Abstract
Background
Dysdercus koenigii Fabricius is a polyphagous agricultural pest of paramount importance. Farnesol is a plant-derived open-chain aliphatic sesquiterpenoid alcohol. It is an intermediate in the metabolic pathway of juvenile hormone biosynthesis. The effects of Farnesol on haemocytes of fifth instar nymphs of Dysdercus koenigii were assessed in the present investigation. The early fifth instar nymphs were treated with doses of 0.05 μL, 0.1 μL and 0.2 μL Farnesol topically and day wise change in the total haemocytes was reported. Different types of haemocytes were identified in the haemolymph based on their morphological characteristics. Difference in day wise distribution pattern of haemocytes was evaluated in relation to dose of treatment.
Results
In normal fifth instar nymphs, age-related variation in the haemocyte count was evident; the haemocyte number increased up to four days and subsequently decreased till six-day when the nymph moulted. The results indicate that Farnesol had an adverse effect on the total number of haemocytes; in treated nymphs the haemocyte count decreased in a dose-dependent manner. The decrease was most prominent in the nymphs treated with a dose of 0.2 μL Farnesol. Based on the morphological characteristics, six different types of haemocytes, viz. prohaemocyte, plasmatocyte, adipohaemocyte, granulocytes, oenocytoids and disintegrated cells were identified in the haemolymph. The percent composition of different haemocytes showed variation depending on the age of the fifth instar nymph and the dose of treatment. In control, the percentages of plasmatocytes decreased up to day 5 and then increased up to 7 days. The percentage of adipohaemocytes increased throughout the nymphal period of the fifth instar, except for a small dip on the fourth day. Granulocytes and oenocytoids showed no clear trend. In Farnesol-treated nymphs, an increase in percent of prohaemocytes was observed in all the treatments. Dose-dependent effects were more prominent after day 5. An increase in plasmatocytes was seen after day 3. A distinct dose-related decrease was reported in adipohaemocytes on all days. Percent of disintegrated cells also showed an increase in all the tested doses of Farnesol on all the days. Granulocytes and oenocytoids showed no definitive trend of change.
Conclusions
Farnesol is a plant sesquiterpenoid and acts as a precursor in juvenile hormone biosynthesis. The application of Farnesol exogenously may disturb haemocyte patterns which may challenge the immune response of insects. Therefore, Farnesol can potentially be used as an alternative approach in pest management.
Similar content being viewed by others
Background
Red cotton bug, Dysdercus koenigii Fabricius (Heteroptera: Pyrrhocoridae), is a widely distributed insect pest in South-East Asia including India (Kapur & Vazirani, 1956; Stehlík & Kerzhner, 1999). It is a destructive pest of cotton and causes severe lint staining problems (Ahmad & Mohammad, 1983; Ashfaq et al., 2011). Both the adults and the nymphs damage the cotton crop by sucking sap from emerging cotton bolls and mature cotton seeds which results in the destruction of cotton bolls, a decrease in oil contents and poor germinating ability of the seeds. The pest is also responsible for the transmission of cotton staining fungi, Nematospora gossypii, that develops on the immature lint and seed (Ahmad & Khan, 1980; Maxwell-Lefroy, 1908; Schaefer & Panizzi, 2000; Srinivasan, 2016). Chemical control is the most widely used method of insect pest control as it is reliable, fast and effective in its action (Rathee et al., 2018. However, excessive use of chemicals for pest control leads to bioaccumulation and biomagnification in crop fields and inhabiting organisms. Due to the low biodegradability of the chemical insecticides, they remain for a longer period in the environment and pose health hazards to humans and other non-target organisms. They also pose risk to the beneficial insects. Pest resistance to insecticides and pest resurgence is another serious concern of indiscriminate use of chemical insecticides for insect control (Boudh & Singh, 2019). Integrated Pest Management (IPM) is a holistic approach to insect pest management. It aims to suppress the pest population below the economic injury level (EIL) by using a wide range of pest control methods, economic use of pesticides and outreach programs for awareness (Hagstrum & Flinn, 2018). Recent strategies for the development of new eco-friendly pesticide aim at targeting the pest without affecting the environment adversely (Bhattacharyya et al., 2016). For this purpose, plants offer better options, as they possess a variety of bioactive chemicals, which can be used for the development of eco-friendly insecticides against a variety of target insect pests (Salunke et al., 2009; Zoubiri & Baaliouamer, 2014. Botanicals have the potential to affect various life processes of the insect adversely (Asawalam & Adesiyan, 2010; Schmidt et al., 1991) and, therefore, can be employed in integrated pest management.
Farnesol is a plant-derived open-chain aliphatic sesquiterpenoid alcohol; widely distributed as an odoriferous component of leaves, fruits, and roots of many higher plants (Genovese et al., 2005; Kayesth et al., 2017, 2019; MacLeod & Pieris, 1984; Medeiros et al., 2003). It is a 15 carbon isoprenoid alcohol, a linear sesquiterpene signalling molecule having four geometric isomers due to the double bonds at the C-2 and C-6 positions: 2-cis,6-cis (2c-c), 2-cis, 6-trans (2c-6t), 2-trans,6-cis (2t-6c) and 2-trans, 6-trans (2t-6t) isomers (Hisaya et al., 1982). Farnesol is an intermediate in Juvenile hormone biosynthesis in insects. It is converted to JH by Mevalonic acid pathway (Bellés et al., 2005; Goldstein & Brown, 1990; Kaneko et al., 2011; Lombard & Moreira, 2011).
Farnesol has been reported to affect various life processes of insects. Trans, trans-Farnesol is a very strong deterrent to Myzus persicae; application of this terpenoid caused the avoidance of plants by the peach potato aphid. Farnesol has also been reported to have a significant effect on various biological activities such as the growth, development and morphogenesis of Spodoptera litura (Dancewicz & Gliszczyńska, 2010; Ghoneim et al., 2020). Two structurally related sesquiterpenes Farnesol and bisabolene had strong antifeedant activity against the Colorado potato beetle Leptinotarsa decemlineata (Gonzalez-Coloma et al., 1995). Treatment of Diploptera punctata with exogenous farnesoic acid or Farnesol stimulated JH-III synthesis by the corpora allata (Feyereisen et al., 1981a, 1981b). β-farnesene, and (E, E)-farnesol are the main plant volatiles that increased the attraction of both male and female codling moth Cydia pomonella (Gökçe et al., 2018).
In insects, haemolymph contains plasma, organic and inorganic substances and a variety of haemocytes. The main functions of haemolymph are to transport nutrients, excretory products, hormones and various other substances to tissues (Ayyangar & Rao, 1990; Kanost et al., 1990; Ragan et al., 2009; Vommaro et al., 2021). The haemocytes play a crucial role in the defence system of the insects (Rantala et al., 2020). The distribution pattern of the haemocytes depends on the age, sex and physiological conditions of the insects (Perveen & Ahmad, 2017). Depending on the structure and functions performed, a variety of haemocytes has been reported in insects. Prohaemocytes are undifferentiated haemocytes that commonly occur in the haemolymph; these probably serve as a source of other differentiated haemocytes (Ghoneim & History, 2019; Ragan et al., 2009). Granulocytes are the haemocytes responsible for phagocytosis of small pathogens such as bacteria and viruses (Kurihara et al., 1992; Ling et al., 2005; Strand, 2008). Plasmatocytes, adipohaemocytes, and oenocytoids are some other types of haemocytes present in insect haemolymph (Mannakkara, 2022; Siddiqui & Al-khalifa, 2012).
Present research work is focused on the effect of Farnesol on the haemocytes of D. koenigii. This work will highlight the response of the insect to the exogenous Farnesol.
Methods
Rearing and maintenance of culture
Present work was conducted on the fifth instar nymphs of Dysdercus koenigii. In order to get a sustained supply of good quality insects, a culture of D. koenigii was maintained in the ‘Insect reproduction Laboratory’ at Deshbandhu College, University of Delhi, New Delhi, India, under optimum conditions of temperature, humidity and photoperiod on a natural diet. For this they were kept in BOD incubators with ambient conditions of temperature 27 ± 1 ºC, relative humidity 70 ± 5% and 12 h dark and 12 h light photophase. The insects were reared in glass jars and provided with cotton seeds for feeding and wet cotton swabs for drinking water (Kayesth et al., 2021a, 2021b).
Identification of fifth instar nymph of Dysdercus koenigii
The fifth instar nymphs were identified by presence of a white collar behind the head and prominent wing pads. Their body was cylindrical shaped and crimson red coloured. The proboscis was also red while the antennae and legs were black. The antennae of the fifth instar nymphs were five segmented, divided into the scape, pedicel, and flagellum. Inter-segmental membranes of abdomen had opening of stink gland which secrete smelling liquid. The fifth instar nymphs take 6 to 7 days to moult finally into adult.
Treatment of fifth instar nymphs with Farnesol
Newly emerged (0–6 h old) fifth instar nymphs D. koenigii were separated and isolated from the stock culture and used for experimental purposes. Farnesol was diluted with ethanol and 2 μL Farnesol of concentrations of 2.5%, 5% and 10% was applied topically on the meta-thoracic pleuron of the test insect. This made the application doses of 0.05 μL, 0.1 μL, and 0.2 μL of Farnesol per insect. In control, the nymphs were treated with 2 μL of ethanol (Leonardi et al., 1996). Effect of Farnesol was studied on day wise change in the ‘Total Haemocyte Counts’ and change in the relative percent of different types of haemocytes in relation to the time period after exposure for seven days.
Haemolymph collection and study of total haemocyte count
The haemolymph of both the control and treated fifth instar nymphs of D. koenigii was obtained by cutting the tip of the flagellum of the antenna. A drop of haemolymph was exuded at the cut end of the antenna; this was collected with the help of a micropipette and placed in a microcentrifuge tube containing thiourea. After this, 2 μl haemolymph was taken and diluted with 10 μl diluting fluid of composition 8 μl of 1.5% glacial acetic acid and 2 μL Giemsa stain. The haemolymph was mixed properly with diluting fluid by vortexing for 5 min. The processed haemolymph was poured in between the Neubauer counting chamber and coverslip. Haemocytes were counted in four corners of the chamber under a microscope. The total number of haemocytes present in 1 mm.3 haemolymph was calculated by the formula used by (Jones, 1962; Mannakkara, 2022; Pugazhvendan & Soundararajan, 2009)
Fifth instar nymphs treated with a dose of 0.05 µL, 0.01 µL and 0.2 µL of Farnesol were used after 1 day, 2 days, 3 days, 4 days, 5 days, 6 days and 7 days of treatment for the study of total haemocyte count. In control, the fifth instar nymphs of corresponding age treated with 2 µL of ethanol were used for total haemocyte count. All the experiments were replicated ten times.
Identification of different types of haemocytes and differential haemocyte count
A drop of haemolymph was collected by cutting the tip of the antenna of experimental and control fifth instar nymphs of D. koenigii as described earlier. The haemolymph exuded from the cut antenna was collected with the help of a micropipette. It was placed on a clean glass slide and a uniform smear was made. The smear was air-dried and then covered with a few drops of methyl alcohol for 2–5 min in order to fix the haemocytes. After this, the haemolymph smear was covered with the diluted Giemsa stain for 45 min. The Giemsa's stain was mixed with phosphate buffer (pH 7) in the ratio of 1: 9 before use. Subsequently, the slides were rinsed with distilled water; the stained slides were air-dried at room temperature and mounted in Dibutylphthalate Polystyrene Xylene (DPX).
The slides were observed under oil emersion of Nikon Eclipse E200 microscope. Different types of haemocytes were identified based on their morphological characteristics such as shape and size of cell and nucleus, presence of granules or vacuoles in the cytoplasm, etc. (Berger & Klára, 2008; Perveen & Ahmad, 2017; Vommaro et al., 2021). Different types of haemocytes were counted in a region of slide having at least 100–110 cells.
The experiments were conducted with the fifth instar nymphs treated with 0.05 μL, 0.1 μL, and 0.2 μL doses of Farnesol. Also, for each treatment, different types of cells were counted after 1 day, 2 days, 3 days, 4 days, 5 days, 6 days and 7 days of treatment in order to relate the change in the haemocyte pattern with the days after treatment. All the experiments were replicated seven times.
Statistical analysis
Results of total haemocyte count were presented as mean ± S.E. For differential haemocyte count, relative percent of each type of haemocyte was calculated. IBM SPSS 19.1software was used for statistical analysis of the results. The validity of the hypothesis was confirmed by one way ANOVA followed by post hoc TUKEY test at a 95% of confidence limit.
Results
Effect of Farnesol on total haemocyte count of fifth instar nymphs of D. koenigii
The results of total haemocytes count of fifth instar nymphs of Dysdercus koenigii are presented in Table 1. The results indicate day wise variation in the total haemocyte count in both the treated and the control insects. In a one day old fifth instar nymph, the total number of haemocytes was 6060 haemocytes/mm3; the number of haemocyte increased up to four day; approximately 40% increase was observed in four days. Subsequently, there was a decrease in the number of haemocyte on 5–7 day; the number increased again on day 8. Minimum number of haemocytes was observed on day 7.
Treatment of fifth instar nymph with Farnesol showed decrease in the haemocyte count in dose dependant manner. After day 1 of the treatment the number of haemocytes, in treated nymphs it was 6630 haemocytes/mm3, 6115 haemocytes/mm3, and 4045 haemocytes/mm3 at treatment doses of 0.05 µL, 0.1 µL, and 0.2 µL, respectively; in control it was 6970 haemocytes/mm3. The difference in the total haemocyte count in control and treatments at a doses of 0.2 µL after day 1 was statistically significant. Similar trends in the number of total haemocytes were observed for six days of treatment (Table 1). The decrease in the total haemocyte count as a consequence of Farnesol treatment was most pronounced after four days. The number of haemocytes in the nymphs treated with 0.2 µL was reduced to almost half to that of control. The results also showed that highest haemocyte count was observed on day 4 in control i.e. 8405 haemocytes/mm3 and lowest value of haemocyte count was 2980 haemocytes/mm3 in the nymphs treated with a dose of 0.2 µL Farnesol per insect on day 6. In control, the fifth instar nymphs usually moulted after sixth day; therefore, the figures of haemocyte on seventh and eighth day represent the haemocyte count of adults.
Different types of haemocytes present in the fifth instar nymphs of D. koenigii
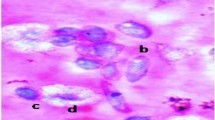
Six different types of haemocytes were observed in the haemolymph of fifth instar nymph of D. koenigii. These were Prohaemocytes, Plasmatocytes, Adipocytes, Granulocytes, and Disintegrated cells (Fig. 1). The characteristic features of these haemocytes are described in Table 2. Prohaemocytes were small (3–10 μm), round, oval, or elliptical in shape with large nucleus. These haemocytes were characterized by high nuclear/cytoplasmic ratio. Plasmatocytes appeared as elongated or spindle-shaped cells with a size ranged 4–25 μm. The nucleus of plasmatocytes was rounded or elongated, 5–11 μm in diameter and contains a large nucleolus. Granulocytes were the most common type of haemocyte. They were small to medium in size (4–20 μm) and elongated, rounded, or spindle-shaped. The nucleus (1–4 μm) was generally located in the cell centre. Adipohaemocytes were large (22–48 μm), and rounded or ovoid with a large, often eccentric nucleus (12–15 μm in diameter) surrounded by relatively little cytoplasm and large vesicles. Oenocytoids were large round or oval shaped cells. The nucleus of these cells was small with large nucleolus. They contain abundant cytoplasm. Disintegrated cells were also seen in the haemolymph of fifth instar nymphs of D. koenigii.
Influence of Farnesol on relative percentage of different types of haemocytes
Difference in relative abundance of different types of haemocytes was evident in control and experiments. Also, the results indicate that change in relative percent of different haemocytes varies according to type of haemocyte, day after treatment and dose of Farnesol applied to the insect.
Prohaemocyte
In control, the percent of prohaemocytes was 12.57% on day 1. Their percentage increased up to four day and subsequently decreased on fifth and sixth days. In the adult males and females, the percent prohaemocytes remained less in comparison to their percentage in the nymphs after day 1. Treatment with Farnesol promoted the percent prohaemocyte count. A distinct dose dependant increase in the percent prohaemocyte was seen on day five, six and seven. The increase was most conspicuous on day seven; in comparison to control, almost five times increase was observed in the nymphs treated with a dose of 0.2 µL. However, in earlier days there was no direct correlation between the dose of Farnesol applied to the nymphs and percent increase in the prohaemocytes (Fig. 2).
Effect of Farnesol on Prohaemocytes of fifth instar nymphs of Dysdercus koenigii. Each bar represents Mean ± SE, Seven replicates were taken for calculation of mean Bars on a specific day after treatment with different letter are significantly different (p < 0.05), computed by one-way ANOVA followed by Tukey’s all pairwise multiple comparison test
Plasmatocytes
Plasmatocytes were the most common haemocytes in the haemolymph of fifth instar nymph of D. koenigii. In control, they constitute 52.29% on day 1. Subsequently, their percentage in the haemolymph gradually decreased to 34.57% on day 6. The fifth instar nymph moult after six days and the percent of plasmatocytes increased again in the adult males and the adult females. Treatment of insect with a dose of 0.2 µL Farnesol had adverse effect on the relative percent of plasmatocytes for two days; the relative percent decreased, and the difference were statistically significant. It was also observed that after two days the relative percent of plasmatocytes increased in Farnesol-treated fifth instar nymphs in a dose-dependent manner up to seven days. Increase in percent plasmatocytes was most conspicuous on day 5; the nymphs treated with 0.2 µL Farnesol showed 46.86% plasmatocytes in contrast to insects in control which had only 32.86% plasmatocytes (Fig. 3).
Effect of Farnesol on Plasmatocytes of fifth instar nymphs of Dysdercus koenigii. Each bar represents Mean ± SE, Seven replicates were taken for calculation of mean Bars on a specific day after treatment with different letter are significantly different (p < 0.05), computed by one-way ANOVA followed by Tukey’s all pairwise multiple comparison test
Adipohaemocyte
Results summarized in Fig. 4 indicate that in control, there was gradual increase in the percent of adipohaemocytes with age of the fifth instar nymph; percent adipohaemocytes increased from 23 to 41.43% from day 1 to day 6. Day wise change in the percent adipohaemocytes was not consistent in the nymphs treated with Farnesol; on a specific dose, the percent adipohaemocyte, did not show any specific trend. However, on a specific day, when compared, a distinct dose-dependent decrease was observed on all the days. The decrease in the adipohaemocytes fraction was most prominent in the treatment with 0.2 µL/insects (Fig. 4).
Effect of Farnesol on Adipohaemocytes of fifth instar nymphs of Dysdercus koenigii Each bar represents Mean ± SE, Seven replicates were taken for calculation of mean Bars on a specific day after treatment with different letter are significantly different (p < 0.05), computed by one-way ANOVA followed by Tukey’s all pairwise multiple comparison test
Granulocyte
In control, the percent granulocytes first decreased from 7.71% on day 1 to 4.57% on day 2; subsequently they increase up to day 4 and then there was marginal dip on day 5 and day 6 of the fifth instar nymphal period. No distinct dose-related change was observed in the percent of granulocytes as a result of farnesol treatment (Fig. 5).
Effect of Farnesol on Granulocytes of fifth instar nymphs of Dysdercus koenigii. Each bar represents Mean ± SE, Seven replicates were taken for calculation of mean Bars on a specific day after treatment with different letter are significantly different (p < 0.05), computed by one-way ANOVA followed by Tukey’s all pairwise multiple comparison test
Oenocytoids
The percent count of oenocytoids is presented in Fig. 6. The results indicate that in control, the percent decrease from 4.43% on day 1 to 1.57% on day 4; the value subsequently increased on day 5. The treatment of Farnesol did not show any specific trend on the increase or decrease in percent oenocytoids in relation to dose of treatment and days after treatment.
Effect of Farnesol on Oenocytoids of fifth instar nymphs of Dysdercus koenigii. Each bar represents Mean ± SE, Seven replicates were taken for calculation of mean Bars on a specific day after treatment with different letter are significantly different (p < 0.05), computed by one-way ANOVA followed by Tukey’s all pairwise multiple comparison test
Disintegrated cells
The results shown in Fig. 7 indicate that in control, the percent disintegrated cells remained less throughout fifth instar nymphal period of D. koenigii. Farnesol treatment enhances the percent of disintegrated cells in a dose-dependent manner. On day 1 in comparison to control, an almost four time increase in the percent of disintegrated cells was observed in the nymphs treated with 0.2 µL. Similar trends of increase in disintegrated cells percent was seen on other days (Fig. 7).
Effect of Farnesol on Disintegrated cells of fifth instar nymphs of Dysdercus koenigii. Each bar represents Mean ± SE, Seven replicates were taken for calculation of mean Bars on a specific day after treatment with different letter are significantly different (p < 0.05), computed by one-way ANOVA followed by Tukey’s all pairwise multiple comparison test
Discussion
Haemolymph is the circulating fluid of insects. It moves through the open circulatory system, directly bathing the organs and tissues and facilitates transfer of resources to different organs. Volume of haemolymph varies in different developmental stages; usually the larval stages have larger haemolymph volume than adults. The haemolymph contains circulating cells called haemocytes, which contribute to the immune functions to the insects (Kanost, 2009; Kanost et al., 1990). Haemocyte count is affected both the internal factors of insect such as age, developmental stage, sex and size of insect (Jones, 1962; Ragan et al., 2009; Rantala et al., 2020), and external factor such as nutritional status, physiological state and exposure to xenobiotics (Beetz et al., 2008; Ghoneim & History, 2019; Jones, 1962). Change in the number of haemocytes has also been reported after treatment with insecticide; dose, duration of exposure and time after exposure to insecticide affect the haemocyte count (George & Ambrose, 2004; Morya et al., 2010; Perveen & Ahmad, 2017; Tiwari et al., 2006).
Our studies revealed that the total number of haemocytes in one day old fifth instar nymph of D. koenigii was 6060 cells/ mm3. The number of haemocytes increased to 8405cells/ mm3 on day 4, which subsequently decreased to 4900cells/ mm3 on day seven. Qamar (1998) reported an increase in that haemocyte count from 4557.0cells/ mm3 in one day old to 9499.5cells/ mm3 in 4 day old nymphs of D. cingulatus; decreased in total haemocyte count in 6 days old nymphs was also observed (Qamar, 1998). Similar trends of change in total haemocyte count were also observed in other insects such as Chrysocoris purpureus (Pugazhvendan & Soundararajan, 2009). Farnesol has an adverse effect on the number of haemocytes; the decrease in total haemocyte count was dose dependent. The results were consistent with other studies (Mannakkara, 2022; Qamar, 1998; Qamar & Jamal, 2009).
Five types of haemocytes are reported in Dysdercus koenigii based on their morphological characteristics. These were Prohaemocytes, Plasmatocytes, Adipohaemocytes, Granulocytes and Oenocytoids. Besides, disintegrated cells were also observed; these cells were characterized by fragmentation to vary extant and usually absence of nucleus. Similar types of haemocytes were reported in control and treated fifth instar nymphs of D. cingulatus (Qamar & Jamal, 2009) and in Apis dorsata (Perveen & Ahmad, 2017).
Prohaemocytes were the smallest of all haemocytes; they had round shape and high nucleo-cytoplasmic ratio. Plasmatocytes were pleiomorphic, varying in size and shape from round (discoid), to spindle-shaped and had a single nucleus that occupies about 40–50% of the granular, basophilic cytoplasm. Kanost (2009) reported high RER and Golgi apparatus in plasmatocytes indicating a high synthetic activity of plasmatocytes. Also, these cells are phagocytic and provide the primary line of defence against microbial infection in the insects. These cells release substances that induce degranulation of the granular haemocytes during the final stage of encapsulation (Capinera, 2005; Capinera et al., 2008). Adipohaemocytes vary in size and were ovoid in shape with well-defined lipid droplets and granular cytoplasm. Presence of abundant rough endoplasmic reticulum and Golgi complexes indicated their role in synthetic and secretory activity. Granulocytes vary in size and shape and have a small nucleo-cytoplasmic ratio. The cytoplasm of granulocytes contains small, uniform acidophilic cytoplasmic inclusions and possesses an elaborate network of RER, Golgi vesicles and other organelles (Gupta, 2009). Oenocytoids were large cells, may contain two eccentric nuclei and acidophilic cytoplasm. Numerous microtubules and few synthetic organelles were reported in oenocytoids (Capinera et al., 2008; Kanost, 2009; Ragan et al., 2009). They also exhibit endogenous phenoloxidase activity; which suggest their role in the process of melanization, wound healing and encapsulation (Capinera, 2005).
Our study revealed age-related differences in the relative percent of various types of haemocytes in the fifth instar nymphs. The mean percentage of prohaemocytes in one day old fifth instar nymphs was 12.57% which progressively increased to 16.14% on the 4th day. After this, the population of these cells decreased to 7.86% in 6 day old nymphs. In the adult males, the mean percentage of prohaemocytes was 12.57 and in adult females it was 16.43%. The relative percentage of plasmatocytes in newly moulted fifth instar nymphs was 52.29% and by fourth day it decreased to 36.29%. In adult males and females it increased to 52.29%; in female the increase was marginal i.e.39.29%. The population of adipohaemocytes increased from 23% in 1 day old to 41.33% in 6 days old nymphs. In one day old adult males the population of adipohaemocytes was maximum (46.29%). The population of adipohaemocytes fluctuated inconsistently during development of fifth instar nymph. The percent granulocytes remained less throughout the development, ranging between 7% and 11%. Similarly, the oenocytoids were poorly represented in comparison to other haemocytes. These reports on the relative distribution of haemocytes are in agreement with previous studies on Dysdercus cingulatus (Qamar, 1998). However, percent prohaemocytes and plasmatocytes of one day old male and female were found less in the present study.
The change in percent distribution of different types of haemocytes in response to Farnesol was seen in the present research work. Percent count of prohaemocyte is promoted in response to treatment with Farnesol; a distinct dose dependant increase was seen on day five, six and seven. The increase in percent prohaemocyte was most conspicuous on day seven. However, in earlier days there was no direct correlation between the dose of Farnesol in treated fifth instar nymphs and percent increase in the prohaemocytes. Farnesol treatment was responsible for increase in percent of plasmatocytes in a dose-dependent manner after 2 days. Adipohaemocytes showed distinct decrease in their percent count on all the days; this trend was dose dependent and seen more profound at a dose of 0.2 µL. Granulocytes and oenocytoids did not show any specific trend in response to the dose of treatment and days after treatment. Percent of disintegrated cell increased in all the treatments on all the days. Increase in the percent disintegration of cells was dose dependent on all days. Similar type of changes in the haemocyte pattern was observed in D. koenigii treated with imidacloprid, deltamethrin, lambda cyhalothrin, gamma cyhalothrin and cyfluthrin (Sarwar et al., 2018).
Farnesol is an intermediate in juvenile hormone biosynthesis. There are evidences that JH also influences immune function. For example, high titres of JH have been implicated in humoral immunosuppression in insects (Hiruma & Riddiford, 1993). In addition, hormone titres of JH and ecdysone may be responsible for disintegration of cells as seen in Farnesol-treated nymphs (Nunes, 2021; Rantala et al., 2003, 2020; Rolff & Siva-Jothy, 2002).
Conclusions
Our results interpret that the Farnesol had a profound effect on the total haemocyte count in a dose-dependent manner. The effects were cumulative in relation to the days after treatment. It was also observed that the effect of Farnesol on different types of haemocytes was different. Percent of prohaemocytes and plasmatocytes usually showed increase, adipohaemocytes showed decrease, granulocytes and oenocytoids did not show any specific trend. It was also observed that Farnesol is responsible for disintegration of haemocytes, as reflected by increase in the percent of disintegrated cell in the farnesol-treated fifth instar nymphs of Dysdercus koenigii.
Availability of data and materials
All the data are available with corresponding author, Professor Kamal Kumar Gupta.
Abbreviations
- ANOVA::
-
Analysis of variance
- EIL::
-
Economic injury level
- IPM::
-
Integrated pest management
- JH::
-
Juvenile hormone
- Mean ± S.E.:
-
Mean ± Standard error
References
Ahmad, M., & Khan, N. H. (1980). Chemical repellents for Dysdercus koenigii (Fabr.). Indian J Entomol, 42, 820-821.
Ahmad, I., & Mohammad, F. (1983). Biology and immature systematics of red cotton stainer dysdercus koenigii (Fabr.)(hemiptera: pyrrohocoridae) with a note on their phylogenetic value. Bull Zool, 1, 1–9.
Asawalam, E., & Adesiyan, S. (2010). Potential of ocimum basilicum(Linn) for the control of maize weevil sitophilus zeamais (Motsch). Nigerian Agricultural Journal. https://doi.org/10.4314/naj.v32i1.49381
Ashfaq, S., Khan, I. A., Saeed, M., Ur, A., & Saljoqi, R. (2011). Population dynamics of insect pests of cotton and their natural enimies. Sarhad J Agric, 27, 2009–2011.
Ayyangar, G. S. G., & Rao, P. J. (1990). Changes in haemolymph constituents of Spodoptera litura (Fabr.) under the influence of azadirachtin. Indian Journal of Entomology, 52, 69–83.
Beetz, S., Holthusen, T. K., Koolman, J., & Trenczek, T. (2008). Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Archives of Insect Biochemistry and Physiology, 67, 63–75. https://doi.org/10.1002/ARCH.20221
Bellés, X., Martín, D., & Piulachs, M. D. (2005). The mevalonate pathway and the synthesis of juvenile hormone in insects. Annual Review of Entomology, 50, 181–199. https://doi.org/10.1146/annurev.ento.50.071803.130356
Berger, J., & Klára, S. (2008). Morphological characterization of hemocytes in the adult linden bug, Pyrrhocoris apterus (L.)(Heteroptera). Zool Stud, 47, 466–472.
Bhattacharyya, A., Duraisamy, P., Govindarajan, M., Buhroo, A. A., & Prasad, R. (2016). Nano-biofungicides: emerging trend in insect pest control. Advances and Applications through Fungal Nanobiotechnology. https://doi.org/10.1007/978-3-319-42990-8_15
Boudh, S., & Singh, J. S. (2019). Pesticide contamination: environmental problems and remediation strategies. Emerg Eco-Friendly Approaches Waste Manag. https://doi.org/10.1007/978-981-10-8669-4_12
Capinera, J. L. (2005). Encyclopedia of entomology. USA: Springer.
Capinera, J. L., Crist, T. O., Heppner, J. B., Tzanakakis, M. E., Gayubo, S. F., Tartar, A., Lawrence, P. O., Hangay, G., Shukle, R. H., Skevington, J. H., Maimala, S., Boucias, D. G., Fasulo, T. R., Shanower, T. G., Brown, S. J., Shippy, T. D., Denell, R. E., Beeman, R. W., Webster, T. C., … Hernández, S. (2008). Hemocytes of insects: their morphology and function. Encycl Entomol. https://doi.org/10.1007/978-1-4020-6359-6_1302
Dancewicz, K., & Gliszczyńska, A. (2010). Effect of farnesol and its synthetic derivatives on the settling behaviour of the peach potato aphid Myzus persicae (Sulz.). Pestycydy/Pesticides, 1–4, 51–57.
Feyereisen, R., Friedel, T., & Tobe, S. S. (1981a). Farnesoic acid stimulation of C16 juvenile hormone biosynthesis by corpora allata of adult female Diploptera punctata. Insect Biochem, 11, 401–409. https://doi.org/10.1016/0020-1790(81)90073-1
Feyereisen, R., Koener, J., & Tobe, S. S. (1981b). In vitro studies with C2, C6 and C15 precursors of C16 JH biosynthesis in the corpora allata of adult female Diploptera punctata. Dev Endocrinol, 15, 81–92.
Genovese, A., Dimaggio, R., Lisanti, M. T., Piombino, P., & Moio, L. (2005). Aroma composition of red wines by different extraction methods and gas chromatography-SIM/MASS spectrometry analysis. Annales De Chimie, 95, 383–394. https://doi.org/10.1002/ADIC.200590045
George, P. E., & Ambrose, D. P. (2004). Impact of insecticides on the haemogram of rhynocoris kumarii ambrose and Livingstone (Hem., Reduviidae). Journal of Applied Entomology, 128, 600–604. https://doi.org/10.1111/j.1439-0418.2004.00896.600-604
Ghoneim K, History R (2019) Characterization of qualitative and quantitative haemogram parameters in insects: current concepts and future prospects. Egypt Acad J Biol Sci A Entomol https://doi.org/10.21608/EAJBSA.2019.25088.
Ghoneim K, Hamadah K, Waheeb H (2020) Bioefficacy of farnesol, a common sesquiterpene, on the survival, growth, development, and morphogenesis of spodoptera littoralis (Lepidoptera: Noctuidae). Egyptian Academic Journal of Biological Scienceshttps://doi.org/10.21608/EAJBSF.2020.78671.
Gökçe, A., Stelinski, L. L. & Whalon, M. E. (2018) The effects of non-host plant extracts on electroantennogram responses, behavior and egg hatching of codling moth Cydia pomonella. Journal of Pest Science, 91, 681–690. https://doi.org/10.1007/S10340-018-0953-5.
Goldstein, J. L., & Brown, M. S. (1990). Regulation of the mevalonate pathway. Nature, 343, 425–430. https://doi.org/10.1038/343425a0
Gonzalez-Coloma, A., Reina, M., Cabrera, R., Castañera, P., & Gutierrez, C. (1995). Antifeedant and toxic effects of sesquiterpenes fromSenecio palmensis to colorado potato beetle. Journal of Chemical Ecology, 21, 1255–1270. https://doi.org/10.1007/BF02027560
Gupta, A. (2009). Insect hemocytes development, forms, functions, and techniques. Cambridge University Press.
Hagstrum, D. W., & Flinn, P. W. (2018). Integrated pest management. Integr Manag Insects Stored Prod. https://doi.org/10.1201/9780203750612-9
Hiruma, K., & Riddiford, L. M. (1993). Molecular mechanisms of cuticular melanization in the tobacco hornworm, Manduca sexta (L.) (Lepidoptera : Sphingidae). International Journal of Insect Morphology and Embryology, 22, 103–117. https://doi.org/10.1016/0020-7322(93)90003-J
Hisaya, S., Akira, K., Kiyoshi, M., & Yasuyuki, T. (1982). Separation of farnesol isomers by liquid chromatography. Journal of Chromatography A, 237, 178–182.
Jones, J. C. (1962). Current concepts concerning Insect Hemocytes. American Zoologist, 246, 209–246.
Kaneko, M., Togashi, N., Hamashima, H., Hirohara, M., & Inoue, Y. (2011). Effect of farnesol on mevalonate pathway of Staphylococcus aureus. The Journal of Antibiotics (tokyo), 64, 547–549. https://doi.org/10.1038/ja.2011.49
Kanost, M. R. (2009). Hemolymph. Encycl Insects. https://doi.org/10.1016/B978-0-12-374144-8.00126-0
Kanost MR, Kawooya JK, Law JH, Ryan RO, Van Heusden MC, Ziegler R (1990) Insect haemolymph proteins. In: Advances in insect physiology. Elsevier, USA.
Kapur, A. P., & Vazirani, T. G. (1956). The identity and geographical distribution of the Indian species of the genus Dysdercus boisduval (Hemiptera: Pyrrhocoridae). Rec Indian Mus, 54, 159–175.
Kayesth S, Shazad M, Kumar S, Kumar K (2017) Effect of ethanol extract of Catharanthus roseus , Ocimum sanctum and Lantana camara on fecundity and fertility of red cotton bug , Dysdercus koenigii Fabricius ( heteroptera : pyrrhoc . Effect of ethanol extract of Catharanthus roseus , Ocimum sanctum
Kayesth, S., Gupta, K. K., Kumar, S., & Shazad, M. (2019). Effects of Ocimum sanctum hexane extract on survival and development of Dysdercus koenigii Fabricius (Heteroptera: Pyrrhocoriedae). Archives of Phytopathology and Plant Protection, 51, 993–1007. https://doi.org/10.1080/03235408.2018.1541148
Kayesth, S., Kumar, S., Shazad, M., & Gupta, K. K. (2021b). Diminished fitness of red cotton bug Dysdercus koenigii Fabricius induced by the volatiles in the hexane extract of Catharanthus roseus. International Journal of Tropical Insect Science., 41, 389–399. https://doi.org/10.1007/s42690-020-00217-5
Kayesth S, Shazad M, Kumar S, Gupta KK (2021a) Decreased reproductive fitness of Dysdercus koenigii Fabricius (Heteroptera: Pyrrhocoreidae) in response to hexane leaf extract of Ocimum sanctum Linn. (Lamiaceae). International Journal of Tropical Insect Science. https://doi.org/10.1007/s42690-021-00669-3.
Kurihara, Y., Shimazu, T., & Wago, H. (1992). Classification of hemocytes in the common cutworm, spodoptera litura(Lepidoptera:Noctuidae): II.possible roles of granular plasmatocytes and oenocytoids in the cellular defense reactions. Applied Entomology and Zoology, 27, 237–242. https://doi.org/10.1303/AEZ.27.237
Leonardi, M. G., Cappellozza, S., Ianne, P., Cappellozza, L., Parenti, P., & Giordana, B. (1996). Effects of the topical application of an insect growth regulator (fenoxycarb) on some physiological parameters in the fifth instar larvae of the silkworm Bombyx mori. Comparative Biochemistry and Physiology, Part B: Biochemistry & Molecular Biology, 113, 361–365.
Ling, E., Shirai, K., Kanekatsu, R., & Kiguchi, K. (2005). Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: Prohemocytes have the function of phagocytosis. Cell and Tissue Research, 320, 535–543. https://doi.org/10.1007/S00441-004-1038-8
Lombard, J., & Moreira, D. (2011). Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Molecular Biology and Evolution, 28, 87–99.
MacLeod, A. J., & Pieris, N. M. (1984). Volatile aroma constituents of Sri Lankan ginger. Phytochemistry, 23, 353–359. https://doi.org/10.1016/S0031-9422(00)80332-5
Mannakkara, A. (2022). Study of haemocytes of the rice brown planthopper, nilaparvata lugens stål (Hemiptera: Delphacidae). J Agric Sci - Sri Lanka, 17, 111–121. https://doi.org/10.4038/JAS.V17I1.9614
Maxwell-Lefroy, H. (1908). The red cotton bug (Dysdercus cingulatus Fabr.). Mem Dept Agric India (entomol Ser), 2, 47–58.
Medeiros, J. R., Campos, L. B., Mendonça, S. C., Davin, L. B., & Lewis, N. G. (2003). Composition and antimicrobial activity of the essential oils from invasive species of the Azores, Hedychium gardnerianum and Pittosporum undulatum. Phytochemistry, 64, 561–565. https://doi.org/10.1016/S0031-9422(03)00338-8
Morya, K., Pillai, S., & Patel, P. (2010). Effect of powdered leaves of lantana camara, clerodendrum inerme and citrus limon on the rice moth, corcyra cephalonica. Bull Insectology, 63, 183–189.
Nunes, C. (2021). Endocrine regulation of immunity in insects. The FEBS Journal, 288, 3928–3947. https://doi.org/10.1111/febs.15581
Perveen, N., & Ahmad, M. (2017). Toxicity of some insecticides to the haemocytes of giant honeybee, Apis dorsata F. under laboratory conditions. Saudi Journal of Biological Science, 24, 1016–1022. https://doi.org/10.1016/j.sjbs.2016.12.011
Pugazhvendan, S. R., & Soundararajan, M. (2009). Effect of penfluron on total haemocyte count of chrysocoris purpureus. Middle-East Journal of Scientific Research, 4, 338–340.
Qamar, A. (1998). Observations on the haemocytes of certain insect pests affected by some chemical. Aligarh Muslim University.
Qamar, A., & Jamal, K. (2009). Differential haemocyte counts of 5th instar nymphs and adults of Dysdercus cingulatus Fabr (Hemiptera: Pyrrhocoridae) treated with acephate, an organophosphorus insecticide. Biologie Et Médecine, 1, 116–121.
Ragan EJ, An C, Jiang H, Kanost MR (2009) Roles of haemolymph proteins in antimicrobial defences of Manduca sexta. In: Insect Infection and Immunity. Oxford University Press, pp 34–48
Rantala, M. J., Vainikka, A., & Kortet, R. (2003). The role of juvenile hormone in immune function and pheromone production trade-offs: A test of the immunocompetence handicap principle. Proceedings of the Royal Society of London. Series b: Biological Sciences, 270, 2257–2261. https://doi.org/10.1098/rspb.2003.2472
Rantala, M. J., Dubovskiy, I. M., Pölkki, M., Krama, T., Contreras-garduño, J., & Krams, I. A. (2020). Effect of juvenile hormone on resistance against entomopathogenic fungus metarhizium robertsii Di ff ers between sexes. 1–8.
Rathee, M., Dalal, P. K., Mehra, S. (2018). Integrated pest management under protected cultivation: A review. Journal of Entomology and Zoology Studies, 6(2), 1201–1208.
Rolff, J., & Siva-Jothy, M. T. (2002). Copulation corrupts immunity: A mechanism for a cost of mating in insects. Proc Natl Acad Sci U S A, 99, 9916–9918. https://doi.org/10.1073/PNAS.152271999/ASSET/8CEEE6BB-F6E1-4E64-AE87-C0D89680AB13/ASSETS/GRAPHIC/PQ1522719003.JPEG
Salunke, B. K., Prakash, K., Vishwakarma, K. S., & Maheshwari, V. L. (2009). Plant metabolites: An alternative and sustainable approach towards post harvest pest management in pulses. Physiology and Molecular Biology of Plants, 15, 185–197. https://doi.org/10.1007/s12298-009-0023-9
Sarwar, Z. M., Ijaz, M., Sabri, M. A., Yousaf, H., & Mohsan, M. (2018). Effects of selected synthetic insecticides on the total and differential populations of circulating haemocytes in adults of the red cotton stainer bug Dysdercus koenigii (Fabricius) (Hemiptera: Pyrrhocoridae). Environmental Science and Pollution Research, 25, 17033–17037. https://doi.org/10.1007/s11356-018-1898-1
Schaefer, C. W., & Panizzi, A. R. (2000). Heteroptera of economic importance. CRC Press.
Schmidt, G. H., Ibrahim, N. M. M., & Abdallah, M. D. (1991). Toxicological studies on the long-term effects of heavy metals (Hg, Cd, Pb) in soil on the development of Aiolopus thalassinus (Fabr.) (Saltatoria: Acrididae). Science of the Total Environment, 107, 109–133. https://doi.org/10.1016/0048-9697(91)90254-C
Siddiqui, M. I., & Al-khalifa, M. S. (2012). Circulating haemocytes in insects. Phylogenic Review of Their Types., 44, 1743–1750.
Srinivasan, R. (2016). Integrated Pest Management in Tropical Vegetable Crops. In D. P. Abrol (Ed.), Integrated pest management in the tropics (pp. 219–247). New India Publishing Agency.
Stehlík, J. L., & Kerzhner, I. M. (1999). On taxonomy and distribution of some palaearctic and oriental largidae and Pyrrhocoridae (Heteroptera). Zoosystematica Ross, 8, 121–128.
Strand, M. R. (2008). Insect hemocytes and their role in immunity. Insect Immunol, 32, 25–47.
Tiwari, R. K., Pandey, J. P., & Kumar, D. (2006). Effects of neem-based insecticides on metamorphosis, haemocytes and reproductive behavior in the red cotton bug, Dysdercus koenigii (Fab.) (Heteroptera: Pyrrhocoridae). Entomon, 31, 257–267.
Vommaro, M. L., Kurtz, J., & Giglio, A. (2021). Morphological characterisation of haemocytes in the mealworm beetle tenebrio molitor (Coleoptera, Tenebrionidae). Insects. https://doi.org/10.3390/INSECTS12050423
Zoubiri, S., & Baaliouamer, A. (2014). Potentiality of plants as source of insecticide principles. Journal of Saudi Chemical Society, 18, 925–938. https://doi.org/10.1016/J.JSCS.2011.11.015
Acknowledgements
The authors acknowledge Professor Rajiv Aggarwal, Principal, Deshbandhu College, University of Delhi, for providing necessary infrastructure during research work. Shailendra Kumar acknowledge receive of Junior Research Fellowship and Senior Research Fellowship from University Grants Commission, India
Funding
Partial funding was provided by University Grants Commission to Mr Shailendra Kumar in the form of Research Fellowship and Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. KKG contributed to conceptualization and designing the study, data interpretation and finalization of the manuscript. Material preparation, data collection and analysis were performed by SK, SK1 and MS. The first draft of the manuscript was written by SK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, S., Shazad, M., Kayesth, S. et al. Evaluation of farnesol-induced changes in the haemocyte pattern of red cotton bug Dysdercus koenigii Fabricius (Heteroptera: Pyrrhocoridae). JoBAZ 83, 44 (2022). https://doi.org/10.1186/s41936-022-00308-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00308-4