Abstract
The tropical armyworm, Spodoptera litura Fabricius (Noctuidae; Lepidoptera), is among the most harmful pests causing economic loss in the quality and production of a variety of crops, particularly cotton. Entomopathogens play an important role in insect pest management. The nuclear polyhedrosis virus (NPV) isolate of S. litura (V-SpltNPV) was isolated from infected larvae in a cotton crop, and viral occlusion bodies were confirmed, using an inverted microscope. The pathogenicity of V-SpltNPV against 2nd, 3rd, and 4th larval instars of S. litura was evaluated at various concentrations (1 × 104 to 1 × 108 OBs/ml). Mortality rate was high (37.65–96.82%) in early instar larvae against tested concentrations. LC50 and LT50 values increased with increasing larval age. There was 689,865 times increase in LC50 value (1.35 × 102 OBs/ml) for 2nd instar larvae to LC50 value (6.90 × 105 OBs/ml) for 4th instar larvae. LT50 values enhanced from 4.99 days for 2nd instar larvae to 7.49 days for 4th instar larvae, due to a decrease in efficacy of NPVs with the increasing age of larvae. In a greenhouse experiment, a combined application of spinosad with V-SpltNPV (1 × 108 OBs/ml) caused (100%) mortality of 2nd instar larvae. A single application of V-SpltNPV (1 × 108 OBs/ml) resulted to mean mortality (52.63%) of tested larvae. The native isolate V-SpltNPV seems to have a potential to be used in integrated manner with other IPM tactics to significantly reduce the use of toxic chemical pesticides.
Similar content being viewed by others
Background
The tropical armyworm, Spodoptera litura Fabricius (Noctuidae; Lepidoptera), which is one of the important species of genus Spodoptera attacks a variety of agricultural crops, such as horticultural plants, fiber crops, vegetables, and miscellaneous wild plants as well as weeds (Zhou et al. 2010). Its common hosts are cotton, cabbage, lucerne, chickpea, beet, soybean, tobacco, and okra (Ellis 2005). The larvae of S. litura can cause 26–100% yield loss in field (Tuan et al. 2014). It is distributed worldwide, especially in North America, Oceania islands, Africa, and Asia, (El-Helaly and El-bendary 2013) in the sub-continent (Kranthi et al. 2002).
S. litura is an emerging insect pest of Pakistan as it causes a heavy loss in various regions such as the northern and southern districts of Punjab (Ahmad et al. 2007). In Pakistan, S. litura can be found on the cotton crop at the beginning of the cotton season (Saleem et al. 2016). As a sporadic pest, it can infest the cotton crop at any stage. Its overlapping generations throughout cotton cultivated areas of Pakistan decimated this crop in 2003 (Ahmad et al. 2007). The increase in cultivation of succulent crops like cotton, soybean, cabbage, mung bean, and vegetables provides ideal conditions for S. litura to vigorously reproduce, resulting in a rapid increase of generations and population size (Gao et al. 2004).
Despite advancement in pest management techniques, only a few control strategies have been recommended to manage the S. litura populations (Prayogo et al. 2005). Excessive pesticide use resulted to resistance development and environmental and human health issues (Sabir et al. 2011). Increased pest resistance development and environmental problems open opportunities for bio-pesticides (Jacobson et al. 2009). Microbial insecticides, especially virus-based insecticides, are effective biological agents against various agricultural as well as forest insect pests due to their specificity. These viruses are especially used to control lepidopteran insect pests (Liu et al. 2006) and considered as the most intensively studied insect pathogenic viruses (Inceoglu et al. 2006).
Nuclear polyhedrosis viruses (NPVs) belong to the Baculoviridae family (O’Reilly et al. 1992) and are the pathogens which infect many insect pests and other arthropods. NPVs are rod-shaped double-stranded DNA viruses which infect arthropods (Jehle et al. 2006). Many lepidopteran pests, like S. litura (Fabricius), S. exigua (Hübner), S. littoralis (Boisduval), and Helicoverpa armigera (Hübner) have shown susceptibility to NPV isolates (Kumar et al. 2011; Khattab 2013; Ahmad et al. 2018). NPVs can persist in the environment and formulate and package in a similar way to chemical pesticides.
Propagation of NPVs is primarily done in vivo and in vitro (Ikonomou et al. 2003). The most commonly used method is in vivo, simply infecting the healthy larvae by NPV contaminated artificial diet and harvesting the infected larvae for virus propagation (Eberle et al. 2012). The pathogenicity of V-SpltNPV on cotton leaves against the larvae of S. litura was assessed because there is no record about the use of NPV against S. litura on cotton crop in Pakistan. Only a few studies showed the use of NPV (not on cotton) against pests in Pakistan in which the commercially available products were used. Some commercial products have become available in the market.
This current study aimed to evaluate effective control tools of S. litura and to provide basis for research and development on indigenous NPV-based biopesticide in Pakistan.
Materials and methods
Rearing of Spodoptera litura
Larvae of S. litura, collected from various cotton fields in the district Faisalabad, were brought into the laboratory for rearing under controlled conditions. Larvae were reared on an artificial diet (Saljoqi et al. 2015) under controlled conditions (26 ± 2 °C, 70 ± 5 RH, 12:12 h light:dark photoperiod) in IPM laboratory, Department of Entomology, University of Agriculture Faisalabad, Pakistan. The artificial diet consisted of yeast powder (24 g), kidney bean flour (150 g), methyl-4-hydroxy benzoate (1.5 g), ascorbic acid (2.35 g), sorbic acid (0.75 g), formaldehyde solution, agar (8.4 g), streptomycin (0.75 g), and distilled water 550 ml. Newly hatched larvae were transferred individually to plastic vials (3.2-cm height, 3-cm diameter) containing a piece of artificial diet.
Insect virus
The NPV-infected larvae of S. litura were collected from district Vehari, Punjab, Pakistan. Infected larvae were stored in labeled plastic vials and brought to the laboratory where they were placed in a freezer at − 40 °C. Larvae showed infection symptoms were brought to the laboratory and observed to confirm the presence of NPV by inverted microscope (× 40) with Giemsa staining (Yaman et al. 2001). The collected isolate V-SpltNPV (V, Vehari; SpltNPV, S. litura NPV) was used in the laboratory experiment. The name was given to the isolate with reference to the location from which it was collected (V, Vehari; SpltNPV, S. litura NPV). Thereafter, virus isolation and propagation were carried out in vivo as described by (Monobrullah and Nagata 2000). Purified occlusion bodies (OBs/ml) were counted 5 times using a hemocytometer under inverted microscope. A dilution of various concentrations (1 × 104 to 1 × 108 OBs/ml) of V-SpltNPV was prepared in distilled water from stock suspension (Cory and Myers 2003).
Laboratory bioassay
S. litura larvae were obtained from the laboratory rearing colony. Cotton leaves 3 cm in diameter were cut and placed in a plastic container (7-cm height and 3 cm in diameter). Various concentrations (1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 OBs/ml) were prepared and 5–10 μl viral concentration was applied on leaf disks with a micropipette. Control treatment were applied, using only distilled water. Newly molted 30 larvae of 2nd, 3rd, and 4th instars (30 for each instar) were placed in a container having treated leaf disk and allowed to feed on contaminated leaf disks. After 24 h, larvae were shifted on fresh leaves. Fresh leaves were provided daily until pupation. All plastic containers were placed in a growth chamber under controlled conditions (25 ± 2 °C, 70 ± 5% R. H, and 14:10 (D:L) photoperiod). LC50 and LT50 values were calculated from mortality data after every 48 h. All experiments were replicated 3 times.
Greenhouse evaluation of NPV alone and in combination with spinosad
The bioassay was performed under greenhouse conditions, using potted cotton plants of the same age (50 days). Three concentrations (1 × 106, 1 × 107, and 1 × 108 OBs/ml) of V-SpltNPV were used for greenhouse experiments. For evaluation of the effectiveness of spinosad, (Tracer 240 SC, Dow AgroSciences) it was used alone and in binary combination at the 3 different concentrations of V-SpltNPV (1 × 106, 1 × 107, and 1 × 108 OBs/ml). The 20 ml water was calibrated for spraying the whole cotton plant. For the greenhouse experiment, 1 ml of each virus concentration was mixed with 19 ml distilled water to make 20 ml final volume. For treatment applications of spinosad (recommended dose; 1%), 20 ml formulation was prepared and sprayed on potted cotton plants. For the combined application of spinosad with different concentrations (1 × 106, 1 × 107, and 1 × 108 OBs/ml) of NPV (the recommended dose of spinosad was mixed with different concentrations of NPV) comprised of a 20-ml solution, applied along with 0.1% Tween-80 as adjuvant. Twenty larvae of 2nd instar S. litura were released separately on each potted cotton plant with a camel hair brush. Cotton plants were covered by a 0.5 mm2 meshed mosquito net to avoid larval escape. The concentrations were sprayed on cotton plants by a hand sprayer. Mortality rate was observed daily until pupation.
Statistical analysis
Corrected mortality rates were calculated by Abbott’s formula (1925) and data were analyzed using Minitab software for LT50 and LC50 values. For laboratory and greenhouse trials, data were analyzed, using Statistica 8.1 software and means were separated by Tukey’s HSD test at α = 5%.
Results and discussion
LC50 values of different larval instars of S. litura, when exposed to various concentrations of V-SpltNPV, are presented in Table 1. Larval mortality was high in early instars and decreased in full-grown tested larvae. Maximum mortality rate (88.08%) for 2nd instar larvae was observed, while the minimum mortality rate (65.52%) was recorded for 4th instar larvae of S. litura, when exposed to various concentrations. The calculated LC50 were 1.3 × 102, 5.4 × 104, and 6.9 × 105 for 2nd, 3rd and 4th larval instar, respectively. The increase in the LC50 values, with an increase in the age of larvae, may be due to the dilution effect of virus inoculum associated with the increase in weight of larvae. LC50 values also showed that 2nd instar larvae was (53,865 and 689,865) times more susceptible than 3rd and 4th larval instars, respectively, while 3rd instar larvae was (636,000) times suceptible than 4th instar larvae.
LT50 for various larval instars of S. litura, when exposed to various V-SpltNPV concentrations, are presented in Table 2. Data in the table showed the concentration and larval instar dependent on LT50 values. The decreasing trend of LT50 values was observed by increase in concentration used in the experiment. Likewise, with the increase in larval age, more time was required to kill the tested population. LT50 of 2nd instar larvae were 4.99 days against the highest concentration (1 × 108 OBs/ml), which increased up to 11.13 days against the lowest concentration (1 × 104 OBs/ml). LT50 values of 3rd instar larvae were 6.61 and 12.35 days and were observed against maximum and minimum concentrations, respectively. The same trend was observed for 4th instar larvae, where LT50 value was 7.49 days against (1 × 108 OBs/ml) concentration, while the LT50 value was increased to 11.11 days against 1 × 104 OBs/ml concentration.
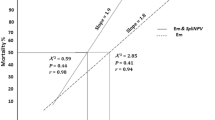
It was evident from the results that the combined application gave better results than each application of tested concentration alone (Fig. 1). The sole V-SpltNPV concentration (1 × 108 OBs/ml) caused 52.63% mean mortality of tested larvae. Similarly, sole application of spinosad caused 46.58% mean mortality. V-SpltNPV (1 × 108 OBs/ml) and spinosad combination caused a maximum mortality of 100%. The combination of a low concentration V-SpltNPV with spinosad also gave effective results and caused 75–78% (1 × 106 OBs/ml) and 91.31% (1 × 107 OBs/ml) mortality rates of S. litura larvae.
The V-SpltNPV isolate was effective for managing the larval population of S. litura. The LT50 and LC50 were gradually enhanced by increase in larval instar. The increase in the LC50 values with an increase in the age of larvae was due to the dilution effect of virus inoculum associated with an increase in weight of larvae. Monobrullah and Nagata (2000) also noticed an increasing trend of LD50 values for second (224 PIBs/larva) to 5th (519,381 PIBs/larva) instar larvae of S. litura. They also noticed an increase of LT50 for 2nd instar larvae (6.9 days) to 5th instar larvae (9.3 days), when exposed to the same concentration. Similarly, Trang and Chaudhari (2002) reported increasing trends of LC50 (1 × 103 to 1.5 × 109 PIB/ml) and LT50 (4.4–9.4 days) values with the increase in larval instars. The variation of LC50 values may be due to the change in diet as they used coaster leaves in the experiment instead of cotton. In addition, the larvae were exposed at different time intervals to calculate LC50, while in the present study, the larvae were exposed only once. The LT50 values were inversely proportional to dose, while directly proportional to larval instar (Subramanian et al. 2005; Kouassi et al. 2009; Bhutia et al. 2012). A similar trend was observed in other studies as well (Cherry et al. 1997). The LT50 values may deviate to present findings due to change of Spodoptera species which were exposed to NPV.
Mature larvae were more resistant to the concentration of V-SpltNPV due to physiological changes (body mass) related to pupation. Kumar et al. (2011) noticed increasing trends in the LC50 and LT50 values of SpltNPV for 2nd to 3rd larval instars of S. litura. Similar findings were documented by Teakle et al. (1986) who noted the increasing in LC50 values of NPV commercial formulation with an increase in larval instar of Heliothis punctigera (Wallengren). Ahmad et al. (2018) also recorded the increase in LC50 values (2.64 × 103 to 2.15 × 106 OB/ml) and LT50 values (72.50–144.64 h) for 2nd and 5th larval instars of S. litura against various concentrations of SpltNPV. They also stated that the same trend of LT50 value as (72.50 h) for 2nd instar larvae, which increased up to (144.64 h) for 5th instar larvae. Similarly, Evans (1981) reported variation in LC50 values among 1st and 5th larval instars of S. litura. LC50 value was (34,000 times) higher for 5th larval instar, than the 1st instar larvae of Mamestra brassicae (Linnaeus), while 5th and 6th instars larvae were almost at par for resistant to virus infectivity.
The physiological changes (increase in body mass) associated with pupation might not allow infection at the late developmental stage as the mature larvae (10 days) were found to be more resistant to SlNPV (Kumar et al. 2011). The possibility of biovirus not getting sufficient time to replicate or kill the larvae may not be ruled out. Such suggestion gets support from the findings of Evans (1981) and Teakle et al. (1986). In addition, Tuan et al. (1998) and Jayanthi (1992) also observed significant differences in LC50 values among different larval instars of S. litura and also in Trichoplusia ni (Milks et al. 1998).
The combination of spinosad with EPV proved to be suitable because spinosad has no antiviral, antifungal, or antibacterial activity (Bret et al. 1997). Spinosad has been distinguished as a biopesticide, as spinosyns, which are produced by fermentation of soil bacterium actinomycete (Copping and Menn 2000). Spinosad has insecticidal properties that differentiate it from other entomopathogenic bio-pesticides (Salgado et al. 1998).
Bret et al. (1997) noted that the combination of spinosad with SlNPV resulted in better control of S. furgiperda population. The present findings are in agreement with El-Helaly and El-bendary (2013) that combined treatment of SlNPV and spinosad exhibited a maximum larval mortality (55%) of S. littoralis. While alone treatment with SlNPV caused 20.11% mortality and spinosad gave 26.66% mortality of S. littoralis. These findings showed an additive correlation of spinosad with NPV. The combined application of AgMNPV and spinosad also increased the mortality of pickleworms larvae up to 78% (Jackson et al. 2014). While significantly lower mortality rate 32 and 24% was observed against AgMNPV and Spinosad, respectively, when each was applied alone. The additive effects of spinosad and SfMNPV combination was also reported in previous findings of Mendez et al. (2002) against larvae of S. furgiperda as combined treatment caused a high mortality rate than the alone application. Similarly, 40% more control of S. litura was reported, when exposed to the combined formulation of SpltNPV and azadirachtin (Cook et al. 1996). In addition, Nathan and Kalaivani (2005) observed 92.7% mortality rate, when a combined application of azadirachtin (AZA) and NPV was used against S. litura larvae than individual application of NPV (28.5%) and AZA (36.3%). Similarly, Shaurub et al. (2014) suggested that the mixture application of NPV with AZA enhanced larval mortality of S. littoralis significantly as compared to individual treatment.
Conclusions
It is the time to use baculoviruses to manage insect pests in agricultural fields of Pakistan, to reduce the use of synthetic toxic chemicals, especially in cotton-growing regions, where more than 80% of total imported pesticides are being used to manage pests. The isolated strain of NPV (V-SpltNPV) can be effectively used to manage S. litura population. Furthermore, the combined efficacy can be enhanced by evaluating their pathogenicity with new chemistry insecticides without causing damage to non-target organisms.
Availability of data and materials
The data used and analyzed during this project are available from the corresponding author on reasonable request.
Abbreviations
- IPM:
-
Integrated pest management
- Kbp:
-
Kilobase pare
- LC50:
-
Lethal concentration to kill 50% population
- LD50:
-
Lethal dose to kill 50% population
- LT50:
-
Lethal time to kill 50% population
- NPV:
-
Nuclear polyhedrosis virus
- OBs/ml:
-
Occlusion bodies/milliliter
- PIB/ml:
-
Polyhedral inclusion bodies/milliliter
- S.E:
-
Standard error
- V-SpltNPV:
-
Vehari-Spodoptera litura nuclear polyhedrosis virus
- χ2:
-
Chi square
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Ahmad JN, Mushtaq R, Ahmad SJN, Maqsood S, Ahuja I, Bones AM (2018) Molecular identification and pathological characteristics of NPV isolated from Spodoptera litura (Fabricius) in Pakistan. Pak J Zool 50:2229–2237
Ahmad M, Arif MI, Ahmad M (2007) Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot 26:809–817
Bhutia KC, Chakravarthy AK, Doddabasappa B, Narabenchi GB, Lingaraj VK (2012) Evaluation and production of improved formulation of nucleopolyhedrosis virus of Spodoptera litura. Bull Insectol 65:247–256
Bret BL, Larson LL, Schoonover JR, Sparks TC, Thompson GD (1997) Biological properties of spinosad. Down Earth 52:6–13
Cherry AJ, Parnell MA, Grzywacz D, Jones KA (1997) The optimization of in vivo nuclear polyhedrosis virus production in Spodoptera exempta (Walker) and Spodoptera exigua (Htbner). J Invertebr Pathol 70:50–58
Cook SP, Webb RE, Thorpe KW (1996) Potential enhancement of the gypsy moth (Lepidoptera: Lymantriidae) nuclear polyhedrosis virus with the Triterpene azadirachtin. Environ Entomol 25:1209–1214
Copping LG, Menn JJ (2000) Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci 56:651–676
Cory JS, Myers JH (2003) The ecology and evolution of insect baculoviruses. Annu Rev Ecol Evol Syst 34:239–272
Eberle KE, Wennmann JT, Kleespies RG, Jehle JA (2012) Basic techniques in insect virology. In: Manual Tech. Invertebr. Pathol, 2nd edn, pp 15–74
El-Helaly AA, El-bendary HM (2013) Impact of Spinosad and Nucleopolyhedrovirus alone and in combination against the cotton leaf worm Spodoptera littoralis under laboratory. App Sci Rep 2:17–21
Ellis SE (2005) New pest response guidelines: Spodoptera USDA. APHIS/PPQ/PDMP.
Evans HF (1981) Quantitative assessment of the relationship between dosage and response of the nuclear polyhedrosis virus of Mamestra brassicae. J Invertebr Pathol 37:10l–109l
Gao C, Bei Y, Chen T, Gu X (2004) On factors causing outbreak of Spodoptera litura (Fabricius). Acta Agri Zhejiangensis 16:332–335
Ikonomou L, Schneider YJ, Agathos SN (2003) Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol 62:1–20
Inceoglu AB, Kamita SG, Hammock BD (2006) Genetically modified Baculoviruses: a historical overview and future outlook. Adv Virus Res 68:323–360
Jackson DM, Shapiro M, Shepard BM (2014) Effects of spinosad and neem on the efficacy of a nucleopolyhedrovirus on pickleworm larvae. J Agri Urban Entomol 30:28–37
Jacobson A, Foster R, Krupke C, Hutchison W, Pittendrigh B, Weinzierl R (2009) Resistance to pyrethroids insecticides in Helicoverpa zea (Lepidoptera: Noctuidae) in Indiana and Illinois. J Econ Entomol 102:2289–2295
Jayanthi PDK (1992) Studies on the microbial and chemical pesticides in the control of Spodoptera litura (Fabricius) (Noctuidae: Lepidoptera). (M. Sc. Dissertation) Andhra Pradesh Agricultural University, Hydcrabad
Jehle JA, Blissard GW, Bonning BC, Cory JS, Herniou EA, Rohrmann GF, Theilmann DA, Thiem SM, Vlak JM (2006) On the classification and nomenclature of baculoviruses: a proposal for revision. Arch Virol 151:1257–1266
Khattab M (2013) Isolation of Nucleopolyhedrovirus (NPV) from the beet armyworm Spodoptera exigua (Hubner)(SpexNPV). Int J Environ Sci Eng 4:75–83
Kouassi LNG, Tsuda K, Goto C, Mukawa S, Sakamaki Y, Nakamura M (2009) Biological activity and identification of nucleopolyhedroviruses isolated from Mythimna separata and Spodoptera litura in Japan. Biol Control 54:537–548
Kranthi KR, Jadhav DR, Kranthi S, Wanjari RR, Ali SS, Russell DA (2002) Insecticide resistance in five major insect pests of cotton in India. Crop Prot 21:449–460
Kumar CS, Rao GR, Sireesha K, Kumar PL (2011) Isolation and characterization of baculoviruses from three major lepidopteran pests in the semi-arid tropics of India. Ind J Virol 22:29–36
Liu X, Zhang Q, Xu BL, Li JC (2006) Effects of Cry1Ac toxin of Bacillus thuringiensis and nuclear polyhedrosis virus of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on larval mortality and pupation. Pest Manag Sci 62:729–737
Mendez WA, Valle J, Ibarra JE, Cisneros J, Penagosand DI, Williams T (2002) Spinosad and nucleopolyhedrovirus mixtures for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Biol Control 25:195–206
Milks ML, Burnstyn I, Myers JH (1998) Influence of larval age on the lethal and sub lethal effect of the nuclear polyhedrosis virus of Trichoplusia ni in the cabbage looper. Biol Control 12:119–126
Monobrullah M, Nagata M (2000) Effects of larval age on susceptibility of Spodoptera litura (Lepidoptera: Noctuidae) to Spodoptera litura multiple nuclear polyhedrosis virus. Can Entomol 132:337–340
Nathan S, Kalaivani K (2005) Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Biol Control 34:93–98
O’Reilly DR, Miller LK, Luckow VA (1992) Baculovirus expression vectors: a laboratory manual. W. H. Freeman & Co., New York
Prayogo Y, Tengkano W, Marwoto D (2005) Prospect of entomo-pathogenic fungus Metarhizium anisopliae to control Spodoptera litura on soybean. J Litbang Pertanian 24:19–26
Sabir HM, Tahir SH, Khan MB (2011) Bt cotton and its impact on cropping pattern in Punjab. Pak J Soc Sci 31:127–134
Saleem M, Hussain D, Ghouse G, Abbas M, Fisher SW (2016) Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot 79:177–184
Salgado VL, Sheets JJ, Watson GB (1998) Studies on the mode of action of spinosad: the internal effective concentration and the concentration dependence of neural excitation. Pestic Biochem Physiol 60:103–110
Saljoqi AR, ul Haq R, Khan J, Ali G (2015) Rearing of Spodoptera litura (Fabricius) on different artificial diets and its parasitization with Trichogramma chilonis (Ishii). Pak J Zool 47:169–175
Shaurub EH, Meguid AA, Aziz NMA (2014) Effect of individual and combined treatment with Azadirachtin and Spodoptera littoralis multicapsidnucleopolyhedro virus (SpliMNPV, Baculoviridae) on the Egyptian cotton leaf worm Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Ecol Balkanica 6:93–100
Subramanian S, Rabindra RJ, Palaniswami S, Sathiah N, Rajasekaran B (2005) Impact of granulovirus infection on susceptibility of Spodoptera litura to insecticides. Biol Control 33:165–172
Teakle RE, Jensen JM, Giles JE (1986) Age-related susceptibility of Heliothis punctigera to a commercial formulation of nuclear polyhedrosis virus. J Invertebr Pathol 36:281–282
Trang TK, Chaudhari S (2002) Bioassay of nuclear polyhedrosis virus (NPV) and in combination with insecticide on Spodoptera litura (Fab). Omon Rice 10:45–53
Tuan SJ, Chen WL, Kao SS (1998) In vivo mass production and control efficacy of Spodoptera litura (Lepidoptera: Noctuidae) nucleopolyhedrosis virus. Chinese J Entomol 18:101–116
Tuan SJ, Li NJ, Yeh CC, Tang LC, Chi H (2014) Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J Econ Entomol 107:897–905
Yaman M, Nalçacioğlu R, Demirbağ Z (2001) Viral control of the european pine sawfly, Neodiprion sertifer (Geoffroy) in Turkey. Turk J Biol 25:419–425
Zhou Z, Chen Z, Xu Z (2010) Potential of trap crops for integrated management of the tropical armyworm, Spodoptera litura in tobacco. J Insect Sci 10:1–11
Acknowledgements
The authors highly acknowledged Dr. Laura L Ingwell from Purdue University, Indiana, USA, for review and her valuable comments for this article.
Funding
Funding Agency: Higher Education Commission of Pakistan
Funding Number: No.20-3209/NRPU/R&D/HEC/13/460
Author information
Authors and Affiliations
Contributions
MBA and AN designed the experiment. MBA conducted the experiment and wrote the article. AN helped in statistical analysis. MJA and LA revised the article. All authors approved the final article after reading.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ayyub, M.B., Nawaz, A., Arif, M.J. et al. Individual and combined impact of nuclear polyhedrosis virus and spinosad to control the tropical armyworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae), in cotton in Pakistan. Egypt J Biol Pest Control 29, 67 (2019). https://doi.org/10.1186/s41938-019-0170-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-019-0170-4