Abstract
Background
Lactate is a well-known marker to estimate prognosis after cardiac surgery and critically ill patients. The liver and kidney have a major role in lactate metabolism; however, there was less characterized about the change of lactate and threshold to predict in-hospital mortality in dialysis-dependent patients undertaking cardiac surgery. We conducted this retrospective observational study to characterize when and how lactate values after cardiac surgery affected in-hospital mortality.
Methods
This two-center retrospective study included dialysis-dependent patients who underwent cardiac surgery with a cardiopulmonary bypass from January 2014 to December 2018. Lactate values were collected at three points: at ICU admission (T1), the maximum level of lactate within 24 h postoperatively (T2), and 24 h after ICU admission (T3). We determined hyperlactatemia as more than 2 mmol/L following previous studies.
Results
We enrolled 122 dialysis-dependent patients. The mean age was 73 ± 8 years and hyperlactatemia was observed in 100 patients (81.9%). In-hospital mortality was 11.4%. Univariate analysis and area under curve in ROC suggested that T2 lactate was the most significantly associated with in-hospital mortality (AUC = 0.845). Multivariate logistic analysis showed a significant association between in-hospital mortality when patients showed early peak lactate levels of > 4.5 mmol/L after ICU admission (adjusted OR 8.35; 95% CI: 1.44–57.13).
Conclusions
In dialysis-dependent patients after cardiac surgery, the early-onset of a maximum arterial lactate concentration of > 4.5 mmol/L was significantly associated with in-hospital mortality.
Similar content being viewed by others
Introduction
The number of dialysis-dependent patients is increasing in Japan due to the increased longevity and prevalence of diabetes mellitus. In 2016, it reached > 300,000 patients with chronic dialysis, and > 39,000 new patients were registered yearly [1]. Along with aging, dialysis-dependent patients require cardiac surgery because calcification and continuous inflammation lead to valve stenosis and coronary artery disease, respectively [2]. Of 13,887 coronary artery bypass grafts (CABGs) in 2016 in Japan, 1608 (12%) were dialysis-dependent patients [3], and the number continues to rise. However, dialysis-dependent patients have higher mortality after cardiac surgery than non-dialysis patients [4,5,6,7,8]. The European system for cardiac operative risk evaluation II included dialysis as a risk factor for mortality [9]. They are more vulnerable to infection, pneumonia, abdominal ischemia, and infraction than non-dialysis patients [5, 7].
The kidney is the second most important organ after the liver for metabolizing and removing lactate from the circulation [10, 11]. It converts blood lactate to glucose in the Cori cycle and gluconeogenesis, and transformed glucose is utilized in tissue again [12]. If patients have chronic kidney disease, more hyperlactatemia and metabolic acidosis is anticipated. In previous studies, 20–80% of patients presented with hyperlactatemia after cardiac surgery, and increased lactate levels were associated with high mortality and long stays in the intensive care unit [13,14,15]. In those studies, the threshold of hyperlactatemia for in-hospital mortality was 3.0-4.4 mmol/L. A recent study showed that temporary hyperlactatemia less than 2.0 mmol/L within 24 h after cardiac surgery had an adverse impact on prognosis [16]; however, dialysis-dependent patients were few or excluded in these studies. We speculated that dialysis-dependent patients have higher blood lactate level due to lack of kidney function, and this high lactate level affects the mortality.
This two-center, retrospective, observational study including dialysis-dependent patients after cardiac surgery had two aims: to explore how the changes in arterial lactate levels are associated with in-hospital mortality and to find a threshold of lactate levels that predicts in-hospital mortality. Identifying the lactate trend and threshold help us deciding treatment strategies.
Methods
Setting and ethical approval
This two-center, retrospective, observational study was conducted at two middle-sized private hospitals (hospitals A and B). It was reviewed and approved by the ethics committee of New Tokyo Hospital (Matsudo, Chiba, Japan, approval ID: 0175) and that of the Saitama Red Cross Hospital (Omiya, Saitama, Japan, approval ID: 18- AO). This study was reported in accordance with the strengthening the reporting of observational studies in epidemiology statement [17].
Patients
We enrolled dialysis-dependent patients who underwent elective open cardiac surgery from January 2014 to December 2018. The inclusion criteria were (1) age > 20 years, (2) chronically dependent on dialysis prior to cardiac surgery, (3) undergoing elective cardiac surgery with cardiopulmonary bypass (CPB). We excluded patients who required mechanical circulatory support, preoperative inotropes, and emergency surgery. Neither hospital performed cardiac transplantation or ventricular assisted device implantation. When patients underwent more than one cardiac surgery during the study period, we collected the data concerning the first operation.
Intraoperative anesthesia and surgery
Anesthesia was administered according to the protocol of each hospital. Propofol or midazolam, and fentanyl were used for anesthesia induction; sevoflurane, fentanyl, and remifentanil were used for anesthesia maintenance. Rocuronium or vecuronium was used to achieve neuromuscular blockade. For all patients, arterial blood pressure was monitored using a radial or brachial artery catheter and central venous pressure was monitored using a central venous catheter. Transesophageal echocardiography was used to monitor the procedure and hemodynamic status. After heparinization (300 unit/kg), CPB was maintained under an activated coagulation time of > 400 s and at a cardiac index of 2.4-2.6 L/min/m. Intermittent cold cardioplegia was used for all patients. Hospital A used blood cardioplegia and hospital B primarily used crystalloid cardioplegia. Hemodialysis or extracorporeal ultrafiltration was combined with CPB to remove excess fluid. Mild systemic hypothermia (32–34 °C) was most often used; however, deep systemic hypothermia (25 °C in hospital A and 18 °C in hospital B) was used for circulatory arrest. The dose of inotropes and vasopressors to maintain hemodynamic stability depended on the attending anesthesiologist. Adrenaline, dopamine, dobutamine, noradrenaline, phenylephrine, and vasopressin were mainly used. All patients were intubated and admitted to the ICU along with mechanical ventilation support. After ICU admission, continuous hemodiafiltration (CHDF) or intermittent hemodiafiltration was performed depending on the patient’s hemodynamic and electrolyte status. We started intermittent hemodiafiltration in the morning on the day after the operation, whereas CHDF was implemented several hours after the operation, and the initiation time and duration of CHDF depended on each patient.
Data collection
The main outcome of this study was in-hospital mortality of any causes. And blood lactate was measured from arterial blood gas samples (RAPIDLab 1200 Systems, Siemens Healthcare, Germany) after ICU admission. Blood gas samples were evaluated at least every 4 h until the patients were extubated or their hemodynamic status reached stability. In hospital A, the unit of arterial lactate measurement was mg/dl, thereby we divided the data by 9 to align mmol/L used in hospital B. We collected lactate measurements at three-time points: at ICU admission (T1), the maximum level of lactate within 24 h postoperatively (T2), and 24 h after ICU admission (T3). The time of T2 was also recorded.
The following data were collected as covariates for all patients from electronic medical records: age; sex; body mass index; preoperative complications including diabetes mellitus (DM), atrial fibrillation (Afib), cerebral ischemia (CI), coronary artery disease, and arteriosclerosis obliterans (ASO); year on dialysis; preoperative ejection fraction on transthoracic echocardiography; the cause of renal failure including DM, glomerulonephritis, nephrosclerosis; type of procedure including valve surgery, CABG, aortic surgery, complicated surgery with > 2 procedures; operating time; CPB time; aortic cross-clamp time; and use of adrenaline at ICU admission.
Statistical analysis
Categorical and dichotomous variables were summarized as frequencies and percentages, respectively, and continuous variables as mean and standard deviation or median and interquartile range (IQR). To determine the most predictable arterial lactate levels for in-hospital mortality, we plotted receiver operating characteristic (ROC) curves and obtained the area under the curve (AUC). After deciding the most predictable arterial lactate levels from the AUC, univariate and multivariate logistic regression model with a stepwise variable selection method (inclusion and exclusion criteria: 0.2, respectively) were used for the analyses. We used log-transformed data of each lactate value because they did not follow a normal distribution. For the multivariate analysis, variables shown in Table 2 in addition to age and sex were used as covariates. We analyzed the association between arterial lactate and in-hospital mortality following 3 steps. First of all, we calculated the odds ratio in a multivariate logistic regression analysis using the continuous variable of lactate to explore whether the lactate is independently associated with in-hospital mortality. Secondly, we conducted additional multivariate logistic regression analyses to determine a threshold, each with lactate at different binary cutoff as a target value for intervention. Thirdly, we added the T2 time (< 12 h or > 12 h after ICU admission) on the second multivariate logistic regression analysis with the binary cutoff lactate level showing the highest odds ratio. All statistical analyses were performed using R Statistics version 3.3.0 (Institute for Statistics and Mathematics, Wirtschaftsuniversität Wien). A two-tailed p value < 0.05 was considered statistically significant.
Results
Patient characteristics
In hospital A, 120 dialysis-dependent patients underwent cardiac surgery during the study period. We excluded 7 patients who required emergency surgery and 10 patients because they did not require CPB for off-pump CABG. In hospital B, 36 dialysis-dependent patients were initially included and 17 patients were subsequently excluded (4 for emergency surgery and 13 for off-pump CABG). Therefore, we enrolled a total of 122 patients in this study. The patient characteristics are presented in Table 1. The patients had a mean age of 73 ± 8 years and a median year on dialysis of 9 (IQR 4–14) years. DM (35%) and nephrosclerosis (20%) were the primary reasons for dialysis.
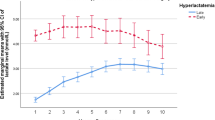
In-hospital mortality and lactate levels, the in-hospital mortality was 11% (14 patients). Among those patients, the median hospital stay was 46 (IQR 21–78) days, and six out of fourteen patients (43%) died before the 30th postoperative day. Three patients with intestinal ischemia and one with cerebral ischemia were also included. Of all enrolled patients, 100 patients (82%) presented with hyperlactatemia (> 2 mmol/L) within 24 h postoperatively. The median arterial lactate levels at each time point were 1.57 mmol/L at T1, 3.12 mmol/L at T2, and 1.67 mmol/L at T3 (Table 2). The median time of T2 (the maximum lactate) was 8 h after ICU admission (Fig. 1).
In the ROC analysis, T2 lactate levels showed the largest AUC (0.848, p < 0.001) (Fig. 2); therefore, we used the T2 lactate value in the logistic regression analysis. The univariate analysis of preoperative and intraoperative data revealed that age, preoperative history of CI and ASO, operating time, CPB time, aortic cross-clamp time, adrenaline use, and arterial lactate levels were associated with in-hospital mortality (Table 2). In multivariate logistic regression analyses, the T2 arterial lactate level was significantly associated with in-hospital mortality [odds ratio (OR), 6.01; 95% confidence interval (95% CI), 1.47–33.0] (Table 3). Table 4 displays additional multivariate logistic regression models at different binary thresholds. There appears to be a threshold effect at T2 lactate level of > 4.5 mmol/L. We also examined the association between in-hospital mortality and the combination of the T2 lactate (< 4.5 mmol/L or > 4.5 mmol/L) and the T2 time (< 12 h or > 12 h after ICU admission). The result indicated a significant association between in-hospital mortality when patients showed early peak lactate levels of > 4.5 mmol/L after ICU admission (adjusted OR 8.35; 95% CI: 1.44–57.13).
Discussion
In-hospital mortality and lactate levels
This study was conducted to elucidate the changes and the threshold in arterial lactate levels associated with in-hospital mortality in dialysis-dependent patients. The main findings of our study indicated frequent hyperlactatemia in those patients and it was associated with in-hospital mortality when the maximum value was > 4.5 mmol/L within 12 h after cardiac surgery.
Mechanisms of hyperlactatemia in dialysis patients
In general, lactate is a well-known marker that affects the prognosis after cardiac surgery. Although tissue hypoxia has been considered as the main causality of hyperlactatemia [18], accelerated glycolysis to create lactate to transform glucose also contributes to hyperlactatemia [4]. These two mechanisms of hyperlactatemia mean if a tissue needs more energy, a tissue itself and the whole metabolic system boost blood lactate level. While patients who died within 30th postoperative day had the highest lactate levels, we notice that late in-hospital deaths also had significantly higher lactate levels than survivors. This result indicates that hyperlactatemia within 24 h after cardiac surgery explains the comprehensive ability to manage tissue damage by hemodynamic change. Aside from these mechanisms, several factors should be considered for dialysis-dependent patients. Firstly, renal dialysis improves acidosis and controls hyperlactatemia in experimental situations [19], but its effect was controversial. In this study, although we used dialysis for all patients during CPB and after ICU admission, the blood lactate levels remained high in > 80% of patients. This suggests that hemodialysis was pragmatically inefficient in controlling overproduced lactate after cardiac surgery. Our result shows that CHDF after surgery was associated with higher mortality than intermittent hemodiafiltration because CHDF was prone to be chosen in patients with unstable hemodynamics. Efficacy of lactate removal between continuous or intermittent hemodiafiltration should be examined in future studies. Secondly, longer years on dialysis renders the arteries stiffer and leads to comorbidities such as peripheral arterial disease and DM [8], which may cause hypoxia-induced hyperlactatemia. This study did not reveal any association between peripheral arterial diseases such as ASO or DM, and in-hospital mortality. Although DM was a risk factor of perioperative mortality in non-dialysis patients, further investigation with a larger sample size is necessary to elucidate the causes of hyperlactatemia in dialysis-dependent patients. Lastly, dialysis-dependent patients are likely to have malnutrition because of strictly controlled diets [20]. Dialysis can induce vitamin deficiency, and the lack of vitamin B1 also induces hyperlactatemia [21]. Accelerated glycolysis in the absence of vitamin B1 causes lactate accumulation because pyruvate dehydrogenase cannot function. Considering those characteristics of dialysis-dependent patients, we should manage hyperlactatemia by diversified strategies. Recently, metabolic management [22] and microcirculation improvement [16] are examined for the effectiveness. Combination of thiamine, vitamin C, and corticosteroids was reported to improve lactate clearance and 28-day mortality in critically ill patients [22,23,24]. Luger et al. [25] showed patients without dialysis maintained their vitamin B1 level after cardiac surgery; however, there is no investigation in dialysis-dependent patients. Since the result of randomized clinical trial targeting thiamine intervention is controversial [26], further study is warranted to investigate the association between serum lactate levels and these interventions in dialysis-dependent patients.
Strengths and limitations
This study showed that an arterial lactate level of 4.5 mmol/L was the threshold value above which in-hospital mortality increased linearly in dialysis-dependent patients. This is the first study that elucidates the dynamics of lactate levels in dialysis-dependent patients after cardiac surgery.
Our results also suggest that early-onset hyperlactatemia is associated with worse outcomes than late-onset hyperlactatemia and previous studies had controversial results [27, 28]. Early-onset hyperlactatemia was mainly associated with impairment of tissue oxygen during CPB [29], and late-onset was associated with mainly inotrope usage. Even though a cardiac index was maintained within 2.4-2.6 L/min/m2 in our study, longer CPB time and aortic cross-clamp time were associated with both in-hospital mortality and postoperative hyperlactatemia. Moreover, we used pulsatile flow that was proved to improve hyperlactatemia and reduce inotrope use intra- and post-operatively [30], our study suggests that it was not enough to diminish arterial lactate level in dialysis-dependent patients.
On the other hand, our study has several limitations to consider. Firstly, this was a retrospective study and we presented the association between hyperlactatemia and in-hospital mortality, but causation cannot be inferred. To prove this causation, intensive randomized controlled trial to reduce lactate levels is essential. Secondly, the sample size was limited; thus, we could not include several covariates in our multivariate logistic regression analysis. A larger multi-center study should be considered. We think this study is valuable that this result will give the important information about the effect size for the future RCT study. Thirdly, we could not obtain preoperative blood lactate levels in all enrolled patients. These levels were not measured in hospital A because of the lack of relevant equipment. To assume that preoperative lactate levels were within normal limits, we excluded emergency operations and patients who required inotropes preoperatively, whose preoperative lactate levels were high. By this, it could be expected to minimize the effects of preoperative variability of the lactate level.
Conclusion
Among dialysis-dependent patients after cardiac surgery, the early-onset of a maximum arterial lactate concentration of > 4.5 mmol/L was significantly associated with in-hospital mortality.
Availability of data and materials
Derived data supporting the findings of this study are available from the corresponding author (ME) on request.
References
Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada A, Ogata S, Hasegawa T, Hamano T, Hanafusa N, Hoshino J, Goto S, Yamamoto K, Minakuchi J, Nakamoto H, on behalf of Japanese Society for Dialysis Therapy Renal Data Registry Committee. Annual Dialysis Data Report 2016, JSDT Renal Data Registry. Renal Replacement Therapy. 2018;4:45.
Aoki J, Ikari Y. Cardiovascular disease in patients with end-stage renal disease on hemodialysis. Ann Vasc Dis. 2017;10:327–37.
Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan in 2016: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2019;67:377–411.
Minton J, Sidebotham DA. Hyperlactatemia and cardiac surgery. J Extra Corpor Technol. 2017;49:7–15.
Rahmanian PB, Adams DH, Castillo JG, Vassalotti J, Filsoufi F. Early and late outcome of cardiac surgery in dialysis-dependent patients: single-center experience with 245 consecutive patients. J Thorac Cardiovasc Surg. 2008;135:915–22.
Tanaka K, Tajima K, Takami Y, Okada N, Terazawa S, Usui A, Ueda Y. Early and late outcomes of aortic valve replacement in dialysis patients. Ann Thorac Surg. 2010;89:65–70.
Charytan DM, Kuntz RE. Risks of coronary artery bypass surgery in dialysis- dependent patients--analysis of the 2001 National Inpatient Sample. Nephrol Dial Transplant. 2007;22:1665–71.
Vohra HA, Armstrong LA, Modi A, Barlow CW. Outcomes following cardiac surgery in patients with preoperative renal dialysis. Interact Cardiovasc Thorac Surg. 2014;18:103–11.
Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–44.
Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004;32:1120–4.
Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6:322–6.
Cano N. Bench-to-bedside review: glucose production from the kidney. Crit Care. 2002;6:317–21.
Kogan A, Preisman S, Bar A, Sternik L, Lavee J, Malachy A, Spiegelstein D, Berkenstadt H, Raanani E. The impact of hyperlactatemia on postoperative outcome after adult cardiac surgery. J Anesth. 2012;26:174–8.
Evans AS, Levin MA, Lin HM, Lee K, Weiner MM, Anyanwu A, Adams DH, Mittnacht AJC. Prognostic value of hyperlactatemia and lactate clearance after mitral valve surgery. J Cardiothorac Vasc Anesth. 2018;32:636–43.
Laine GA, Hu BY, Wang S, Thomas Solis R, Reul GJ Jr. Isolated high lactate or low central venous oxygen saturation after cardiac surgery and association with outcome. J Cardiothorac Vasc Anesth. 2013;27:1271–6.
Vellinga NAR, Boerma EC, Koopmans M, Donati A, Dubin A, Shapiro NI, Pearse RM, van der Voort PHJ, Dondorp AM, Bafi T, Fries M, Akarsu-Ayazoglu T, Pranskunas A, Hollenberg S, Balestra G, van Iterson M, Sadaka F, Minto G, Aypar U, Hurtado FJ, Martinelli G, Payen D, van Haren F, Holley A, Gomez H, Mehta RL, Rodriguez AH, Ruiz C, Canales HS, Duranteau J, Spronk PE, Jhanji S, Hubble S, Chierego M, Jung C, Martin D, Sorbara C, Bakker J, Ince C. microSOAP study group. Mildly elevated lactate levels are associated with microcirculatory flow abnormalities and increased mortality: a microSOAP post hoc analysis. Crit Care. 2017;21:255.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7.
Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371:2309–19.
Rocktäschel J, Morimatsu H, Uchino S, Ronco C, Bellomo R. Impact of continuous veno-venous hemofiltration on acid-base balance. Int J Artif Organs. 2003;26:19–25.
Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57:1176–81.
Phypers B, Pierce JM. Lactate physiology in health and disease. Cont Educ Anaesth Crit Care Pin. 2006;6:128–32.
Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. 2018;46:1747–52.
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229–38.
Moskowitz A, Andersen LW, Huang DT, Berg KM, Grossestreuer AV, Marik PE, Sherwin RL, Hou PC, Becker LB, Cocchi MN, Doshi P, Gong J, Sen A, Donnino MW. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care. 2018;22:283.
Luger M, Hiesmayr M, Köppel P, Sima B, Ranz I, Weiss C, König J, Luger E, Kruschitz R, Ludvik B, Schindler K. Influence of intravenous thiamine supplementation on blood lactate concentration prior to cardiac surgery: a double-blinded, randomised controlled pilot study. Eur J Anaesthesiol. 2015;32:543–8.
Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, Deane AM, Shehabi Y, Hajjar LA, Oliveira G, Udy AA, Orford N, Edney SJ, Hunt AL, Judd HL, Bitker L, Cioccari L, Naorungroj T, Yanase F, Bates S, McGain F, Hudson EP, Al-Bassam W, Dwivedi DB, Peppin C, McCracken P, Orosz J, Bailey M, Bellomo R, VITAMINS Trial Investigators. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. JAMA. 2020. https://doi.org/10.1001/jama.2019.22176.
Lopez-Delgado JC, Esteve F, Javierre C, Torrado H, Rodriguez-Castro D, Carrio ML, Farrero E, Skaltsa K, Mañez R, Ventura JL. Evaluation of serial arterial lactate levels as a predictor of hospital and long-term mortality in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29:1441–53.
Maillet JM, Le Besnerais P, Cantoni M, Nataf P, Ruffenach A, Lessana A, Brodaty D. Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest. 2003;123:1361–6.
Inoue S, Kuro M, Furuya H. What factors are associated with hyperlactatemia after cardiac surgery characterized by well-maintained oxygen delivery and a normal postoperative course? A retrospective study. Eur J Anaesthesiol. 2001;18:576–84.
O’Neil MP, Fleming JC, Badhwar A, Guo LR. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Ann Thorac Surg. 2012;94:2046–53.
Acknowledgements
The authors thank Enago for providing editorial assistance.
Funding
Financial support was not provided by any institution.
Author information
Authors and Affiliations
Contributions
ME and NK conceived of and designed the study, ME, KM and JT collected the data. ME wrote the manuscript. KY provided valuable discussion of the results and assisted with statistical analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of New Tokyo Hospital (Matsudo, Chiba, Japan, approval ID: 0175) and that of the Saitama Red Cross Hospital (Omiya, Saitama, Japan, approval ID: 18- AO).
Consent for publication
This study took the opt-out method to obtain consents for publication and participation.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ezaka, M., Tsukamoto, J., Matsuo, K. et al. Hyperlactatemia of dialysis-dependent patients after cardiac surgery impacts on in-hospital mortality: a two-center retrospective study. JA Clin Rep 6, 47 (2020). https://doi.org/10.1186/s40981-020-00348-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40981-020-00348-1