Abstract
Background
Peritonitis is a serious and potentially fatal complication of peritoneal dialysis. We report a case of fatal peritonitis caused by Mycobacterium mageritense that was detected for the first time in peritonitis.
Case presentation
A male patient in his 60 s undergoing peritoneal dialysis was admitted for catheter diversion with exit-site renewal. The patient had a refractory exit-site infection. Mycobacterial culture was not performed at the exit site prior to admission. After the surgery, the patient developed a fever, and a cloudy effluent was observed. Various antibiotics, including anti-tuberculosis drugs, were administered; however, his symptoms did not improve. The catheter was removed on the thirty-seventh day of admission. Bacteria positive for Ziehl–Neelsen staining were found in the peritoneal sample collected during the surgery. Since nontuberculous mycobacteria were considered the cause of peritonitis, the patient was administered imipenem/cilastatin, amikacin, and clarithromycin. However, he died of septic shock on the fifty-first day after admission. Mycobacterium mageritense was detected in the ascites culture after death.
Conclusion
This is, to our knowledge, the first report of peritonitis caused by Mycobacterium mageritense. In patients undergoing peritoneal dialysis, when a refractory exit-site infection is observed, mycobacterial culture is necessary to prevent the development of peritonitis.
Similar content being viewed by others
Background
Peritoneal dialysis (PD)-associated peritonitis is a serious complication of PD that causes withdrawal from dialysis and even death. Nontuberculous mycobacteria (NTM) account for 3% of all culture-positive exit-site infections and cases of peritonitis [1], and previous studies have reported that the mortality rate of NTM peritonitis was 14–30% [2, 3]. Treatment regimens for NTM peritonitis are not completely understood [4]. In this report, we describe a fatal case of PD-associated peritonitis caused by Mycobacterium mageritense (M. mageritense), which was detected in peritonitis for the first time.
Case presentation
A male patient in his 60 s undergoing PD for 7 years was admitted to our hospital for a catheter diversion procedure with exit-site renewal. The patient had an end-stage kidney disease associated with primary IgA nephropathy. The patient had no history of diabetes mellitus and had not received immunosuppressants. He had granulation at the exit site one year prior to admission. Methicillin-susceptible Staphylococcus aureus was detected in the culture of the exit-site sample. The patient was treated with topical gentamicin, oral first-generation cephems, and quinolones. However, the granulation increased, and methicillin-resistant Staphylococcus aureus was detected at the site 6 months later. Moreover, the external cuff had moved closer to the exit site. No mycobacterial culture test was performed at the exit site before admission. The patient had received vancomycin 5 days prior to admission. On admission, the patient’s body temperature was 38 °C. The white blood cell count and C-reactive protein (CRP) levels were 3100/μL and 0.3 mg/dL, respectively. Laboratory data on admission are summarized in Table 1.
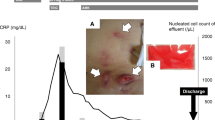
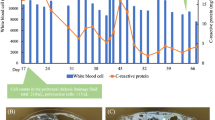
The patient’s fever was believed to be due to viral infection, and on the second day of admission, the catheter diversion surgery was performed as planned (Fig. 1). Culture tests of blood collected on admission were negative. Methicillin-resistant coagulase-negative Staphylococci were detected in the pus cultures from the exit site and the resected catheter during catheter diversion surgery. On the tenth day of admission, the patient received 1 g of additional vancomycin. The next day, his body temperature reached 39 °C, and a rash appeared, which was considered a side effect of vancomycin. However, since the patient’s CRP level had increased to 3.7 mg/dL and bacterial infection could not be ruled out, 4.5 g tazobactam/piperacillin twice daily was initiated on the thirteenth day of admission. On the twenty-second day of admission, the patient’s effluent was observed to be cloudy, without symptoms of peritoneal irritation. The nucleated cell count of the effluent was 200 cells/μL (62% neutrophils and 7% lymphocytes). The patient’s antibiotics were changed to cefazolin and cefepime, both at a dose of 1 g once daily, which were intraperitoneally administered. Bacterial culture, mycobacterial culture, and tuberculosis PCR tests of the effluent were all negative. On the twenty-eighth day after admission, the turbidity of the effluent persisted, and the nucleated cell count of the effluent increased to 2300 cells/μL (57% neutrophils and 12% lymphocytes). Cefazolin and cefepime were replaced with 0.25 g doripenem twice daily (a carbapenem antibiotic) and 0.3 g (6 mg/kg) daptomycin every 48 h. However, peritoneal irritation symptoms appeared on the thirty-first day after admission. Computed tomography revealed an increase in the density of the mesenteric fat tissue and thickening of the visceral peritoneum, indicating peritonitis (Fig. 2). We believed that the patient had developed tuberculous peritonitis because the bacterial culture of the effluent was negative, and antibiotics had no effect. Anti-tuberculosis drugs (0.3 g isoniazid daily, 0.45 g rifampicin daily, 1 g ethambutol every other day, and 1.5 g pyrazinamide every other day) were administered on the thirty-second day after admission. Doripenem and daptomycin were continued at the same volume. However, the patient’s abdominal symptoms worsened, and the CRP level increased to 20 mg/dL. On the thirty-seventh day after admission, the PD catheter was removed laparoscopically (Fig. 1). Laparoscopy revealed fibrotic adhesions in the peritoneum, which indicated peritonitis. Bacteria positive for Ziehl–Neelsen staining were found in the peritoneal sample collected during the surgery. As the anti-tuberculosis drugs were ineffective, we believed that NTM caused the peritonitis. On the forty-sixth day after admission, doripenem and daptomycin were replaced with 0.25 g imipenem/cilastatin twice daily, 0.3 g amikacin on dialysis days only, and 0.5 g clarithromycin daily, which were believed to be effective against NTM. However, on the fifty-first day of admission, the patient died of septic shock. The postmortem ascites culture collected at the time of catheter removal surgery was positive for M. mageritense. The susceptibility results are shown in Table 2.
Discussion
We encountered a fatal case of PD-associated peritonitis caused by M. mageritense, a type of NTM. The vast majority of NTM peritonitis cases are caused by Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum [1, 2, 11,12,13]; however, in this case, the peritonitis was caused by M. mageritense, which has not been reported until now.
Diagnosing NTM peritonitis is difficult because the associated symptoms are fever, abdominal pain, and cloudy effluent, which are indistinguishable from those of bacterial and tuberculous peritonitis [2, 13]. In this case, anti-NTM drugs were initiated 25 days after the appearance of the cloudy effluent; according to previous reports, this duration is not long enough to affect the efficacy of the drugs [2, 12]. Here, we used imipenem/cilastatin, amikacin, and clarithromycin as anti-NTM drugs, to which the causative bacterium, M. mageritense, is susceptible. However, the patient still died.
ISPD Guideline 2022 recommends prompt catheter removal in cases of refractory peritonitis [4]. The guideline also notes that NTM peritonitis requires both effective antibiotics and catheter removal, although no specific antibiotic regimen has been presented. In this case, the patient could not be saved despite catheter removal and the administration of effective antibiotics. The mortality rate of NTM peritonitis has been reported to be as high as 14–30% [2]. Therefore, we believe that it is important to prevent the development of NTM peritonitis to avoid fatal outcomes. NTM peritonitis is caused mainly by the progression of exit-site or tunnel infections [14]. Mycobacterial cultures at the exit site, early surgical intervention, and antibiotic administration prevent the development of NTM peritonitis [14, 15]. Mycobacterial cultures should be performed to check for an NTM infection if the patient has a history of refractory exit-site infections. Early identification of the species and susceptibility allows the selection of an appropriate antibiotic. In this case, early diagnosis of the NTM infection would have allowed for earlier administration of antibiotics and possibly saved the patient.
The incidence of infections caused by M. mageritense, a nontuberculous mycobacterium first discovered in 1997, is growing rapidly [16]. Similar to this case, M. mageritense primarily infects the skin and soft tissues [5, 8, 9, 17,18,19]. In a previous report that investigated M. mageritense antibiotic susceptibility, all 23 specimens were susceptible to imipenem, amikacin, linezolid, ciprofloxacin, and trimethoprim-sulfamethoxazole [20]. However, our review of papers revealed that the susceptibility of M. mageritense was inconsistent with those findings (Table 2). In addition, most of the cases, such as this case, required some type of surgical intervention.
As mentioned above, in such cases, it is important to prevent the development of peritonitis to avoid fatal outcomes. Furthermore, since treatment regimens for peritonitis and catheter-related infection due to NTM have not been established [4, 21], it is necessary to accumulate many cases and establish a standard treatment regimen for infections caused by NTM.
Conclusion
Here, we report, to the best of our knowledge, the first case of peritonitis caused by M. mageritense. Since peritonitis can be fatal, physicians should perform mycobacterial culture in cases of refractory exit-site infections. Furthermore, if NTM infection is detected, appropriate treatment, including the use of antibiotics with high bacterial sensitivity, should be administered to prevent the development of peritonitis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- NTM:
-
Nontuberculous mycobacteria
- CRP:
-
C-reactive protein
- M. mageritense :
-
Mycobacterium mageritense
References
Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in Asians: a case series and literature review. Nephrology (Carlton). 2011;16:174–9.
Song Y, Wu J, Yan H, Chen J. Peritoneal dialysis-associated nontuberculous mycobacterium peritonitis: a systematic review of reported cases. Nephrol Dial Transplant. 2012;27:1639–44.
Fung WW, Chow KM, Li PK, Szeto CC. Clinical course of peritoneal dialysis-related peritonitis due to non-tuberculosis mycobacterium—a single centre experience spanning 20 years. Perit Dial Int. 2022;42:204–11.
Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–53.
Okabe T, Sasahara T, Suzuki J, Onishi T, Komura M, Hagiwara S, et al. Mycobacterium mageritense parotitis in an immunocompetent adult. Indian J Microbiol. 2018;58:28–32.
Caravedo Martinez MA, Blanton LS. Mycobacterium mageritense prosthetic joint infection. Case Rep Infect Dis. 2020;2020:8845430.
Niitsu T, Kuge T, Fukushima K, Matsumoto Y, Abe Y, Okamoto M, et al. Pleural effusion caused by Mycolicibacterium mageritense in an immunocompetent host: a case report. Front Med (Lausanne). 2021;8:797171.
Yamaguchi Y, Kitano T, Onishi T, Takeyama M, Suzuki Y, Nogami K. A case of pediatric subcutaneous abscess caused by Mycobacterium mageritense Infection. Jpn J Infect Dis. 2021;74:377–80.
Koyama T, Funakoshi Y, Imamura Y, Nishimura S, Fujishima Y, Toyoda M, et al. Device-related Mycobacterium mageritense infection in a patient treated with nivolumab for metastatic breast cancer. Intern Med. 2021;60:3485–8.
Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, et al. CLSI Standards: Guidelines for Health Care Excellence (2011) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes [Internet]. 2nd edn. Clinical and Laboratory Standards Institute, Wayne. Report No.: M24-A2 PMID: 31339680
Lo MW, Mak SK, Wong YY, Lo KC, Chan SF, Tong GM, et al. Atypical mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Dial Int. 2013;33:267–72.
Ono E, Uchino E, Mori KP, Yokoi H, Toda N, Koga K, et al. Peritonitis due to Mycobacterium abscessus in peritoneal dialysis patients: case presentation and mini-review. Ren Replace Ther. 2018;4:1–10.
Bnaya A, Wiener-Well Y, Soetendorp H, Einbinder Y, Paitan Y, Kunin M, et al. Nontuberculous mycobacteria infections of peritoneal dialysis patients: a multicenter study. Perit Dial Int. 2021;41:284–91.
Jo A, Ishibashi Y, Hirohama D, Takara Y, Kume H, Fujita T. Early surgical intervention may prevent peritonitis in cases with Tenckhoff catheter infection by nontuberculous mycobacterium. Perit Dial Int. 2012;32:227–9.
Chamarthi G, Kamboj M, Archibald LK, Shukla AM. Mycobacterium abscessus exit-site infection in peritoneal dialysis patients: should we ever aim to salvage the catheter? CEN Case Rep. 2021;10:12–6.
Domenech P, Jimenez MS, Menendez MC, Bull TJ, Samper S, Manrique A, et al. Mycobacterium mageritense sp. nov. Int J Syst Bacteriol. 1997;47:535–40.
Wallace RJ Jr, Brown-Elliott BA, Hall L, Roberts G, Wilson RW, Mann LB, et al. Clinical and laboratory features of Mycobacterium mageritense. J Clin Microbiol. 2002;40:2930–5.
Gira AK, Reisenauer AH, Hammock L, Nadiminti U, Macy JT, Reeves A, et al. Furunculosis due to Mycobacterium mageritense associated with footbaths at a nail salon. J Clin Microbiol. 2004;42:1813–7.
Appelgren P, Farnebo F, Dotevall L, Studahl M, Jonsson B, Petrini B. Late-onset posttraumatic skin and soft-tissue infections caused by rapid-growing mycobacteria in tsunami survivors. Clin Infect Dis. 2008;47:e11–6.
Gordon Huth R, Brown-Elliott BA, Wallace RJ Jr. Mycobacterium mageritense pulmonary disease in patient with compromised immune system. Emerg Infect Dis. 2011;17:556–8.
Szeto CC, Li PK, Johnson DW, Bernardini J, Dong J, Figueiredo AE, et al. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37:141–54.
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
NH was primarily involved in patient treatment and wrote the manuscript. NK, SM, TN, HM, KO, RU, SB, RI, YU, and TH contributed to patient treatment and related discussions and reviewed the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was written in compliance with the Declaration of Helsinki.
Consent for publication
Consent for the publication of this case report was obtained from a family member of the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hashimoto, N., Kani, N., Makino, S. et al. Fatal peritoneal dialysis-associated peritonitis caused by Mycobacterium mageritense: a case report with review. Ren Replace Ther 9, 5 (2023). https://doi.org/10.1186/s41100-023-00457-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00457-4