of granulovirus infection on susceptibility of Spodoptera litura to insecticides. Biol Control 33:165–172
Sun XL, Wang HL, Sun XC, Chen XW, Peng CM, Pan DM, Jehle JA, Van der Werf W, Vlak JM, Hu ZH (2004) Biological activity and field efficacy of a genetically modified Helicoverpa armigera SNPV expressing an insect-selective toxin from a chimeric promoter. Biol Control 29:124–137
Wolterink G, van Kesteren P, McGregor D (2012) Emamectin benzoate. Pestic Residues Food 2011:211
Yang MM, Li ML, Zhang Y, Wang YZ, Qu LJ, Wang QH, Ding JY (2012) Baculoviruses and insect pests control in China. Afr Microbiol Res 6(2):214–218
Yoon JH, Lee CS, O’Connor TR, Yasui A, Pfeifer GP (2000) The DNA damage spectrum produced by simulated sunlight. J Mol Biol 299:681–693
Zamora-Avilés N, Alonso-Vargas J, Pineda S, Isaac-Figueroa J, Lobit P, Martínez-Castillo AM (2013) Effects of a nucleopolyhedrovirus in mixtures with azadirachtin on Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) larvae and viral occlusion body production. Biocontrol Sci Technol 23(5):521–534
Zhu J, He Y, Gao M, Zhou W, Hu J, Shen J, Zhu YC (2011) Photodegradation of emamectin benzoate and its influence on efficacy against the rice stem borer, Chilo suppressalis. Crop Prot 30(10):1356–1362
Acknowledgements
The authors wish to express their gratitude to Prof. Dr. Adel Hatem and Dr. Gamal Hassan from Plant Protection Institute Research, Ministry of Agriculture, Giza, Egypt, for helpful discussion and technical support of the manuscript.
Funding
No specific fund was indicated in this study.
Author information
Authors and Affiliations
Contributions
AE and WS carried out the bioassay and biological studies. AE and HE conducted the isolation and propagation of the virus. WS carried out the filed treatment and analyzed the data. AE, WS, and HE contributed in the experimental design and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Helaly, A.A., Sayed, W.A.A. & El-Bendary, H.M. Impact of emamectin benzoate on nucleopolyhedrosis virus infectivity of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt J Biol Pest Control 30, 111 (2020). https://doi.org/10.1186/s41938-020-00314-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00314-0