Abstract
Background
Peritoneal dialysis (PD)-associated peritonitis caused by nontuberculous Mycobacterium is rare; however, the number of cases has increased over the past decades. Mycobacteroides massiliense is a subspecies of the Mycobacteroides abscessus complex. It has different clinical characteristics compared to the other subspecies of the complex. Previous case reports of PD-associated peritonitis caused by Mycobacteroides abscessus complex have not distinguished the subspecies in detail.
Case presentation
A 40-year-old man presented with an exit-site and tunnel infection refractory to antibiotic therapy. Peritonitis occurred after simultaneous catheter removal and reinsertion. The Mycobacteroides abscessus complex was detected in the culture of the dialysis effluent. Removal of the PD catheter combined with antibiotics, including macrolides, resulted in a good clinical course. Further analysis of multiplex PCR and the hsp65 gene sequence identified the bacterium as Mycobacteroides massiliense.
Conclusions
The Mycobacteroides abscessus complex is classified into three subspecies; Mycobacteroides abscessus, Mycobacteroides massiliense, and Mycobacteroides bolletii. These have different characteristics, particularly antibiotic susceptibility. Therefore, clear identification of the subspecies of the Mycobacteroides abscessus complex is necessary for definitive treatment.
Similar content being viewed by others
Background
Peritoneal dialysis (PD)-associated peritonitis is a major complication leading to PD failure. The most common organisms causing peritonitis are S. aureus, Enterococcus, Escherichia. coli, and Pseudomonas aeruginosa. Relatively fewer cases caused by nontuberculous mycobacterium (NTM) have been reported [1, 2]. A mycobacteroides abscessus complex is a group of NTM that can cause peritonitis related to poor prognosis than the other NTM [2]. Therefore, appropriate treatment is necessary for peritonitis caused by bacteria. The M. abscessus complex has three subspecies: Mycobacteroides abscessus, Mycobacteroides massiliense, and Mycobacteroides bolletii [3]. Since the three subspecies have different susceptibilities to antibiotics, an increasing number of investigations have emphasized the importance of their definite identification. In this report, we present the first case of M. massiliense peritonitis with a review of previous case reports describing peritonitis caused by M. abscessus complex.
Case presentation
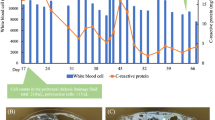
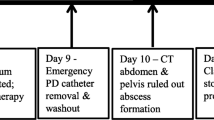
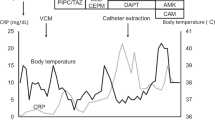
A 40-year-old male patient with PD was admitted to our hospital because of a persisting exit-site and tunnel infection of the PD catheter. He was started on PD due to IgA nephropathy 8 months before admission. He had no comorbid disorders and was not receiving corticosteroids. He visited the outpatient unit because of the pain at the exit-site of the PD catheter 1 month before admission. He was administered levofloxacin (250 mg once every other day) orally for 7 days, followed by oral cefpodoxime proxetil (100 mg once every other day) for 8 days and oral minocycline (100 mg twice daily) for 8 days, combined with topical nadifloxacin for 1 month. However, his symptoms worsened. On admission, his blood pressure was 130/90 mmHg, pulse was 79 beats/min and body temperature was 36.6 °C. The skin at the exit-site of the PD catheter and the subcutaneous cuff was red, painful, swollen, and purulent discharge from the exit-site was observed, indicating exit-site infection and catheter-tunnel infection. He did not have rebound tenderness. Laboratory data from the whole blood showed a white blood cell count of 4900/μL with 84% segmented neutrophils and a C-reactive protein level of 0.11 mg/dL. The dialysis effluent was clear, and the cell count of the dialysate effluent was 16/μL. Therefore, peritonitis was not suspected. An increased density around the PD catheter was observed on abdominal computed tomography (Fig. 1). Cultures of pus and dialysis effluent were negative. On the second day, the patient underwent simultaneous PD catheter removal and reinsertion. Gram staining of the removed catheter showed negative results. On day 6, abdominal pain and a fever of 38.3 °C appeared. The dialysis effluent became turbid, and the cell count of the dialysate increased to 379/μL with 40% neutrophils; thus, PD-associated peritonitis was strongly suspected. Empirical therapy with intravenous cefazolin (1 g once daily) and ceftazidime (1 g once daily) was initiated, although the antibiotics were switched to intravenous meropenem (0.5 g once daily) because of exacerbation of the abdominal pain and an increase in the cell count of the effluent to 13,272/μL. On day 10, Cutibacterium acnes was cultured from the subcutaneous cuff, deep cuff and infected tissue around the exit-site. In addition, acid-fast bacilli were detected in the same specimens. The culture from dialysis effluent collected on day 6 was positive for M. abscessus by the Bruker MALDI Biotyper (Bruker Daltonics, Billerica, MA) matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) system. On day 12, all three cultures, including the dialysis effluent collected on day 6, subcutaneous cuff and deep cuff removed on day 2, turned out to be positive for M. abscessus. Therefore, meropenem was changed to oral clarithromycin (200 mg twice daily), intravenous imipenem/cilastatin (250 mg once daily), and intravenous amikacin (100 mg once daily). After the removal of the PD catheter on day 14, his symptoms rapidly improved. Renal replacement therapy was switched to hemodialysis (HD), and the dosage of amikacin was changed to 300 mg after each HD session. On day 55, imipenem/cilastatin was changed to oral moxifloxacin (400 mg once daily) due to mild hepatic dysfunction; aspartate aminotransferase, 63 IU/L; alanine aminotransferase, 20 IU/L; and lactate dehydrogenase, 300 IU/L. The patient was discharged from the hospital on day 59. Combined therapy with clarithromycin, amikacin, and moxifloxacin was continued until day 114, and moxifloxacin was discontinued according to the susceptibility of the bacterium (Table 1) [4]. We further analyzed the bacterium by multiplex PCR and the hsp65 gene sequence and identified it as M. massiliense [5]. Clarithromycin and amikacin were continued 17 weeks. The patient does not plan to re-start PD concerning for recurrence of peritonitis and encapsulation peritoneal sclerosis, and is receiving maintenance hemodialysis without recurrence of peritonitis (Fig. 2).
Discussion and conclusions
Herein, we report the first case of PD-associated peritonitis caused by M. massiliense, a subspecies of the M. abscessus complex. The susceptibility to antibiotics and pathogenicity varies in each individual. Therefore, it is necessary to identify these subspecies and pay attention to their susceptibility especially macrolides.
The M. abscessus complex belongs to the Runyon classification group IV, which rapidly grows within 7 days [6]. The M. abscessus complex has been isolated from surface water, tap water, and soil [7]. The major clinical manifestations of M. abscessus complex are skin and soft-tissue infections and respiratory infections, and only a few cases of peritonitis have been documented [8]. Rapidly growing NTM comprises approximately 3% of the causative pathogens of PD-associated infections [9]. The M. abscessus complex accounts for 8.8% of NTM-caused peritonitis and is associated with poor outcomes [2]. Considering the number of NTM peritonitis cases is increasing [10, 11], it is important to appropriately manage peritonitis caused by M. abscessus complex.
The M. abscessus complex is resistant to several antibiotics. In addition to surgical removal of the infected foci, it is recommended to treat patients with multiple agents including macrolides [8]. Yoshimura et al. reported that 82.1% of peritonitis or exit-site infections require catheter removal [12]. Furthermore, a case of exit-site infection requiring catheter removal after the termination of antibiotic therapy has been reported [13]. Considering that almost all the peritonitis cases failed to continue PD and that some cases resulted in patient death [9, 12], it seems necessary to remove the PD catheter when the M. abscessus complex is isolated.
Recently, M. abscessus complex has been classified into three subspecies; M. abscessus, M. massiliense, and M. bolletii [3, 14]. These subspecies cannot be distinguished by MALDI-TOF MS, which is usually used for clinical isolates; therefore, previous reports of M. abscessus complex are a mixture of three subspecies. Multiplex PCR targeting several primer sets enabled clear identification of the M. abscessus complex [5]. Clinical behavior, particularly susceptibility to antibiotics, differs among each subspecies [15]. M. massiliense responded well to macrolides, whereas M. abscessus was resistant [16]. Similarly, M. abscessus lead to poor outcomes due to resistance to clarithromycin, whereas M. massiliense was susceptible [15]. M. abscessus possesses a gene responsible for inducible resistance to macrolides [17]; thus, treatment with macrolides must be carefully determined when treating M. abscessus complex. Furthermore, M. massiliense has been reported to cause outbreaks [18]. Therefore, a clear identification of M. massiliense is necessary.
In the present case, the patient showed exit-site and trans-catheter infections without any signs of peritonitis. Peritonitis appeared after simultaneous removal and reinsertion of the catheter. The patient was not immunocompromised. The long duration of oral antibiotics before admission may have influenced the emergence of NTM. As the culture of the pus and dialysate effluent was negative on admission, it is important to suspect acid-fast bacilli when the culture-negative infection persists. In the present case, M. abscessus was initially recovered from the culture; however, it was later identified as M. massiliense. To the best of our knowledge, this is the first case report of PD-associated peritonitis caused by M. massiliense. Further consideration of this organism would lead to better treatment of peritonitis in the future.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- CRP:
-
C-reactive protein
- HD:
-
Hemodialysis
- MALDI-TOF MS:
-
Matrix-assisted laser desorption ionization-time of flight mass spectrometry
- NTM:
-
Nontuberculous mycobacterium
- PD:
-
Peritoneal dialysis
References
Prasad KN, Singh K, Rizwan A, Mishra P, Tiwari D, Prasad N, et al. Microbiology and outcomes of peritonitis in northern India. Perit Dial Int. 2014;34:188–94.
Song Y, Wu J, Yan H, Chen J. Peritoneal dialysis-associated nontuberculous mycobacterium peritonitis: a systematic review of reported cases. Nephrol Dial Transplant. 2012;27:1639–44.
Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21:1638–46.
Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, et al. M24-A2: susceptibility testing of mycobacteria, Nocardiae, and other aerobic Actinomycetes; approved standard—second edition. Wayne: NCCLS; 2011. p. 31.
Nakanaga K, Sekizuka T, Fukano H, Sakakibara Y, Takeuchi F, Wada S, et al. Discrimination of mycobacterium abscessus subsp. Massiliense from mycobacterium abscessus subsp. Abscessus in clinical isolates by multiplex PCR. J Clin Microbiol. 2014;52:251–9.
Kusunoki S, Ezaki T, Tamesada M, Hatanaka Y, Asano K, Hashimoto Y, et al. Application of colorimetric microdilution plate hybridization for rapid genetic identification of 22 Mycobacterium species. J Clin Microbiol. 1991;29:1596–603.
Brown-Elliott BA, Wallace RJ. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15:716–46.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416.
Renaud CJ, Subramanian S, Tambyah PA, Lee EJC. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in Asians: a case series and literature review. Nephrology. 2011;16:174–9.
Szeto CC, Leung CB, Chow KM, Kwan BCH, Law MC, Wang AYM, et al. Change in bacterial aetiology of peritoneal dialysis-related peritonitis over 10 years: experience from a Centre in South-Asia. Clin Microbiol Infect. 2005;11:837–9.
Kunin M, Knecht A, Holtzman EJ. Mycobacterium chelonae peritonitis in peritoneal dialysis. Literature review. Eur J Clin Microbiol Infect Dis. 2014;33:1267–71.
Yoshimura R, Kawanishi M, Fujii S, Yamauchi A, Takase K, Yoshikane K, et al. Peritoneal dialysis-associated infection caused by Mycobacterium abscessus: a case report. BMC Nephrol. 2018;19:341.
Chamarthi G, Kamboj M, Archibald L, Shukla A. Mycobacterium abscessus exit-site infection in peritoneal dialysis patients: should we ever aim to salvage the catheter? CEN Case Rep. 2020. https://doi.org/10.1007/s13730-020-00506-5.
Adékambi T, Berger P, Raoult D, Drancourt M. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol. 2006;56:133–43.
Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–10.
Morimoto K, Nakagawa T, Asami T, Morino E, Fujiwara H, Hase I, et al. Clinico-microbiological analysis of 121 patients with pulmonary Mycobacteroides abscessus complex disease in Japan – an NTM-JRC study with RIT. Respir Med. 2018;145:14–20.
Nash KA, Brown-Elliott AB, Wallace RJA. Novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53:1367–76.
Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet. 2013;381:1551–60.
Acknowledgments
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
SH drafted the manuscript. TT and TK critically revised and edited the manuscript. CT, ST, YM, TK, and AA treated the patient. HI, HC and SM substantially revised the manuscript and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was waived according to regulations of the Ethical Committee of Tottori University Hospital. Written informed consent was obtained from the patient to publish this case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hamada, S., Takata, T., Kitaura, T. et al. Peritoneal dialysis-associated peritonitis caused by Mycobacteroides massiliense: the first case and review of the literature. BMC Nephrol 22, 90 (2021). https://doi.org/10.1186/s12882-021-02297-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-021-02297-y