Abstract
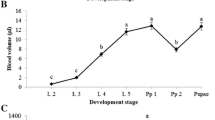
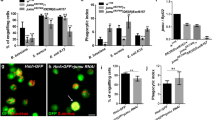
Hemocytes isolated from the larval hematopoietic organs of the silkworm were classified following staining with acridine orange and propidium iodide. Among the hemocytes isolated from the hematopoietic organs of whole fifth larval and wandering stages, most were prohemocytes (60%–70%) and oenocytoids (30%–40%). Granulocytes comprised only about 0.5%–1% at the wandering stage and were even rarer at other stages; no spherulocytes or plasmatocytes were found. Therefore, hemocyte differentiation inside larval hematopoietic organs is not as extensive as previously thought. Following 10–30 min in vitro culture of hemocytes isolated from larval hematopoietic organs, many young granulocytes and plasmatocytes appeared. Furthermore, during phagocytosis assays, prohemocytes were seen to adopt the morphology of plasmatocytes, containing fragments of phagocytosed cells. Our results underline the similarities between Drosophila and Bombyx hematopoiesis.







Similar content being viewed by others
References
Akai H, Sato S (1971) An ultrastructural study of the haemopoietic organs of the silkworm, Bombyx mori. J Insect Physiol 17:1665–1676
Akai H, Sato S (1973) Ultrastructure of the larval hemocytes of the silkworm, Bombyx mori L. (Lepidoptera: Bombycidae). Int J Insect Morphol Embryol 2:207–231
Ashida M, Brey PT (1997) Recent advances in research on the insect prophenoloxidase cascade. In: Brey PT, Hultmark D (eds) Molecular mechanisms of immune responses in insects. Chapman & Hall, London, pp 135–172
Beaulaton J (1979) Hemocytes and hemocytopoiesis in silkworms. Biochimie 61:157–164
Carton Y, Nappi AJ (2001) Immunogenetic aspects of the cellular immune response of Drosophila against parasitoids. Immunogenetics 52:157–164
Dearolf CR (1998) Fruit fly “leukemia”. Biochim Biophys Acta 1377:M13–M23
Evans CJ, Banerjee U (2003) Transcriptional regulation of hematopoiesis in Drosophila. Blood Cells Mol Dis 30:223–228
Evans CJ, Hartenstein V, Banerjee U (2003) Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell 5:673–690
Foglieni C, Meoni C, Davalli AM (2001) Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol 115:223–229
Fossett N, Schulz RA (2001) Functional conservation of hematopoietic factors in Drosophila and vertebrates. Differentiation 69:83–90
Fossett N, Tevosian SG, Gajewski K, Zhang Q, Orkin SH, Schulz RA (2001) The friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci USA 98:7342–7347
Fossett N, Hyman K, Gajewski K, Orkin SH, Schulz RA (2003) Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc Natl Acad Sci USA 100:11451–11456
Gateff E (1994) Tumor-suppressor genes, hematopoietic malignancies and other hematopoietic disorders of Drosophila melanogaster. Ann N Y Acad Sci 712:260–279
Han SS, Lee MH, Kim WK, Wago H, Yoe SM (1998) Hemocytic differentiation in hemopoietic organ of Bombyx mori larvae. Zool Sci 15:371–379
Holz A, Bossinger B, Strasser T, Janning W, Klapper R (2003) The two origins of hemocytes in Drosophila. Development 130:4955–4962
Kawasaki H (1989) Methods for culture of Bombyx mori wing discs. J Tissue Culture Method 12:31–33
Kawasaki H (1995) Ecdysteroid concentration inducing cell proliferation brings about the imaginal differentiation in the wing disc of Bombyx mori in vitro. Dev Growth Differ 37:575–580
Kiguchi K, Agui N (1981) Ecdysteroid levels and developmental events during larval moulting in the silkworm, Bombyx mori. J Insect Physiol 27:805–812
Kiguchi K, Agui N, Kawasaki H, Kobayashi M (1985) Developmental time-table for the last larval and pharate pupal stages in the silkworm, Bombyx mori, with special reference to the correlation between the developmental events and haemolymph ecdysteroid levels. Bull Sericult Exp Sta 30:83–100
Laigo FM, Paschke JD (1966) Variations in total haemocyte counts as induced by a nosemosis in the cabbage looper, Trichoplusia ni. J Invertebr Pathol 8:175–179
Lavine MD, Strand MR (2002) Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Lebestky T, Chang T, Hartenstein V, Banerjee U (2000) Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288:146–149
Ling E, Xu S, Fukamoto K, Shirai K, Kanekatsu R, Kobayashi Y, Tu Z, Funayama T, Watanabe H, Kiguchi K (2001) Hemocytes production and their morphological change with the hemopoietic organs of silkworm, Bombyx mori being cultured (in Japanese). In: Annual Meeting of Japanese Sericultural Science Society, Chubu Branch, 57, p 38
Ling E, Shirai K, Kanekatsu R, Kobayashi Y, Tu Z, Funayama T, Watanabe H, Kiguchi K (2003a) Why does hemocyte density rise at the wandering stage in the silkworm, Bombyx mori? J Insect Biotechnol Sericol 72:101–109
Ling E, Shirai K, Kanekatsu R, Kiguchi K (2003b) Classification of larval circulating hemocytes of the silkworm, Bombyx mori, by acridine orange and propidium iodide staining. Histochem Cell Biol 120:505–511
Meister M, Lagueux M (2003) Drosophila blood cells. Cell Microbiol 5:573–580
Nakahara Y, Kanamori Y, Kiuchi M, Kamimura M (2003) Effects of silkworm paralytic peptide on in vitro hematopoiesis and plasmatocyte spreading. Arch Insect Biochem Physiol 52:163–174
Nittono Y (1960) Studies on the blood cells in the silkworm, Bombyx mori, L (in Japanese). Bull Sericult Exp Sta 16:171–266
Pathak JPN, Soni AK (1990) Impact of diseases on the unfixed total haemocyte counts of B. mori L. during development. In: Promada Kumari J (ed) Recent trends in sericulture. Tirupati University, India, pp 13–26
Ribeiro C, Petit V, Affolter M (2003) Signaling systems, guided cell migration, and organogenesis: insights from genetic studies in Drosophila. Dev Biol 260:1–8
Rizki TM (1956) Blood cells of Drosophila as related to metamorphosis. In: Campbell FL (ed) Physiology of insect development. Chicago University Press, Chicago, pp 91–94
Rizki TM (1962) Experimental analysis of hemocyte morphology in insects. Am Zool 2:247–256
Shapiro M, Stock RD, Ugnoffo CM (1969) Haemocyte changes in larvae of the bollworm, Heliothis zea, infected with a nucleopolyhedrosis virus. J Invertebr Pathol 14:28–30
Tepass U, Fessler LI, Aziz A, Hartenstein V (1994) Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120:1829–1837
Vass E, Nappi AJ (2000) Developmental and immunological aspects of Drosophila-parasitoid relationships. J Parasitol 86:1259–1270
Wago H (1991) Phagocytic recognition in Bombyx mori. In: Gupta AP (ed) Immunology of insects and other arthropods. CRC Press, Boca Raton, pp 215–235
Wyllie A, et al (1998) Apoptosis and cell proliferation, 2nd edn. Biochemica Boehringer, Mannheim
Yamashita M, Iwabuchi K (2001) Bombyx mori prohemocyte division and differentiation in individual microcultures. J Insect Physiol 47:325–331
Acknowledgements
The authors thank V. Hartenstein and J.H. Hillyer for helpful discussions and comments on the manuscript. We also thank two unknown reviewers for critically reading and reviewing this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, E., Shirai, K., Kanekatsu, R. et al. Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: prohemocytes have the function of phagocytosis. Cell Tissue Res 320, 535–543 (2005). https://doi.org/10.1007/s00441-004-1038-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1038-8