Abstract
Background
As there is no established standard of care for non-tuberculous mycobacterium (NTM) peritoneal dialysis (PD)-related peritonitis, its treatments have to be case-dependent, which is often difficult. Additionally, several reported cases were accompanied by encapsulated ascites, adhesive ileus, and encapsulating peritoneal sclerosis, suggesting treatment difficulties. We report two cases of PD-related peritonitis with encapsulated ascites due to Mycobacterium abscessus subsp. massilience and subsp. bolletii. To the best of our knowledge, this is the first case series to report PD-related peritonitis caused by Mycobacterium abscessus subsp. bolletii.
Case presentation
The first case is that of a 74-year-old male patient who started PD six years ago for end-stage renal failure due to diabetic nephropathy. In February 2021, he presented with signs of infection at the exit-site and swelling of the tunnel. Mycobacterium abscessus subsp. massilience was detected in the culture of the exit-site exudate; thus, he was diagnosed with tunnel infection (caused by NTM). Subsequently, fever, abdominal pain, and increased cell counts in the PD drainage fluid were observed, and he was judged to have NTM peritonitis. His general condition improved after PD catheter removal in addition to antimicrobial treatment and encapsulated ascites drainage. The second case is that of a 52-year-old man who commenced PD for end-stage renal failure due to nephrosclerosis 12 years ago. In May 2022, he was diagnosed with PD-related peritonitis based on signs of infection at the exit-site, encapsulated ascites on computed tomography, and a cloudy PD drainage fluid. Mycobacterium abscessus subsp. bolletii was detected in the culture of the exit-site exudate, which led to the diagnosis of NTM peritonitis. In addition to antimicrobial treatment, PD catheter removal and encapsulated ascites drainage were performed. The patient also had adhesive bowel obstruction due to peritonitis and required decompression therapy with the insertion of a gastric tube.
Conclusions
PD catheter removal and encapsulated ascites drainage might have improved inflammation and treatment outcomes. Additionally, Mycobacterium abscessus might be prone to forming encapsulated cavities and/or intestinal adhesions; however, further accumulation of cases clarifying “subspecies” of Mycobacterium abscessus is necessary to confirm this hypothesis.
Similar content being viewed by others
Background
PD-associated peritonitis due to NTM accounts for 0.3%–1.3% of peritonitis cases [1, 2]. Risk factors for PD-related peritonitis have been reported to include diabetes, obesity, race, climate and depression [3]. NTM peritonitis usually occurs in patients with a history of recurrent peritonitis, local trauma, catheter exit-site leaks, and poor aseptic manipulation techniques [4, 5]. Risk factors for NTM peritonitis also include immunodepression, inefficient dialysis, low residual renal function, exposure to environmental sources of the pathogen (such as soil and contaminated water), and a history of long-term broad-spectrum antibiotherapy for recurrent peritonitis [4, 5]. Treatment with clarithromycin and amikacin has been reported; however, no standard of care has been established [6, 7]. The duration of treatment is also unclear and previous reports vary [7]. Therefore, treatment for NTM peritonitis needs to be case-dependent, which is often difficult. Additionally, several reported cases were accompanied by encapsulated ascites, adhesive ileus, and encapsulating peritoneal sclerosis (EPS), all of which suggest treatment difficulties [8,9,10,11,12,13]. In this paper, we report two rare cases of PD-related peritonitis with encapsulated ascites due to Mycobacterium abscessus.
Case presentation
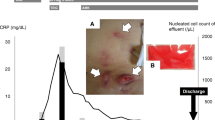
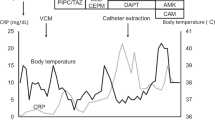
The first case is that of a 74-year-old male patient who had been undergoing PD for diabetic nephropathy-induced end-stage renal failure since 2015. In February 2021, he was diagnosed with exit-site and tunnel infection caused by NTM after signs of exit-site infection and swelling of the tunnel were observed and Mycobacterium abscessus subsp. massilience was detected in the exit-site exudate culture. In July 2021, he underwent simultaneous PD catheter removal and replacement and subcutaneous abscess drainage at another hospital (day 0). However, twelve days after, he developed fever, abdominal pain, and increased cell counts in the PD drainage fluid (total: 210/μL, polynuclear cells: 117/μL); thus, he was diagnosed with PD-related peritonitis due to Mycobacterium abscessus. Antimicrobial treatment (lascufloxacin 75 mg oral + clarithromycin (CAM) 200 mg oral + meropenem (MEPM) 0.5 g intravenous injection (IV) + isepamicin 400 mg IV) was started. Mycobacterium abscessus detected in the PD fluid showed high sensitivity to CAM and amikacin (AMK), intermediate sensitivity to imipenem (IPM), and resistance to MEPM, tobramycin (TOB), moxifloxacin (MFLX), sulfamethoxazole-trimethoprim (ST), doxycycline (DOXY), and linezolid (LZD). Seventeen days after the surgery, he was transferred to our hospital; then, based on the antibiotic sensitivity profile, he was switched to CAM 800 mg oral, IPM/cilastatin (CS) 1 g IV, and IV AMK dosed with an adjusted trough concentration < 10 µg/mg (Fig. 1A). Additionally, from day 18, he was switched to hemodialysis (HD) with a temporary dialysis catheter. HD was performed every day because of his worsening congestive heart failure due to poor ultrafiltration from PD. On day 22, the PD catheter was removed. Due to the lack of improvement in inflammation based on blood test results, a CT scan was performed on day 40, which showed encapsulated ascites (Fig. 1B, C), and the drainage tube was inserted on day 45. The drainage fluid was a cloudy yellow–brown liquid. As inflammation improved with drainage, the drainage tube was removed on day 51, and IPM/CS was discontinued on day 64. He was switched to intramuscular (IM) injections of AMK and discharged on day 70. A few days after his discharge, AMK was discontinued due to new-onset hearing impairment that was probably drug-related. CAM was continued until six months after PD catheter removal, and after the discontinuation, no signs of relapse have been detected.
The second case is that of a 52-year-old man who had been undergoing PD for nephrosclerosis-induced end-stage renal failure since 2010. He had PD-related peritonitis and underwent simultaneous PD catheter removal and replacement in April 2022. In May 2022, he developed an exit-site infection and encapsulated ascites on CT (Fig. 2A, B). Additionally, as he also had severe abdominal pain and nausea that made him ineligible for PD, he was admitted to our hospital for close examination and treatment (day 0). He was diagnosed with PD-related peritonitis because of increased cell counts in the dialysate (total: 100/μL, polynuclear cells: 50/μL), and Mycobacterium abscessus subsp. bolletii was detected in both exit-site exudates and dialysate cultures (Fig. 2C). Based on CT findings, abdominal pain and nausea were considered to be due to adhesive bowel obstruction; therefore, decompression was performed by inserting a gastric tube while avoiding oral feeding. The PD catheter was removed on day 1 and he was switched to HD. On days 4 and 9, encapsulated ascites drainage was performed. Initially, antimicrobial treatment was started with 400 mg of oral CAM, IV IPM/CS (1 g), and IV AMK, which was dosed with an adjusted trough concentration of < 10 µg/mg; however, CAM was changed to azithromycin (AZM) 250 mg IV on day 7 because of adhesive bowel obstruction, which made it difficult for him to take oral medication. Following these treatments, his blood test results and general condition improved, and he resumed oral feeding. As it was possible that he could have developed EPS due to persistent infection, he was temporarily transferred to another hospital where surgical dissection of the adhesions was possible on day 16. Although it was possible for him to develop EPS due to persistent infection, his abdominal symptoms diminished with gradual diet progression, and AZM 250 mg IV was switched to oral CAM (800 mg) on day 31. At this time, the culture results of the PD fluid collected in the early stages of hospitalization showed that Mycobacterium abscessus demonstrated high sensitivity to AMK, intermediate sensitivity to IPM and TOB, and resistance to MEPM, MFLX, ST, DOXY, and LZD. On day 55, antimicrobials were switched to oral sitafloxacin (STFX, 50 mg) every other day, oral clofazimine (CLF, 100 mg) every other day, with IV IPM/CS (1 g) and IV AMK. Subcutaneous debridement of the tunnel area was performed to decrease the bacterial population on day 72. As the patient's general condition had stabilized, IV IPM/CS was rounded up on day 84 and he was discharged on day 93. Six months after starting antimicrobial treatment, the patient is currently continuing antimicrobial treatment with IV AMK (500–600 mg) three times a week, oral STFX (50 mg), and oral CLF (100 mg).
Discussion and conclusions
Herein, we present two cases of peritonitis caused by Mycobacterium abscessus, one by the massilience subsp and the other by the bolletii subsp. In both cases, the patient had encapsulated ascites, resulting in catheter removal and ascites drainage. The removal of the PD catheter is important in the treatment of NTM peritonitis, and treatment response is poor unless the catheter is removed. In three case reports, treatment failure, recurrent peritonitis, and death were observed in cases in which PD catheters were not removed [14,15,16]. Conversely, rapid improvement was observed after PD catheter removal [17]. In the current two cases, especially in case 1, inflammation also improved with PD catheter removal.
We conducted a systematic review of the literature focusing on PD-related peritonitis caused by NTM since May 2011 because there has already been a systematic review of the cases reported in PubMed up to April 2011 [17]. The number of cases with NTM peritonitis reported in PubMed from May 2011 to December 22, 2022, was 61, including our two cases (Tables 1 and 2) [6, 8,9,10,11,12,13, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Therefore, combined with the 57 cases reported up to April 2011 [17], the number of cases with NTM peritonitis reported in PubMed to date is 118. In our review, 24 patients were females and 37 males, and their ages ranged from 2.7 years to 78 years; the geographical spread included reports from Asia (37 cases), Oceania (15 cases), North America (6 cases), and Europe (3 cases). Mycobacterium abscessus was the most commonly reported cause with 25 cases (41.0%), followed by Mycobacterium fortuitum with 12 cases (19.7%), in contrast to the previous review in which Mycobacterium fortuitum was the most common cause (38.6%) [17]. In our review, the catheter was completely removed in 41 cases but it was not removed in 20 cases, including 8 cases with simultaneous or bi-phasic catheter removal and re-insertion. As expected, death was observed in five (12.2%) of the cases with catheter removal, a lower mortality rate than that recorded among cases without catheter removal (4 cases, 20.0%). Additionally, all catheters were removed among cases who underwent simultaneous or bi-phasic catheter removal and re-insertion, indicating that these procedures were not recommended in NTM peritonitis. Therefore, we believe that the catheter should be removed once the causative organism of PD-related peritonitis is identified as NTM.
Next, we compared the characteristics between the deceased (n = 9, 6 men) and non-deceased (n = 52, 31 men) patients. The median age was comparable between the two groups [56 (25th–75th percentile, 50.5–71.5) and 54 (40–64.8) years, respectively]. PD vintage was recorded in 8 cases in the deceased and 38 cases in the non-deceased group and was similar between the two groups [2 (1.5–3) and 1.5 (0.7–4) years, respectively]. Among the patients who died, the timing of PD catheter removal was only described in one case and the catheter was removed on day 7. Conversely, among the patients who survived, the timing of PD catheter removal was described in 26 cases; the catheters were removed at 11.5 (4.8–24.5) days. Furthermore, the antimicrobials used in each group are summarized in Table 3. Although the use of aminoglycosides and macrolides is comparable between the deceased and non-deceased groups, the rate of using new quinolones and beta-lactams seems higher in the non-deceased group than in the deceased group. Therefore, besides the most frequently used aminoglycosides and macrolides, the addition of new quinolones and/or beta-lactams based on the sensitivity of the organisms can yield successful treatment of patients with NTM peritonitis.
Including the current two cases, only five cases of NTM peritonitis with encapsulated ascites were reported [8,9,10], one of which was accompanied by EPS [9]. Interestingly, the organisms in all these cases were Mycobacterium abscessus. Additionally, three cases of EPS [9, 11, 12] and two cases of adhesive ileus [13] (including our second case) were reported. The culprit pathogens were Mycobacterium fortuitum in one case [11] and Mycobacterium abscessus in four cases [9, 12, 13]. These findings suggested that Mycobacterium abscessus tends to form encapsulated cavities and/or intestinal adhesions compared to other NTMs. Regarding the treatment, both of the current two cases underwent encapsulated ascites drainage, and rapid improvement of the persistent inflammation was observed after drainage. Additionally, among the three cases with encapsulated ascites that were previously reported, one recovered after drainage [10] and one case died without drainage [8]. Therefore, it is suggested that encapsulated ascites drainage was effective in improving inflammation; however, it is difficult to determine whether there was a direct causal relationship between drainage and inflammation abatement.
We hereby raise the issue that most reports did not describe the subtypes of Mycobacterium abscessus. Mycobacterium abscessus was first isolated from a knee abscess in 1952 [45]. Later, subspecies such as Mycobacterium massiliense and Mycobacterium bolletii were discovered. Following the consolidation and isolation of subspecies, genomic comparisons of several studies now show that Mycobacterium abscessus is composed of three subspecies: abscessus, massilience, and bolletii [46]. Different subspecies have different expression patterns of the inducible erythromycin ribosome methyltransferase (erm) (41) gene, which determines resistance to macrolides, and this results in different treatment outcomes for each subspecies [47]. Mycobacterium abscessus subsp. massiliense has been proposed to have a non-functional erm (41) gene, macrolide sensitivity, and good therapeutic outcome. In contrast, Mycobacterium abscessus subsp. bolletii, which has a functional erm (41) gene, is macrolide resistant [46, 47]. Indeed, in the present cases, Mycobacterium abscessus subsp. massiliense was sensitive to macrolides while Mycobacterium abscessus subsp. bolletii was resistant to them, making the choice of antimicrobial agent difficult. Therefore, there is a substantial need to clarify the subspecies of Mycobacterium abscessus to predict antimicrobial treatment response and further evaluate the clinical characteristics of each subspecies from a future accumulation of cases. To the best of our knowledge, this is the first case report of PD-related peritonitis caused by Mycobacterium abscessus subsp. bolletii.
In conclusion, we experienced two cases of PD-related peritonitis with encapsulated ascites due to Mycobacterium abscessus subsp. masillience and subsp. bolletii. In both cases, PD catheter removal and encapsulated ascites drainage might have improved inflammation and treatment outcomes. Additionally, Mycobacterium abscessus may tend to form encapsulated cavities and/or intestinal adhesions. However, further accumulation of cases clarifying the “subspecies” of Mycobacterium abscessus is necessary to confirm our hypothesis. Although no absolutes can be drawn from this case report, prompt catheter removal and administration of new quinolones and/or beta-lactams in addition to aminoglycosides and macrolides may be the key to achieve successful treatment of patients with NTM peritonitis.
Availability of data and materials
The patient results used and/or analyzed during the current case report are available from the corresponding author upon reasonable request.
Abbreviations
- NTM:
-
Non-tuberculous mycobacterium
- PD:
-
Peritoneal dialysis
- CAM:
-
Clarithromycin
- MEPM:
-
Meropenem
- IV:
-
Intravenous injection
- AMK:
-
Amikacin
- IPM:
-
Imipenem
- TOB:
-
Tobramycin
- MFLX:
-
Moxifloxacin
- ST:
-
Sulfamethoxazole-trimethoprim
- DOXY:
-
Doxycycline
- LZD:
-
Linezolid
- CS:
-
Cilastatin
- HD:
-
Hemodialysis
- IM:
-
Intramuscular
- AZM:
-
Azithromycin
- EPS:
-
Encapsulating peritoneal sclerosis
- STFX:
-
Sitafloxacin
- CLF:
-
Clofazimine
- erm:
-
Inducible erythromycin ribosome methyltransferase
References
Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int. 2011;31:651–62.
Portolés J, Janeiro D, Lou-Arnal LM, Lopez-Sanchez P, Ortega M, del Peso G, et al. First episodes of peritoneal infection: description and prognostic factors. Nefrologia. 2013;33:316–24.
Ballinger AE, Palmer SC, Wiggins KJ, Craig JC, Johnson DW, Cross NB, et al. Treatment for peritoneal dialysis-associated peritonitis. Cochrane Database Syst Rev. 2014;4:Cd005284.
Youmbissi JT, Malik QT, Ajit SK, al Khursany IA, Rafi A, Karkar A. Non tuberculous mycobacterium peritonitis in continuous ambulatory peritoneal dialysis. J Nephrol. 2001;14:132–5.
Perazella M, Eisen T, Brown E. Peritonitis associated with disseminated Mycobacterium avium complex in an acquired immunodeficiency syndrome patient on chronic ambulatory peritoneal dialysis. Am J Kidney Dis. 1993;21:319–21.
Lin JH, Wang WJ, Yang HY, Cheng M-H, Huang W-H, Lin C-Y, et al. Non-tuberculous and tuberculous mycobacterial peritonitis in peritoneal dialysis patients. Ren Fail. 2014;36:1158–61.
Rho M, Bia F, Brewster UC. Nontuberculous mycobacterial peritonitis in peritoneal dialysis patients. Semin Dial. 2007;20:271–6.
Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in Asians: a case series and literature review. Nephrology. 2011;16:174–9.
Rouhani S, Adunuri N. Refractory peritonitis and small bowel ileus: a case of encapsulating peritoneal sclerosis secondary to Mycobacterium abscessus peritonitis. Eur J Case Rep Intern Med. 2022;9: 003173.
Ueda Y, Okamoto T, Sato Y, Hayashi A, Takahashi T, Kamada K, et al. Kidney transplantation after peritoneal dialysis-associated peritonitis and abdominal abscesses caused by Mycobacterium massiliense: lesson for the clinical nephrologist. J Nephrol. 2022;35:1907–10.
Simbli MA, Niaz FA, Al-Wakeel JS. Encapsulating peritoneal sclerosis in a peritoneal dialysis patient presenting with complicated Mycobacterium fortuitum peritonitis. Saudi J Kidney Dis Transpl. 2012;23:635–41.
Jiang SH, Roberts DM, Clayton PA, Jardine M. Non-tuberculous mycobacterial PD peritonitis in Australia. Int Urol Nephrol. 2013;45:1423–8.
Yoshimura R, Kawanishi M, Fujii S, Yamauchi A, Takase K, Yoshikane K, et al. Peritoneal dialysis-associated infection caused by Mycobacterium abscessus: a case report. BMC Nephrol. 2018;19:341.
Wallace RJ Jr, Swenson JM, Silcox VA, Bullen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985;152:500–14.
Merlin TL, Tzamaloukas AH. Mycobacterium chelonae peritonitis associated with continuous ambulatory peritoneal dialysis. Am J Clin Pathol. 1989;91:717–20.
Pulliam JP, Vernon DD, Alexander SR, Hartstein AI, Golper TA. Nontuberculous mycobacterial peritonitis associated with continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1983;2:610–4.
Song Y, Wu J, Yan H, Chen J. Peritoneal dialysis-associated nontuberculous mycobacterium peritonitis: a systematic review of reported cases. Nephrol Dial Transplant. 2012;27:1639–44.
Jheeta AS, Rangaiah J, Clark J, Makanjuola D, Somalanka S. Mycobacterium abscessus—an uncommon, but important cause of peritoneal dialysis-associated peritonitis—case report and literature review. BMC Nephrol. 2020;21:491.
Kunin M, Knecht A, Holtzman EJ. Mycobacterium chelonae peritonitis in peritoneal dialysis. Literature review. Eur J Clin Microbiol Infect Dis. 2014;33:1267–71.
Imam O, Al-Zubaidi K, Janahi M, Imram A, Leghrouz B, Dobson S, et al. Peritoneal dialysis-associated peritonitis caused by Mycobacterium abscessus in children—a case report. Open Forum Infect Dis. 2021;8:ofaa579.
Mooren VHJF, Bleeker MWP, van Ingen J, Hermans MHA, Wever PC. Disseminated Mycobacterium abscessus infection in a peritoneal dialysis patient. IDCases. 2017;9:6–7.
Jiang SH, Roberts DM, Dawson AH, Jardine M. Mycobacterium fortuitum as a cause of peritoneal dialysis-associated peritonitis: case report and review of the literature. BMC Nephrol. 2012;13:35.
Chan WW, Murray MC, Tang P, Romney MG. Mycobacterium heckeshornense peritonitis in a peritoneal dialysis patient: a case report and review of the literature. Clin Microbiol Infect. 2011;17:1262–4.
Jung JH, Ahn AH. Peritoneal dialysis catheter-related infection due to Mycobacterium abscessus confused with Rhodococcus. J Korean Med Sci. 2020;35: e44.
Haubrich K, Mammen C, Sekirov I, Mitchell H. Mycobacterium fortuitum peritoneal dialysis-related peritonitis in a child: a case report and review of the literature. J Assoc Med Microbiol Infect Dis Can. 2022;7:125–30.
Yokota S, Nishi K, Ishawa S, Uda K, Shoji K, Kamei K. Mycobacterium avium complex peritonitis in a pediatric patient on peritoneal dialysis: a case report. Medicine. 2021;100: e26321.
Sangwan J, Lathwal S, Kumar S, Juyal D. Mycobacterium fortuitum peritonitis in a patient on continuous ambulatory peritoneal dialysis (CAPD): a case report. J Clin Diagn Res. 2013;7:2950–1.
Hamade A, Pozdzik A, Denis O, Tooulou M, Keyzer C, Jacobs F. Mycobacterium fortuitum and polymicrobial peritoneal dialysis-related peritonitis: a case report and review of the literature. Case Rep Nephrol. 2014;2014: 323757.
Hayat A, Sakhrani B, Rubin M. Mycobacterium chelonae-related peritoneal dialysis peritonitis: a case report and its potential complications. Int Urol Nephrol. 2022;54:1769–71.
Inagaki K, Mizutani M, Nagahara Y, Asano M, Masamoto D, Sawada O, et al. Successful treatment of peritoneal dialysis-related peritonitis due to mycobacterium iranicum. Intern Med. 2016;55:1929–31.
Karakala N, Steed LL, Ullian ME. Peritonitis from Mycobacterium wolinskyi in a chronic peritoneal dialysis patient. Int Urol Nephrol. 2013;45:289–91.
Patil R, Patil T, Schenfeld L, Massoud S. Mycobacterium porcinum peritonitis in a patient on continuous ambulatory peritoneal dialysis. J Gen Intern Med. 2011;26:346–8.
Chan GCW, Mok MY, Hung DLL, Chan JFW, Kwan LPY, Ma MKM, et al. Mycobacterium chlorophenolicum: an uncommon cause of peritonitis in a peritoneal dialysis patient. Nephrology. 2017;22:498–9.
Choi HS, Bae E-H, Ma S-K, Kim S-W. Peritoneal dialysis-related peritonitis caused by Microbacterium paraoxydans. Jpn J Infect Dis. 2017;70:195–6.
Hamada S, Takata T, Kitaura T, Teraoka C, Aono A, Taniguchi S, et al. Peritoneal dialysis-associated peritonitis caused by Mycobacteroides massiliense: the first case and review of the literature. BMC Nephrol. 2021;22:90.
Nakano S, Yamamura-Miyazaki M, Michigami T, Yazawa K, Yanagihara I, Yamamoto K. A case of a preschool child with a successful kidney transplant following the long-term administration of antibiotics to treat peritoneal dialysis-related ESI/peritonitis by Mycobacterium abscessus. CEN Case Rep. 2022;11:408–11.
Fujikura H, Kasahara K, Ogawa Y, Hirai N, Yoshii S, Yoshihara S, et al. Mycobacterium wolinskyi peritonitis after peritoneal catheter embedment surgery. Intern Med. 2017;56:3097–101.
Ranganathan D, Fassett R, John GT. Mycobacterium fortuitum peritonitis in a patient receiving continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transpl. 2013;24:1003–4.
Siddiqi N, Sheikh I. Peritonitis caused by Mycobacterium abscesses in patients on continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transpl. 2012;23:321–4.
Lu J, Jiang Z, Wang L, Mou S, Yan H. Mycobacteria avium-related peritonitis in a patient undergoing peritoneal dialysis: case report and review of the literature. BMC Nephrol. 2021;22:345.
Lo MW, Mak S, Wong Y, Lo K, Chan S, Tong GMW, et al. Atypical mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Dial Int. 2013;33:267–72.
Chamarthi G, Modi D, Andreoni K, Shukia AM. Simultaneous catheter removal and reinsertion, is it acceptable in M. abscessus exit site infection? CEN Case Rep. 2021;10:483–9.
Miyashita E, Yoshida H, Mori D, Nakagawa N, Miyamura T, Ohta H, et al. Mycobacterium avium complex-associated peritonitis with CAPD after unrelated bone marrow transplantation. Pediatr Int. 2014;56:e96–8.
Jo A, Ishibashi Y, Hirohama D, Takara Y, Kume H, Fujita T. Early surgical intervention may prevent peritonitis in cases with Tenckhoff catheter infection by nontuberculous mycobacterium. Perit Dial Int. 2012;32:227–9.
Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–46.
Sassi M, Drancourt M. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics. 2014;15:359.
Lee MR, Sheng W-H, Hung C-C, Yu C-J, Li L-N, Hsue P-R, et al. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21:1638–46.
Acknowledgements
Not applicable.
Funding
The authors did not receive any funding for this work.
Author information
Authors and Affiliations
Contributions
T. Nagasaka, KU, R. Shirai, RM, ES, R. Sumura, and T. Nakayama collected and analyzed the clinical data. T. Nagasaka, KU, R. Shirai, RM, TM, EYH, EK, R. Sumura, T. Nakayama, SK, and KM were involved in the clinical care of the patient. T. Nagasaka and KU were involved in drafting and revision of the original manuscript. YI, NW, and HI supervised the manuscript preparation. All authors contributed to the preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable (case report).
Consent for publication
Informed consent was obtained from the patients whose cases are reported in this article for publication of their personal information that appears in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nagasaka, T., Uchiyama, K., Shirai, R. et al. Peritoneal dialysis-related peritonitis with encapsulated ascites due to Mycobacterium abscessus subsp. massilience and subsp. bolletii: a case series and literature review. Ren Replace Ther 9, 15 (2023). https://doi.org/10.1186/s41100-023-00469-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00469-0