Abstract
Antisense oligonucleotides (ASOs) are an important tool for the treatment of many genetic disorders. However, similar to other gene drugs, vectors are often required to protect them from degradation and clearance, and to accomplish their transport in vivo. Compared with viral vectors, artificial nonviral nanoparticles have a variety of design, synthesis, and formulation possibilities that can be selected to accomplish protection and delivery for specific applications, and they have served critical therapeutic purposes in animal model research and clinical applications, allowing safe and efficient gene delivery processes into the target cells. We believe that as new ASO drugs develop, the exploration for corresponding nonviral vectors is inevitable. Intensive development of nonviral vectors with improved delivery strategies based on specific targets can continue to expand the value of ASO therapeutic approaches. Here, we provide an overview of current nonviral delivery strategies, including ASOs modifications, action mechanisms, and multi-carrier methods, which aim to address the irreplaceable role of nonviral vectors in the progressive development of ASOs delivery.
Similar content being viewed by others
Introduction
In human disease treatment, antibody-based and conformation-corrected therapies that focus on the clearance of certain proteins associated with genetic diseases are being developed [1, 2], particularly because the bulk of therapeutic candidate target genes for genetic diseases are not the targets of the vast majority of small-molecule drugs. Accordingly, nucleic acid-based therapeutics have attracted the attention of researchers, and antisense technology is now beginning to deliver on its promise to treat diseases by targeting RNA [3, 4]. However, even though a wide selection of RNA sources, including precision duplex silencers RNA (siRNA), microRNAs, messenger RNA (mRNA), and RNA aptamers, are available for therapeutic use, the efficiency of the final conversion to reliable drugs is not ideal, and the current output of new drugs is limited [5, 6]. In contrast, short oligonucleotides that localize to the nucleus and provide a pathway for gene silencing by the RNase H pathway offer a more direct and reliable option.
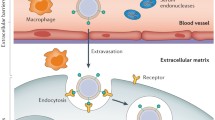
Antisense oligonucleotides (ASOs) are synthetic small single-stranded nucleic acid polymers (approximately 18 ~ 30 nucleotides) with diverse chemical properties that can be employed to regulate gene expression via various mechanisms. Unlike small-molecule drugs, antisense drugs work through Watson–Crick base pairing with the target RNA sequence [7]. This difference is believed to be the underlying reason for the excellent performance of ASOs in treating a variety of genetic disorders for which small-molecule drugs are not available [8]. Meanwhile, compared to RNAs which tolerate only limited modifications to remain RNA-Induced Silencing Complex (RISC) compatibility, one of their critical advantage is higher affinity, as the development of chemical modifications increases affinity, selectivity, and reduces toxicity due to off-target effects [9]. Since Fomiviren was approved by the FDA in 1998 for the treatment of retinitis caused by cytomegalovirus (CMV) infection in immunocompromised AIDS patients [10], several single-stranded antisense oligonucleotide (ASO) drugs belonging to multiple companies administered by four different routes have been approved for commercial use (Table 1), and even more ASOs with varying mechanisms of action and routes of administration are in preparation [4]. However, the extent of drug exploitation using vectors to deliver ASOs is still quite primitive, and this is one of the priorities for the future drug development of ASOs. This review provides an overview of the potentially valuable delivery strategies of ASOs based on nonviral vectors, the graphical overview is presented in Fig. 1.
Modifications of antisense oligonucleotide structure
ASOs are synthetic oligonucleotides or oligonucleotide analogs that can be designed to bind to protein-coding RNAs as well as noncoding RNAs. They regulate RNA function through a variety of different mechanisms, depending on the types of chemical modifications, modification sites, and binding sites by which they target RNAs. Moreover, ASOs can be designed to regulate the processing of RNA molecules, including the regulation of RNA splicing and the selection of polyadenylation sites [11, 12], to disrupt the structure of RNAs used to inhibit the translation of proteins [13], and to promote the degradation of bound RNA by endogenous nucleases [14].
Due to hindered cell uptake [15, 16], off-target effects [17, 18], undesirable on-target effects [19], short half-life, immune clearance, and other disadvantages that free ASOs cannot avoid in vivo, researchers have proposed a variety of modifications to improve the stability and extend the half-life of ASOs [20, 21]. Phosphorothioate allows the nonbridging oxygen of the phosphate group in ASOs to be replaced by a sulfur group, resulting in the formation of a phosphorothioate bond, which is resistant to nuclease-based degradation [22]. In addition, the phosphorodiamidate morpholino modification increases the water solubility of ASOs [23], a peptide nucleic acid is an artificial mimic capable of self-assembly to form a backbone structure [24], and a locked nucleic acid is more commonly used today and can greatly increase the stability of ASOs [25], and 2′-O-methoxyethyl-(2′-O-MOE) and 2′-O-[2-(methylamino)-2-oxoethyl] improve the binding affinity of ASOs and provide resistance to enzymatic degradation [26].
Mechanism of action of antisense nucleotides
ASOs are theoretically designed to regulate the transfer of genetic information to proteins specifically, but the mechanisms by which ASOs induce biological effects are subtle and complex (Fig. 2). Based on the mechanism of action, two major classes of ASOs can be discerned: (a) Degradation by the RNase H and Argonaute 2 (the most widely be adopted two strategies) or some other elements (Fig. 2A), (b) Steric-blocker oligonucleotides, which physically block or inhibit the progression of splicing or translation mechanisms (Fig. 2B, C).
The main mechanisms of ASO regulate genes. A Downregulation mechanism of degradation and steric blockage simultaneously; ①. The ASO-mRNA double strands as a substrate recruit RNase H1, leading to degradation of the target transcript. ②. ASOs enter the RISC including a part in Ago 2, and become the guide strand. Then direct the RISC to mRNA. B Downregulation mechanism of steric blockage; ③. ASOs bind to pre-mRNA to alter polyadenylation position, and decrease mRNA stability and levels. ④. ASOs bind to the most 5ʹ region of mRNAs to avoid the binding of translation initiation factors, inhibiting translation. C Upregulation mechanism of steric blockage; ⑤. ASOs inhibit miRNA function to increase the expression of their target mRNA. ⑥. ASOs can enhance translation by inhibiting upstream open reading frames (uORFs), a translation suppression element
Regulation by degradation and steric blockage together
ASOs bind to target RNA to form a conjugate that recruits RNase H to degrade RNA for silencing [27, 28]. Degradation mediated by RNase H is the most stable and reliable mode of ASO action and is almost unaffected by the multiple modifications imposed on the ASOs themselves. Most FDA-approved ASO drugs work in this way. In addition, ASOs form double strands with the target RNA and then bind to the Argonaute 2 (Ago 2) enzyme to form the RISC. RISC moves to the complementary mRNA region where the Ago 2 enzyme breaks down the mRNA and exerts its gene silencing effect [29].
Regulation by steric blockage only
ASOs with steric blockage function are designed to bind to target transcripts with high affinity. Still, they do not induce degradation of the target transcripts due to their lack of RNase H recruitment capacity [30, 31]. This action is most commonly seen in phosphorodiamidate morpholino antisense-modified oligomers (PMOs), a class of antisense nucleic acid drugs that typically interfere with the expression of target genes by binding and spatially blocking the assembly of the translation machinery [32]. Unlike classical phosphorothioate oligonucleotides (PS-ODNs), PMOs do not induce RNase H activity, they bind to target RNA sequences and spatially block ribosome assembly or intron–exon splice junction sites, leading to translation arrest or splicing alteration. PMO-modified ASOs have different chemical properties from ASOs with other modifications: they are usually neutral rather than carrying charges [33]. Differences in chemically modified structures may lead not only to different mechanisms of action but also unique pharmacokinetics and biosafety of the ASO via steric blockade. As oligonucleotides that do not affect RNA integrity, steric-blocking ASOs have irreplaceable long-term potential and value in nucleic acid pharmaceuticals [34]. Other approaches include that ASOs modulate RNA function to attenuate or augment the translation of corresponding proteins in the cytoplasm. Moreover, ASOs can be designed to affect RNA splicing and polyadenylation site selection to regulate the processing of RNA molecules [35, 36]. In addition, ASOs designed to disrupt translation-suppressing RNA structures, block upstream AUG codons, or bind to microRNA can increase protein translation [13, 37].
Promising delivery system for antisense oligonucleotides
Lipid-based delivery systems
To deliver ASOs to the target site by different routes of administration, nanocarriers of cationic polymers are usually preferred because of their ability to form polyelectrolyte complexes by facilitating ionic interactions between the positively charged functional groups and the negatively charged phosphate fraction [38]. The form of nucleic acids in nanocarriers is complex. ASOs can be encapsulated in the matrix of the nanocarrier or attached to the surface of the carrier by covalent or ionic bonding. Lipid-based nanoparticle (LNP) systems are one of the most promising colloidal nanocarriers for bioactive organic molecules. LNPs for the delivery of ASOs (LNPs-ASOs) typically consist of ionizable cationic lipids, phospholipids, polyethylene glycol (PEG) lipids, and cholesterol due to the negatively charged nature of nucleic acids [39,40,41]. PEG-series materials are structurally similar, but each has a specific structure and unique function (Fig. 3). The LNP-based delivery platform is appreciated as an advanced virus-free delivery system for ASOs for the treatment of a range of diseases [42]. Hitherto three LNP-based RNA drugs have been approved by the FDA, including two COVID-19 mRNA vaccines that play an irreplaceable role in preventing the spread of epidemics [43, 44].
LNPs have a suitable particle size (diameter range of 10–500 nm) combined with their own biocompatible and biodegradable lipids, which enables LNPs-ASOs to escape uptake by the mononuclear phagocyte system (MPS), subsequently prolonging the circulation time of LNPs-ASOs and allowing the particles to passively and efficiently target cells through an enhanced permeability and retention effect to release ASOs [45,46,47]. They also improve cell-to-ASO uptake by inducing lipid fusion between the membranes of LNPs and target cells during structure phase transitions [48,49,50], and help ASOs travel to target genes by promoting endosomal escape after cellular uptake [50, 51]. Examples of lipid-based delivery systems that effectively deliver ASOs are summarized in Table 2.
Several prior studies have reported LNPs to be potentially exploitable. Although some studies have not used LNPs as traditional formulations of nucleic acid drugs, the addition of lipid components alone reduces positive charge toxicity and can significantly improve biocompatibility [47]. However, the optimal composition of LNPs applicable to ASOs will vary, as will the key to successful delivery mechanisms[52, 53]. Hiroki et al. initially hypothesized that the optimal composition of ssPalmO-Phe/Chol for siRNA delivery could be applied to ASOs, but it was revealed that LNPssPalmO-Phe containing ASOs are highly unstable and susceptible to aggregation [54]. With an improved lipid composition and lipid/ASO ratio, an LNP system that could efficiently transport ASOs was obtained.
Liposomes
Liposomes are used in the pharmaceutical and cosmetic industries to transport a wide range of molecules. They are spherical vesicles composed of phospholipids and sterols, usually in the size range of less than 500 nm [61]. Liposomes are classified into several types based on the addition of PEG and ligands [62, 63]. PEG is arguably the most critical component of liposomes, which limits the adsorption of serum proteins and effectively prolongs blood circulation time [64]. While PEG has a recognized effect of improving the pharmacokinetic properties of nucleic acids, it is posing other challenges. The first is the hindrance of tissue penetration, cellular uptake, and endosomal escape behavior [65, 66]. The second is the repeated use of polyethylene glycol-modified liposomes, which inevitably leads to faster serum clearance and severely compromises subsequent therapeutic efficacy [67,68,69]. Despite the apparent disadvantages of PEG, there is still a lack of proven and reliable substitutes.
Liposomes are potentially more enriched in the liver and spleen than other carriers, so it is essential either to develop different types of liposomes to counteract this property or to take advantage of this property to deliver ASOs that are expected to work in these organs [70, 71]. The lipid component of the liposome stabilizes proteins on the surface, making it more advantageous to apply protein modifications. Guan et al. functionalized liposomes with a tumor-homing and -penetrating peptide, iRGD, as a carrier of an ASO against androgen receptor (AR) for prostate cancer treatment, and these iRGD-liposomes markedly improved the ASO efficacy in suppressing the growth of tumor [72]. The modification of liposomes with targeting antibodies improves the affinity of liposomes for cancer cells and optimizes the intratumoral penetration of ASOs [73].
Polymer-based delivery systems
Polymers have been one of the most widely used drug delivery systems since being discovered. In addition to proteins and small molecules, polymer-drug systems are also essential for the delivery of nucleic acid drugs [74, 75]. The classification of polymer systems is also highly complex with numerous categories according to the structural differences of the components. Examples of polymer-based ASO delivery systems are summarized in Table 3. The unique advantage of the polymer system is its stability. Because polymeric materials mostly have rigid shapes, polymeric nanoparticles can retain the ASOs carried in the central cavity of the nanoparticle even after a variety of operations such as long storage, lyophilization, concentration, and so on [76,77,78]. Here, we refer to the traditional classification method and divide them into four categories: early linear polycations, dendrimers, polymeric nanoparticles, and natural polymers [74]. One study claims that cationic micelles offer both the properties of cationic polymers and the benefits of micelles, with the added benefit of reduced toxicities [79]. The molecular structure of several classic materials and the structure of the carriers obtained by assembling them were shown in Fig. 3.
Early linear polycations
Linear cationic compounds have long been shown to be effective in delivering nucleic acids. In the 1960s, these polycationic derivatives of dextran were shown to enhance the transfection of viral RNA and DNA [88, 89]. The advantages of Deae-dextran are chemical simplicity, reproducibility, and low cost, but the disadvantages are low transfection efficiency, cytotoxicity, and inhibition of cell growth in vitro, which limit its use in vivo. The discovery of linear polycations was of epoch-making significance, but linear polycations were soon replaced by dendritic polycations with complex and variable structures due to the insurmountable defects mentioned above.
Micelles
Micelles, self-assembled from block copolymers, have a unique core–shell structure with a size distribution in the range of 10–100 nm [90,91,92]. Although most available cationic polymers can coalesce DNA, they interact weakly with DNA. Thus, the polymers formed in physiological fluids, which contain serum components and salts that tend to break down these complexes, are not very stable. Therefore, they are not the best materials to form micelles for the delivery of ASOs [93, 94]. Furthermore, the synthesis of high molecular weight cationic polymers (e.g. dendrimers) is usually labor intensive and costly, greatly hindering their biomedical applications [95]. The self-assembly of amphiphilic polymers into micelles makes them excellent gene carriers. Amphiphilic cationic polymers such as polylysine (PLL) [96], PEI [97, 98], polyamidoamine (PAMAM) [99], and polydimethylaminoethyl methacrylate (PDMAEMA) [100, 101] are commonly used to construct cationic micelles [102, 103].
Dendrimers
Synthetic polycations such as PEI and PAMAM dendrimers, and some other polycations, such as poly(amine-co-ester) (PACE) are included in this category [104,105,106] (Fig. 4A-C). Due to their extrinsic positive charge, ASO nanocarriers based on electrostatic adsorption are usually prone to nucleic acid leakage through the formation of polyelectrolyte aggregates and induce excessive positive charge-related cytotoxicity and non-specific interactions with serum or plasma proteins, but most of them have been used successively to deliver siRNA and mRNA with good results, however, only a few of which have been used to attempt the delivery of ASOs.
Marcel developed a nanoparticle-based delivery system for ASOs targeting the antibiotic resistance of methicillin-resistant Staphylococcus aureus (MRSA): the system was prepared by the sequential modification of gold nanoparticles with PEI and maintained antibacterial ability with reduced low cytotoxicity [107]. Yoshida succeeded in solving the problem of poor intracellular uptake by target cells by using superparamagnetic iron oxide (SPIO) nanoparticles coated with PEI as a delivery vehicle for ASOs [108].
PAMAM is another cationic dendrimer used to deliver ASOs. A co-delivery system is based on a cationic dendrimer core that encapsulates fluorouracil and oligonucleotides within a hydrophobic lumen, modified with hyaluronic acid and cell-penetrating peptides. The codelivery complex showed efficient cellular uptake and consequently improved intracellular distribution and enhanced cytotoxicity on cells [87, 109].
Polymeric nanoparticles
Polymeric nanoparticles, due to their tunable architecture (10–1000 nm), nontoxicity, biocompatibility, and controlled drug release are promising options for targeted drug delivery platforms [80, 110]. Widely used biodegradable synthetic polymers include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and copolymers such as poly(lactic acid-glycolic acid) (PLGA) [111]. PLGAs have been approved by the FDA for certain transport applications. These materials are difficult to use for nucleic acid delivery because unlike cationic polymers, they cannot rely on charge dominance to hold the nucleic acid [112, 113]. Therefore, PLGA is often used in conjunction with the cationic polymers PEI and PAMAM. The advantage of this is that not only does it rely on the electrical charge to achieve a higher nucleic acid loading, but also the addition of PLGA results in lower surface charge compared to the cationic polymer particles in isolation, resulting in lower toxicity and a lower rate of removal, which facilitates the sustained release of cargo [114]. In addition to dendrimers, other cationic polymers have been used in blends with PLGA, such as Poly-beta-amino-ester (PBAE), which has positively charged groups that can interact with nucleic acids and are simple to synthesize and more readily degraded in vivo [115, 116]. In the blend of PBAE and PLGA, cytotoxicity decreased with the ratio of PBAE to PLGA [117]. PACEs are another commonly used class of cationic polymers with the unique advantage of lower toxicity that results from lower charge density [118, 119]. PACE is more closely associated with plants than the compounds mentioned above, and a variety of PACEs are now derived indirectly or directly from plant-derived components [120]. Cui loaded solid PACE nanoparticles (PACE-NPs) with oligonucleotides designed to knockdown Nogo-B, a protein that has been implicated in the progression of alcoholic liver disease and liver fibrosis, and demonstrates that PACE-NPs can effectively deliver oligonucleotides therapeutics to the liver to mediate protein knockdown in vivo [121].
The synthesis reactions of polymers are quite mature, so chemical precision and flexibility in designing synthetic strategies are considerable; accordingly, a highly functionalized nucleic acid polymer (HFNAP) library is usually designed as needed, and the target compounds are screened by parallel experiments [122, 123]. In addition to the ability to design synthesis methods based on the desired chemical structure, hydrophilicity/hydrophobicity, charge density, and functional domains and structure, it is even possible to select suitable polymers based on the delivered nucleic acid sequence [124, 125].
Natural polymer-based delivery systems
Naturally derived structural proteins and polysaccharides, such as cationic collagen derivatives, cyclodextrins (CDs), and chitosan, have been developed as gene carriers [126,127,128,129]. Collagen, an important component of bone tissue, has a complex structure that makes it available as an artificial scaffold material with an innate drug retentive function. Natural collagen exists in two forms: as a swollen hydrogel and as sparse fibers in lattice-like tissues [130]. When targeting RNA delivery to bone-related cells, collagen should be the first candidate considered as a carrier material that provides good and stable sustained release [131]. In addition to scaffolds, collagen can be used in conjunction with polymers and lipids, as with other organic materials, to make nano preparation suitable for local injection with a slow-release, low systemic circulating drug concentration, and excellent specificity.
Due to the poor specificity, low stability, and low permeability of ASOs through cell membranes, an effective nucleic acid carrier system for most studies usually requires cationic materials. Chitosan is a strong candidate due to its cationic properties, biodegradability, and excellent biocompatibility [132]. Chitosan is a linear polymer formed by α (1 → 4)-linked-2-amino-2-deoxy-β-d-glucopyranose [133]. Various functional groups or molecules can be affixed with chitosan to guarantee the desired function with the nanocarrier system [134]. Kolonko et al. developed a nonviral delivery system based on the natural aminopolysaccharide chitosan (CS) for the transport of ASOs against ENaC to specifically address Na + hyperabsorption and confirmed the successful uptake of the nanocomplex by human airway epithelial cells, demonstrating the possibility of targeted transport of ASOs with chitosan [135].
Extracellular vesicle-based systems
The naming of extracellular vesicles is extremely chaotic. Extracellular vesicles (EVs) are vesicles that are released from cells into the extracellular space, and can be subdivided into microvesicles (100 nm to 500 nm in diameter) or exosomes (30 nm to 100 nm in diameter) by their specific diameter [136, 137], EVs are vesicles that carry nucleic acids and proteins that are secreted by almost all cells into the extracellular fluid and body fluids such as blood, urine, tears, and milk. Because of the propensity of EVs to transfer to recipient cells and the compositional advantages in biocompatibility, they are naturally used as vehicles for the delivery of nucleic acids. Seven classes of exosome isolation strategies have been reported, including stepwise ultracentrifugation (Fig. 5A), gradient density ultracentrifugation (Fig. 5B), ultrafiltration (Fig. 5C), size-exclusion chromatography (Fig. 5D), microfluidic techniques (Fig. 5E), polymer precipitation (Fig. 5F), and immunoaffinity capture (Fig. 5G), each of which has unique advantages and disadvantages. EVs are potent cell-derived nanovesicles that can mediate intracellular communication to achieve nondestructive and efficient delivery.
Schematic diagram of various schemes for collecting extracellular vesicles. A The cell supernatant was separated by repeated multiple ultracentrifugations to obtain EVs. B The supernatant was subjected to sucrose density gradient centrifugation, and EVs with different particle sizes were distributed in different concentrations of sucrose solution. C The separation of exosomes by rotary ultrafiltration technology is based on the principle that the pore size of the ultrafiltration membrane allows and intercepts substances of different relative molecular masses, filtering solvents and some small molecules to the other side of the membrane while retaining substances with high relative molecular mass that are larger than the membrane pore size on the ultrafiltration membrane, thus achieving separation. D Exclusion chromatography separates EVs of different particle sizes due to their different peak emergence times after passing through the column. E The microfluidic technique achieves exosome isolation, concentration, and analysis. F Particles of different sizes are subjected to differentially sized acoustic radiation and viscous forces in the microfluidic acoustic field. Under the combined effect of acoustic radiation and viscous force, particles of different sizes move to different exits, thus achieving separation. G Highly hydrophilic polymers interact with water molecules around exosomes to form a hydrophobic microenvironment, which leads to exosome precipitation. H EVs have specific markers on their surface and are adsorbed onto magnetic beads encapsulated with anti-marker antibodies that bind to exosome vesicles after incubation
The ASO is usually loaded into the exosome by electroporation [138, 139]. The drug loading rate depends on the specific experimental conditions and the type of vesicles, but in general, it is relatively low compared to that of artificial carriers. Although more studies are reporting that EVs carrying ASOs can achieve good therapeutic effects, the mode of administration of the studies appears to be limited to injection, and in one study where ASOs was administered by oral delivery of bovine extracellular vesicles, no significant decrease in target gene expression was seen in vivo [140]. In addition to EVs produced by normal cellular secretion, EVs obtained by various artificial intervention methods have also been used to carry ASOs. Compared to exosomes, apoptotic bodies (ABs) can be produced with much higher efficiency [141].
Biomimetic vesicle-based systems
While delivery systems for artificial materials are not immune to compatibility and clearance problems, biological vesicles represented by exosomes are not immune to another challenge: low nucleic acid loading rates and the potential safety threat of carrying their own nucleic acids. The loading of nucleic acids onto vectors is low in efficiency and their functional activity may be compromised [143]. It was found that the number of nucleic acids loaded into EVs was limited [144]. Therefore, one study pretransfected ASOs into cells and then produced ASO-rich apoptotic vesicles by inducing apoptosis, and good genetic suppression was achieved with these apoptotic vesicles (Fig. 6) [142]. Currently, cell and organelle membranes derived from various cell types have been developed as carriers to deliver ASOs (Fig. 7A). Moreover, we summarized other methods in nanofabrication of artificial EVs, including extrusion (Fig. 7B), promoting secretion (Fig. 7C), and fusion (Fig. 7D). Bionic carriers are a new type of drug delivery system that has been rapidly developed in recent years and has the potential to solve many long-existing challenges at once. Biomimetic vectors are usually composed of endocytic, protein, organelle, microbial or viral structures with artificial nanoparticle materials or individually [145,146,147,148]. The commercialization of biomimetic drug delivery systems presents quality control and ethical issues, but such drug delivery systems are promising in terms of therapeutic efficacy.
The ASOs bound to the cationic material can be made available for cell uptake, and then apoptotic vesicles containing ASOs can be directly produced by cell induction of cells after uptake of the nucleic acid drug. Schematic diagram of the protocol for producing small apoptotic bodies and delivering ASOs into the brain. (Reprinted with permission from Ref [142].
Biomimetic carriers for ASO delivery. A Components from a variety of cells and body fluids have been used to prepare biomimetic nanoparticles. B Cells can be forced to pass through membrane pores to form biomimetic nanoparticles. C Sulfhydryl-blocking can lead to the release of small biomimetic nanoparticles from the cell; UV light can induce apoptosis and produce small apoptotic bodies. D Isolated natural EVs and liposome nanoparticles can be fused into hybrid EVs
Metallic nanoparticles systems
Metal nanoparticles are widely used and recognized in the fields of biotechnology and bioengineering [149]. Currently, metal nanoparticles and conjugates of ligands, drugs, antibodies, peptides, and nucleic acids have been used for targeted drug delivery, diagnostics, and imaging. Among the most studied are gold, silver, and platinum nanoparticles [150, 151]. Gold nanoparticles are the most widely studied and stable, with negligible toxicity and good imaging in vivo [152]. Anna Graczyk et al. invented a conjugate of gold nanoparticles and structural RNA that was successfully used as a tool for gene expression regulation successfully [153]. Gong et al. constructed MALAT1-specific ASO and nucleus-targeting TAT peptide cofunctionalized Au nanoparticles, namely, ASO-Au-TAT NPs, which stabilized fragile ASOs, enhanced nuclear internalization, and exhibited good biocompatibility [154] (Fig. 8A). A multi-layer coated gold nanoparticles (MLGNPs) delivering antisense oligonucleotides (ASOs) were shown to be efficiently internalized into various types of Gram-positive bacteria and may use with conventional antibiotics [107] (Fig. 8B). The biocompatibility of metal nanoparticles and the functionalization of unrestricted nucleic acid structures offer a wide range of potential applications. They have emerged as an outstandingly promising solution for ASO delivery and personalized nanomedicine in the future.

Copyright © 2019, American Chemical Society. Figure 8B reprinted with permission from Ref [107]. Copyright © 2021, Elsevier B.V.)
Schematic illustration of two gold nanoparticle systems. (A) Schematic illustration of nucleus-targeting by ASO-Au-TAT nanocarrier. (B) Schematic illustration for preparation of MLGNPs delivering ASO targeting antibiotic resistance and its application for combinatorial treatment of MRSA infections. (Fig. 8A reprinted with permission from Ref [154].
Potent supporters: specific condition-sensitive materials
Sensitive materials have a rich history of use in the treatment of infections and tumors with superior results [155, 156]. Since the approval of sodium polyphotodynamic therapy as the first photosensitizer (PS) for the treatment of bladder cancer in 1993, photodynamic therapy (PDT) has been widely used in antitumor and anti-infection therapy [157]. Photosensitizers have been tried in many nanoparticle systems [158, 159], and surprising results have been reported. In 2012, a study attempted to address the headache-inducing off-target effects of nucleic acid drugs by using a photosensitizer to trap the RNA carrier/siRNA complex completely within the endosome [160]. A near-infrared (NIR) photocontrolled self-delivery of ASO was designed to suppress hypoxia inducible factor-1α (HIF-1α) and B-cell lymphoma 2 (Bcl-2) for gene therapy [161] (Fig. 9). This precise, light-dependent control will open new possibilities for cellular and molecular biology and therapy.
Design of photolabile spherical nucleic acid (PSNA). (a) Illustration of the preparation of PSNA. (b) Schematic representation of the use of PSNA to deliver siRNA, pASO, and PS for combination cancer therapy. (Reprinted with permission from Ref [161].
Thermosensitive materials can also help retain ASO in local tissues without a serious off-target effect [162]. As confirmed in a study, a type of PLGA-PEG-PLGA thermosensitive hydrogel can increase the residence time of RNA nanoparticles in the eye to prolong the duration of action time of subconjunctival administration [163]. pH-sensitive hydrophobic fragments have been shown to promote the efficiency of oligonucleotide drug delivery by amphiphilic polycationic carriers [164]. The development of multifunctional drug nanoparticles that combine oligonucleotide drugs with different release mechanisms including thermosensitive, photosensitized, ultrasound-responsive [165], redox-responsive [166], and magnetic-responsive material [167], may be useful for specific applications. Sensitive materials can be used in a variety of diseases, perform well in clinical evaluation and assessment, and offer exciting possibilities in moving from the laboratory to real-world use.
Challenges facing ASOs delivery
ASOs are readily degraded by nucleases in body fluids and are enriched in metabolic organs such as the liver and kidney, where they are rapidly cleared, and the half-life of unmodified and unencapsulated ASOs is usually less than 10 min [168,169,170]. In addition, the lack of targeting and the off-target effects of ASOs may lead to serious side effects and consequences, limiting both the dose administered and the therapeutic effect [171,172,173,174]. The negative electrical properties and high molecular weight of ASOs are also important factors, and the chemical structure of single nucleotide chains prevents their active uptake by the cell in any form and therefore makes it difficult to cross the cell membrane to enter the cell [173, 175]. Internalized ASOs are transported out of the body by the endosomal and acidic lysosomal microenvironments, making it difficult for them to enter the nucleus to act on target gene sites [176].
Although ASO drugs continue to come to market, safety has been a stubborn factor preventing them from expanding their impact [177]. An investigator from US-FDA noted that adverse reactions among preclinical and clinical study volunteers tended to occur in those who took ASO drugs intravenously, possibly because the systemic exposure to ASOs via this route was much higher than in other local ways [178]. Another challenge ASOs once got entangled with but now have tackled is that current approved ASO are limited to treating genetic diseases by causing alternative splicing in patients with loss-of-function mutations. Since the validation of new mechanisms of action enhances the versatility of antisense technology [179,180,181].
The human system is way more complex than the in vitro culture systems or even model animals [182, 183]; delivery systems in the human body are not yet fully understood so far, so no surprises that many ASO delivery systems research cases perform well in vitro but poorly in clinical studies [184, 185]. And the accuracy and affordability of synthetic polymers, as well as the safety and stability of biological components, are challenges. Carrier systems demand not only safety, low cost, and ease of manufacturing, but also controllability and stability to advance further toward the clinic [186,187,188]. Many delivery system formulations that perform well in laboratory studies may not always accomplish equally well under the harsh storage conditions of real-world applications, which hinders delivery systems from providing value.
Conclusions
At this time, no ASO drugs using drug delivery systems have been approved by the FDA for marketing, and there will be no substitute for ASO therapeutic technologies for rare diseases for a significant period. It is foreseeable that the emergence of ASO drugs delivered by carriers is inevitable, but the timing depends on innovations in delivery systems. This will require breakthroughs in the development of materials, evaluation systems, synthesis methods, ethical safety, and many other aspects. Given the rapid progress in this field, nonviral delivery systems will certainly play an irreplaceable role in the progressive development of gene therapy.
Availability of data and materials
Not applicable.
Abbreviations
- Abs:
-
Apoptotic bodies
- Ago 2:
-
Argonaute 2
- AML:
-
Acute myeloid leukemia
- AR:
-
Androgen receptor
- ASO:
-
Antisense oligonucleotide
- CDs:
-
Cyclodextrins
- CMV:
-
Cytomegalovirus
- CS:
-
Aminopolysaccharide chitosan
- DDAB:
-
Didecyldimethylammonium bromide
- DOC:
-
Deoxycholate
- DODMA:
-
1,2-Dioleyloxy-3-dimethylaminopropane
- DOPC:
-
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- DOPE:
-
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP:
-
Cationic 1,2-dioleoyl-3-trimethylammonium-propane
- DSPE-PEG2000-Mal:
-
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N[maleimide (polyethylene glycol)-2000]
- Egg PC:
-
Egg L-α-phosphatidylcholine
- EVs:
-
Extracellular vesicles
- FDA:
-
Food and Drug Administration
- HFNAP:
-
Functionalized nucleic acid polymer
- IV:
-
Intravenous
- LNPs:
-
Lipid nanoparticles
- MLGNPs:
-
Multi-layer coated gold nanoparticles
- MO:
-
Monoolein
- mRNA:
-
Messenger RNA
- PACEs:
-
Poly(amine-co-ester)
- PAMAM:
-
Polyamidoamine
- PBAE:
-
Poly-beta-amino-ester
- PDMAEMA:
-
Polydimethylaminoethyl methacrylate
- PEG:
-
Polyethylene glycolylated lipid
- PEG-DMG:
-
1,2-Dimyristoyl-rac-glycero-3-methylpolyoxyethylene
- PEI:
-
Polyethylenimine
- PGD:
-
Poly(glycolic acid)
- PLA:
-
Poly(lactic acid)
- PLGA:
-
Poly(lactic acid-glycolic acid)
- PLL:
-
Polylysine
- PMOs:
-
Phosphorodiamidate morpholino antisense-modified oligomers
- PS-ODNs:
-
Phosphorothioate oligonucleotides
- RISC:
-
RNA-induced silencing complex
- SC:
-
Subcutaneous
- siRNA:
-
Silencers RNA
- SoyPC:
-
L-α-phosphatidylcholine
- Tf:
-
Human holo-transferrin
- TPGS:
-
DL-α-Tocopherol methoxy-polyethylene glycol succinate
References
Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10(3):459–72. https://doi.org/10.1007/s13311-013-0187-4.
Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, Kelly JW. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discovery. 2015;14(11):759–80. https://doi.org/10.1038/nrd4593.
Stein CA, Castanotto D. FDA-Approved Oligonucleotide Therapies in 2017. Mol Ther. 2017;25(5):1069–75. https://doi.org/10.1016/j.ymthe.2017.03.023.
Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20(6):427–53. https://doi.org/10.1038/s41573-021-00162-z.
Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, Feng Z, Ling KKY, Karp GM, Qi H, Woll MG. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345(6197):688–93. https://doi.org/10.1126/science.1250127.
Palacino J, Swalley SE, Song C, Cheung AK, Shu L, Zhang X, Van Hoosear M, Shin Y, Chin DN, Keller CG. SMN2 splice modulators enhance U1–pre-mRNA association and rescue SMA mice. Nat Chem Biol. 2015;11(7):511–7. https://doi.org/10.1038/nchembio.1837.
O’Toole AS, Miller S, Haines N, Zink MC, Serra MJ. Comprehensive thermodynamic analysis of 3’ double-nucleotide overhangs neighboring Watson-Crick terminal base pairs. Nucleic Acids Res. 2006;34(11):3338–44. https://doi.org/10.1093/nar/gkl428.
Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9(1):60. https://doi.org/10.1186/s13073-017-0450-0.
Chery J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 2016;4(7):35–50. https://doi.org/10.14304/surya.jpr.v4n7.5.
Perry CM, Balfour JAB. Fomivirsen. Drugs. 1999;57(3):375–80. https://doi.org/10.2165/00003495-199957030-00010.
Derbis M, Kul E, Niewiadomska D, Sekrecki M, Piasecka A, Taylor K, Hukema RK, Stork O, Sobczak K. Short antisense oligonucleotides alleviate the pleiotropic toxicity of RNA harboring expanded CGG repeats. Nat Commun. 2021;12(1):1265. https://doi.org/10.1038/s41467-021-21021-w.
Naveed A, Cooper JA, Li R, Hubbard A, Chen J, Liu T, Wilton SD, Fletcher S, Fox AH. NEAT1 polyA-modulating antisense oligonucleotides reveal opposing functions for both long non-coding RNA isoforms in neuroblastoma. Cell Mol Life Sci. 2021;78(5):2213–30. https://doi.org/10.1007/s00018-020-03632-6.
Liang XH, Shen W, Sun H, Migawa MT, Vickers TA, Crooke ST. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat Biotechnol. 2016;34(8):875–80. https://doi.org/10.1038/nbt.3589.
Kumar P, Caruthers MH. DNA Analogues Modified at the Nonlinking Positions of Phosphorus. Acc Chem Res. 2020;53(10):2152–66. https://doi.org/10.1021/acs.accounts.0c00078.
Tanaka K, Okuda T, Kasahara Y, Obika S. Base-modified aptamers obtained by cell-internalization SELEX facilitate cellular uptake of an antisense oligonucleotide. Mol Ther Nucleic Acids. 2021;23:440–9. https://doi.org/10.1016/j.omtn.2020.11.016.
Linnane E, Davey P, Zhang P, Puri S, Edbrooke M, Chiarparin E, Revenko AS, Macleod AR, Norman JC, Ross SJ. Differential uptake, kinetics and mechanisms of intracellular trafficking of next-generation antisense oligonucleotides across human cancer cell lines. Nucleic Acids Res. 2019;47(9):4375–92. https://doi.org/10.1093/nar/gkz214.
Scharner J, Ma WK, Zhang Q, Lin KT, Rigo F, Bennett CF, Krainer AR. Hybridization-mediated off-target effects of splice-switching antisense oligonucleotides. Nucleic Acids Res. 2020;48(2):802–16. https://doi.org/10.1093/nar/gkz1132.
Yoshida T, Naito Y, Yasuhara H, Sasaki K, Kawaji H, Kawai J, Naito M, Okuda H, Obika S, Inoue T. Evaluation of off-target effects of gapmer antisense oligonucleotides using human cells. Genes Cells. 2019;24(12):827–35. https://doi.org/10.1111/gtc.12730.
Lima WF, Vickers TA, Nichols J, Li C, Crooke ST. Defining the factors that contribute to on-target specificity of antisense oligonucleotides. PLoS ONE. 2014;9(7): e101752. https://doi.org/10.1371/journal.pone.0101752.
Gupta A, Andresen JL, Manan RS, Langer R. Nucleic acid delivery for therapeutic applications. Adv Drug Deliv Rev. 2021;178: 113834. https://doi.org/10.1016/j.addr.2021.113834.
Bennett CF, Baker BF, Pham N, Swayze E, Geary RS. Pharmacology of Antisense Drugs. Annu Rev Pharmacol Toxicol. 2017;57:81–105. https://doi.org/10.1146/annurev-pharmtox-010716-104846.
Liang XH, Nichols JG, Tejera D, Crooke ST. Perinuclear positioning of endosomes can affect PS-ASO activities. Nucleic Acids Res. 2021;49(22):12970–85. https://doi.org/10.1093/nar/gkab1198.
Echigoya Y, Trieu N, Duddy W, Moulton HM, Yin H, Partridge TA, Hoffman EP, Kornegay JN, Rohret FA, Rogers CS. A Dystrophin Exon-52 Deleted Miniature Pig Model of Duchenne Muscular Dystrophy and Evaluation of Exon Skipping. Int J Mol Sci 2021, 22(23). https://doi.org/10.3390/ijms222313065
Oh SY, Ju Y, Park H. A highly effective and long-lasting inhibition of miRNAs with PNA-based antisense oligonucleotides. Mol Cells. 2009;28(4):341–5. https://doi.org/10.1007/s10059-009-0134-8.
Swayze EE, Siwkowski AM, Wancewicz EV, Migawa MT, Wyrzykiewicz TK, Hung G, Monia BP, Bennett CF. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35(2):687–700. https://doi.org/10.1093/nar/gkl1071.
Prakash TP, Kawasaki AM, Wancewicz EV, Shen L, Monia BP, Ross BS, Bhat B, Manoharan M. Comparing in vitro and in vivo activity of 2’-O-[2-(methylamino)-2-oxoethyl]- and 2’-O-methoxyethyl-modified antisense oligonucleotides. J Med Chem. 2008;51(9):2766–76. https://doi.org/10.1021/jm701537z.
Yin W, Rogge M. Targeting RNA: A Transformative Therapeutic Strategy. Clin Transl Sci. 2019;12(2):98–112. https://doi.org/10.1111/cts.12624.
Anderson BA, Freestone GC, Low A, De-Hoyos CL, Iii WJD, Østergaard ME, Migawa MT, Fazio M, Wan WB, Berdeja A. Towards next generation antisense oligonucleotides: mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides. Nucleic Acids Res. 2021;49(16):9026–41. https://doi.org/10.1093/nar/gkab718.
Castanotto D, Lin M, Kowolik C, Wang L, Ren XQ, Soifer HS, Koch T, Hansen BR, Oerum H, Armstrong B. A cytoplasmic pathway for gapmer antisense oligonucleotide-mediated gene silencing in mammalian cells. Nucleic Acids Res. 2015;43(19):9350–61. https://doi.org/10.1093/nar/gkv964.
Bizot F, Vulin A, Goyenvalle A. Current Status of Antisense Oligonucleotide-Based Therapy in Neuromuscular Disorders. Drugs. 2020;80(14):1397–415. https://doi.org/10.1007/s40265-020-01363-3.
Watts JK, Corey DR. Silencing disease genes in the laboratory and the clinic. J Pathol. 2012;226(2):365–79. https://doi.org/10.1002/path.2993.
Amantana A, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr Opin Pharmacol. 2005;5(5):550–5. https://doi.org/10.1016/j.coph.2005.07.001.
Ghosh C, Stein D, Weller D, Iversen P. Evaluation of antisense mechanisms of action. Methods Enzymol. 2000;313:135–43. https://doi.org/10.1016/s0076-6879(00)13008-3.
Faria M, Spiller DG, Dubertret C, Nelson JS, White MR, Scherman D, Hélène C, Giovannangeli C. Phosphoramidate oligonucleotides as potent antisense molecules in cells and in vivo. Nat Biotechnol. 2001;19(1):40–4. https://doi.org/10.1038/83489.
Jafar-Nejad P, Powers B, Soriano A, Zhao H, Norris DA, Matson J, DeBrosse-Serra B, Watson J, Narayanan P, Chun SJ. The atlas of RNase H antisense oligonucleotide distribution and activity in the CNS of rodents and non-human primates following central administration. Nucleic Acids Res. 2021;49(2):657–73. https://doi.org/10.1093/nar/gkaa1235.
Lee JS, Mendell JT. Antisense-Mediated Transcript Knockdown Triggers Premature Transcription Termination. Mol Cell. 2020;77(5):1044-1054.e1043. https://doi.org/10.1016/j.molcel.2019.12.011.
Liang XH, Sun H, Shen W, Wang S, Yao J, Migawa MT, Bui HH, Damle SS, Riney S, Graham MJ. Antisense oligonucleotides targeting translation inhibitory elements in 5’ UTRs can selectively increase protein levels. Nucleic Acids Res. 2017;45(16):9528–46. https://doi.org/10.1093/nar/gkx632.
Nimesh S, Gupta N, Chandra R. Cationic polymer based nanocarriers for delivery of therapeutic nucleic acids. J Biomed Nanotechnol. 2011;7(4):504–20. https://doi.org/10.1166/jbn.2011.1313.
Hayes ME, Drummond DC, Kirpotin DB, Zheng WW, Noble CO, Park JW, Marks JD, Benz CC, Hong K. Genospheres: self-assembling nucleic acid-lipid nanoparticles suitable for targeted gene delivery. Gene Ther. 2006;13(7):646–51. https://doi.org/10.1038/sj.gt.3302699.
Colombani T, Peuziat P, Dallet L, Haudebourg T, Mével M, Berchel M, Lambert O, Habrant D, Pitard B. Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery. J Control Release. 2017;249:131–42. https://doi.org/10.1016/j.jconrel.2017.01.041.
Kulkarni JA, Darjuan MM, Mercer JE, Chen S, van der Meel R, Thewalt JL, Tam YYC, Cullis PR. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano. 2018;12(5):4787–95. https://doi.org/10.1021/acsnano.8b01516.
Eygeris Y, Gupta M, Kim J, Sahay G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc Chem Res. 2022;55(1):2–12. https://doi.org/10.1021/acs.accounts.1c00544.
Huang H, Zhang C, Yang S, Xiao W, Zheng Q, Song X. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J Control Release. 2021;335:449–56. https://doi.org/10.1016/j.jconrel.2021.05.024.
Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R, Banothu AK, Chhabra D, Joshi K, Bharani KK. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38: 101142. https://doi.org/10.1016/j.nantod.2021.101142.
Yu M, Zheng J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano. 2015;9(7):6655–74. https://doi.org/10.1021/acsnano.5b01320.
Li W, Szoka FC Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24(3):438–49. https://doi.org/10.1007/s11095-006-9180-5.
Yang L, Ma F, Liu F, Chen J, Zhao X, Xu Q. Efficient Delivery of Antisense Oligonucleotides Using Bioreducible Lipid Nanoparticles In Vitro and In Vivo. Mol Ther Nucleic Acids. 2020;19:1357–67. https://doi.org/10.1016/j.omtn.2020.01.018.
Li H, Liu Y, Chen L, Liu Q, Qi S, Cheng X, Lee YB, Ahn CH, Kim DJ, Lee RJ. Folate receptor-targeted lipid-albumin nanoparticles (F-LAN) for therapeutic delivery of an Akt1 antisense oligonucleotide. J Drug Target. 2018;26(5–6):466–73. https://doi.org/10.1080/1061186x.2018.1433678.
Cheng X, Yu D, Cheng G, Yung BC, Liu Y, Li H, Kang C, Fang X, Tian S, Zhou X. T7 Peptide-Conjugated Lipid Nanoparticles for Dual Modulation of Bcl-2 and Akt-1 in Lung and Cervical Carcinomas. Mol Pharm. 2018;15(10):4722–32. https://doi.org/10.1021/acs.molpharmaceut.8b00696.
Yuan Y, Zhang L, Cao H, Yang Y, Zheng Y, Yang XJ. A Polyethylenimine-Containing and Transferrin-Conjugated Lipid Nanoparticle System for Antisense Oligonucleotide Delivery to AML. Biomed Res Int. 2016;2016:1287128. https://doi.org/10.1155/2016/1287128.
Guan J, Pan Y, Li H, Zhu Y, Gao Y, Wang J, Zhou Y, Guan Z, Yang Z. Activity and Tissue Distribution of Antisense Oligonucleotide CT102 Encapsulated with Cytidinyl/Cationic Lipid against Hepatocellular Carcinoma. Mol Pharm. 2022. https://doi.org/10.1021/acs.molpharmaceut.2c00026.
Lou G, Anderluzzi G, Schmidt ST, Woods S, Gallorini S, Brazzoli M, Giusti F, Ferlenghi I, Johnson RN, Roberts CW. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: The impact of cationic lipid selection. J Control Release. 2020;325:370–9. https://doi.org/10.1016/j.jconrel.2020.06.027.
Kulkarni JA, Witzigmann D, Leung J, Tam YYC, Cullis PR. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11(45):21733–9. https://doi.org/10.1039/c9nr09347h.
Tanaka H, Takata N, Yoshida Y, Inoue T, Tamagawa S, Nakai Y, Tange K, Yoshioka H, Maeki M. Maeki M. Delivery of Oligonucleotides Using a Self-Degradable Lipid-Like Material. Pharmaceutics. 2021;13(4):544. https://doi.org/10.3390/pharmaceutics13040544.
Sicard G, Paris C, Giacometti S, Rodallec A, Ciccolini J, Rocchi P, Fanciullino R. Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer. Pharmaceutics. 2020;12(12):1166. https://doi.org/10.3390/pharmaceutics12121166.
Kim ST, Lee KM, Park HJ, Jin SE, Ahn WS, Kim CK. Topical delivery of interleukin-13 antisense oligonucleotides with cationic elastic liposome for the treatment of atopic dermatitis. J Gene Med. 2009;11(1):26–37. https://doi.org/10.1002/jgm.1268.
Araújo D, Gaspar R, Mil-Homens D, Henriques M, Silva BFB, Silva S. Cationic lipid-based formulations for encapsulation and delivery of anti-EFG1 2' OMethylRNA oligomer. Med Mycol 2022, 60(5). https://doi.org/10.1093/mmy/myac030
Benizri S, Gaubert A, Soulard C, Gontier É, Svahn I, Rocchi P, Vacher G, Barthélémy P. Hydrogel based lipid-oligonucleotides: a new route to self-delivery of therapeutic sequences. Biomater Sci. 2021;9(10):3638–44. https://doi.org/10.1039/d1bm00273b.
Ma Y, Zhao W, Li Y, Pan Y, Wang S, Zhu Y, Kong L, Guan Z, Wang J, Zhang L. Structural optimization and additional targets identification of antisense oligonucleotide G3139 encapsulated in a neutral cytidinyl-lipid combined with a cationic lipid in vitro and in vivo. Biomaterials. 2019;197:182–93. https://doi.org/10.1016/j.biomaterials.2018.12.033.
Li H, Xu S, Quan J, Yung BC, Pang J, Zhou C, Cho YA, Zhang M, Liu S, Muthusamy N. CD33-Targeted Lipid Nanoparticles (aCD33LNs) for Therapeutic Delivery of GTI-2040 to Acute Myelogenous Leukemia. Mol Pharm. 2015;12(6):2010–8. https://doi.org/10.1021/mp5008212.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–99. https://doi.org/10.2147/ijn.S68861.
Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188. https://doi.org/10.1016/j.addr.2022.114416.
Algarni A, Pilkington EH, Suys EJA, Al-Wassiti H, Pouton CW, Truong NP. In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression. Biomater Sci. 2022;10(11):2940–52. https://doi.org/10.1039/d2bm00168c.
Xia Y, Tian J, Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016;79:56–68. https://doi.org/10.1016/j.biomaterials.2015.11.056.
Friedl JD, Steinbring C, Zaichik S, Le NN, Bernkop-Schnürch A. Cellular uptake of self-emulsifying drug-delivery systems: polyethylene glycol versus polyglycerol surface. Nanomedicine (Lond). 2020;15(19):1829–41. https://doi.org/10.2217/nnm-2020-0127.
Allen RJ, Mathew B, Rice KG. PEG-Peptide Inhibition of Scavenger Receptor Uptake of Nanoparticles by the Liver. Mol Pharm. 2018;15(9):3881–91. https://doi.org/10.1021/acs.molpharmaceut.8b00355.
Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172(1):38–47. https://doi.org/10.1016/j.jconrel.2013.07.026.
Emam SE, Elsadek NE, Abu Lila AS, Takata H, Kawaguchi Y, Shimizu T, Ando H, Ishima Y, Ishida T. Anti-PEG IgM production and accelerated blood clearance phenomenon after the administration of PEGylated exosomes in mice. J Control Release. 2021;334:327–34. https://doi.org/10.1016/j.jconrel.2021.05.001.
Kozma GT, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154–155:163–75. https://doi.org/10.1016/j.addr.2020.07.024.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. https://doi.org/10.1186/1556-276x-8-102.
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–9. https://doi.org/10.1038/sj.clpt.6100400.
Guan J, Guo H, Tang T, Wang Y, Wei Y, Seth P, Li Y, Dehm SM, Ruoslahti E, Pang HB. iRGD-liposomes enhance tumor delivery and therapeutic efficacy of antisense oligonucleotide drugs against primary prostate cancer and bone metastasis. Adv Funct Mater 2021, 31(24). https://doi.org/10.1002/adfm.202100478
Sicard G, Paris C, Giacometti S, Rodallec A, Ciccolini J, Rocchi P, Fanciullino R. Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer. Pharmaceutics 2020, 12(12). https://doi.org/10.3390/pharmaceutics12121166
Zoulikha M, Xiao Q, Boafo GF, Sallam MA, Chen Z, He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sin B. 2022;12(2):600–20. https://doi.org/10.1016/j.apsb.2021.08.009.
Mirzaei S, Gholami MH, Ang HL, Hashemi F, Zarrabi A, Zabolian A, et al. Pre-clinical and clinical applications of Small Interfering RNAs (siRNA) and co-delivery systems for pancreatic cancer therapy. Cells. 2021;10(12):3348. https://doi.org/10.3390/cells10123348.
Xu CF, Iqbal S, Shen S, Luo YL, Yang X, Wang J. Development of “CLAN” Nanomedicine for Nucleic Acid Therapeutics. Small. 2019;15(16): e1900055. https://doi.org/10.1002/smll.201900055.
Chen H, Fang X, Jin Y, Hu X, Yin M, Men X, Chen N, Fan C, Chiu DT, Wan Y. Semiconducting Polymer Nanocavities: Porogenic Synthesis, Tunable Host-Guest Interactions, and Enhanced Drug/siRNA Delivery. Small. 2018;14(21): e1800239. https://doi.org/10.1002/smll.201800239.
Date T, Nimbalkar V, Kamat J, Mittal A, Mahato RI, Chitkara D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J Control Release. 2018;271:60–73. https://doi.org/10.1016/j.jconrel.2017.12.016.
Li M, Li Y, Li S, Jia L, Wang H, Li M, Deng J, Zhu A, Ma L, Li W. The nano delivery systems and applications of mRNA. Eur J Med Chem. 2022;227: 113910. https://doi.org/10.1016/j.ejmech.2021.113910.
Min HS, Kim HJ, Naito M, Ogura S, Toh K, Hayashi K, Kim BS, Fukushima S, Anraku Y, Miyata K. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew Chem Int Ed Engl. 2020;59(21):8173–80. https://doi.org/10.1002/anie.201914751.
Araújo D, Braz J, Dencheva NV, Carvalho I, Henriques M, Denchev ZZ, Malfois M, Silva S. Polyamide Microsized Particulate Polyplex Carriers for the 2’-OMethylRNA EFG1 Antisense Oligonucleotide. ACS Appl Bio Mater. 2021;4(5):4607–17. https://doi.org/10.1021/acsabm.1c00334.
Yang S, Wang D, Sun Y, Zheng B. Delivery of antisense oligonucleotide using polyethylenimine-based lipid nanoparticle modified with cell penetrating peptide. Drug Deliv. 2019;26(1):965–74. https://doi.org/10.1080/10717544.2019.1667453.
Springate CM, Jackson JK, Gleave ME, Burt HM. Clusterin antisense complexed with chitosan for controlled intratumoral delivery. Int J Pharm. 2008;350(1–2):53–64. https://doi.org/10.1016/j.ijpharm.2007.08.018.
Kilicay E, Karahaliloglu Z, Alpaslan P, Hazer B, Denkbas EB. In vitro evaluation of antisense oligonucleotide functionalized core-shell nanoparticles loaded with α-tocopherol succinate. J Biomater Sci Polym Ed. 2017;28(15):1762–85. https://doi.org/10.1080/09205063.2017.1354670.
Li Y, Chen Y, Li J, Zhang Z, Huang C, Lian G, Yang K, Chen S, Lin Y, Wang L. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017;108(7):1493–503. https://doi.org/10.1111/cas.13267.
Zhang Y, Lai L, Liu Y, Chen B, Yao J, Zheng P, Pan Q, Zhu W. Biomineralized Cascade Enzyme-Encapsulated ZIF-8 Nanoparticles Combined with Antisense Oligonucleotides for Drug-Resistant Bacteria Treatment. ACS Appl Mater Interfaces. 2022;14(5):6453–64. https://doi.org/10.1021/acsami.1c23808.
Venuganti VV, Saraswathy M, Dwivedi C, Kaushik RS, Perumal OP. Topical gene silencing by iontophoretic delivery of an antisense oligonucleotide-dendrimer nanocomplex: the proof of concept in a skin cancer mouse model. Nanoscale. 2015;7(9):3903–14. https://doi.org/10.1039/c4nr05241b.
Vaheri A, Pagano JS. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965;27(3):434–6. https://doi.org/10.1016/0042-6822(65)90126-1.
McCutchan JH, Pagano JS. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968;41(2):351–7.
Wang H, Ding S, Zhang Z, Wang L, You Y. Cationic micelle: A promising nanocarrier for gene delivery with high transfection efficiency. J Gene Med. 2019;21(7): e3101. https://doi.org/10.1002/jgm.3101.
Lundy BB, Convertine A, Miteva M, Stayton PS. Neutral polymeric micelles for RNA delivery. Bioconjug Chem. 2013;24(3):398–407. https://doi.org/10.1021/bc300486k.
Pereira P, Barreira M, Queiroz JA, Veiga F, Sousa F, Figueiras A. Smart micelleplexes as a new therapeutic approach for RNA delivery. Expert Opin Drug Deliv. 2017;14(3):353–71. https://doi.org/10.1080/17425247.2016.1214567.
Tamboli V, Mishra GP, Mitrat AK. Polymeric vectors for ocular gene delivery. Ther Deliv. 2011;2(4):523–36. https://doi.org/10.4155/tde.11.20.
Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, Jiang Z, Saltzman WM. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat Mater. 2011;11(1):82–90. https://doi.org/10.1038/nmat3187.
Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57(15):2215–37. https://doi.org/10.1016/j.addr.2005.09.019.
Xie L, Liu R, Chen X, He M, Zhang Y, Chen S. Micelles Based on Lysine, Histidine, or Arginine: Designing Structures for Enhanced Drug Delivery. Front Bioeng Biotechnol. 2021;9: 744657. https://doi.org/10.3389/fbioe.2021.744657.
Ozturk N, Kara A, Gulyuz S, Ozkose UU, Tasdelen MA, Bozkir A, Yilmaz O, Vural I. Exploiting ionisable nature of PEtOx-co-PEI to prepare pH sensitive, doxorubicin-loaded micelles. J Microencapsul. 2020;37(7):467–80. https://doi.org/10.1080/02652048.2020.1792566.
Chen Y, Huang Y, Huang H, Luo Z, Zhang Z, Sun R, Wan Z, Sun J, Lu B, Li S. Farnesylthiosalicylic acid-derivatized PEI-based nanocomplex for improved tumor vaccination. Mol Ther Nucleic Acids. 2021;26:594–602. https://doi.org/10.1016/j.omtn.2021.09.006.
Qiu Z, Huang J, Liu L, Li C, Cohen Stuart MA, Wang J. Effects of pH on the Formation of PIC Micelles from PAMAM Dendrimers. Langmuir. 2020;36(29):8367–74. https://doi.org/10.1021/acs.langmuir.0c00598.
Zhou Z, Guo F, Wang N, Meng M, Li G. Dual pH-sensitive supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int J Biol Macromol. 2018;116:911–9. https://doi.org/10.1016/j.ijbiomac.2018.05.092.
Zhu C, Jung S, Meng F, Zhu X, Park TG, Zhong Z. Reduction-responsive cationic biodegradable micelles based on PDMAEMA-SS-PCL-SS-PDMAEMA triblock copolymers for gene delivery. J Control Release. 2011;152(Suppl 1):e188-190. https://doi.org/10.1016/j.jconrel.2011.08.081.
Whitfield CJ, Zhang M, Winterwerber P, Wu Y, Ng DYW, Weil T. Functional DNA-Polymer Conjugates. Chem Rev. 2021;121(18):11030–84. https://doi.org/10.1021/acs.chemrev.0c01074.
Li J, Men K, Gao Y, Wu J, Lei S, Yang Y, Pan H. Single Micelle Vectors based on Lipid/Block Copolymer Compositions as mRNA Formulations for Efficient Cancer Immunogene Therapy. Mol Pharm. 2021;18(11):4029–45. https://doi.org/10.1021/acs.molpharmaceut.1c00461.
Zhang X, Liu B, Yang Z, Zhang C, Li H, Luo X, Luo H, Gao D, Jiang Q, Liu J. Micelles of enzymatically synthesized PEG-poly(amine-co-ester) block copolymers as pH-responsive nanocarriers for docetaxel delivery. Colloids and Surfaces B: Biointerfaces. 2014;115:349–58. https://doi.org/10.1016/j.colsurfb.2013.12.029.
Loughrey D, Dahlman JE. Non-liver mRNA Delivery. Acc Chem Res. 2022;55(1):13–23. https://doi.org/10.1021/acs.accounts.1c00601.
Zhang D, Atochina-Vasserman EN, Lu J, Maurya DS, Xiao Q, Liu M, Adamson J, Ona N, Reagan EK, Ni H. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity. J Am Chem Soc. 2022;144(11):4746–53. https://doi.org/10.1021/jacs.2c00273.
Beha MJ, Ryu JS, Kim YS, Chung HJ. Delivery of antisense oligonucleotides using multi-layer coated gold nanoparticles to methicillin-resistant S. aureus for combinatorial treatment. Mater Sci Eng C Mater Biol Appl. 2021;126:112167. https://doi.org/10.1016/j.msec.2021.112167.
Yoshida S, Duong C, Oestergaard M, Fazio M, Chen C, Peralta R, Guo S, Seth PP, Li Y, Beckett L. MXD3 antisense oligonucleotide with superparamagnetic iron oxide nanoparticles: A new targeted approach for neuroblastoma. Nanomedicine. 2020;24: 102127. https://doi.org/10.1016/j.nano.2019.102127.
Jiang K, Chen J, Tai L, Liu C, Chen X, Wei G, Lu W, Pan W. Inhibition of post-trabeculectomy fibrosis via topically instilled antisense oligonucleotide complexes co-loaded with fluorouracil. Acta Pharm Sin B. 2020;10(9):1754–68. https://doi.org/10.1016/j.apsb.2020.03.002.
Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv Sci (Weinh). 2021;8(10):2003937. https://doi.org/10.1002/advs.202003937.
Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Ther Deliv. 2015;6(1):41–58. https://doi.org/10.4155/tde.14.91.
Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. Int J Pharm. 2012;427(1):3–20. https://doi.org/10.1016/j.ijpharm.2011.07.013.
Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol Ther. 2019;27(4):710–28. https://doi.org/10.1016/j.ymthe.2019.02.012.
Piotrowski-Daspit AS, Kauffman AC, Bracaglia LG, Saltzman WM. Polymeric vehicles for nucleic acid delivery. Adv Drug Deliv Rev. 2020;156:119–32. https://doi.org/10.1016/j.addr.2020.06.014.
Bishop CJ, Abubaker-Sharif B, Guiriba T, Tzeng SY, Green JJ. Gene delivery polymer structure-function relationships elucidated via principal component analysis. Chem Commun (Camb). 2015;51(60):12134–7. https://doi.org/10.1039/c5cc04417k.
Karlsson J, Rhodes KR, Green JJ, Tzeng SY. Poly(beta-amino ester)s as gene delivery vehicles: challenges and opportunities. Expert Opin Drug Deliv. 2020;17(10):1395–410. https://doi.org/10.1080/17425247.2020.1796628.
Kaczmarek JC, Patel AK, Rhym LH, Palmiero UC, Bhat B, Heartlein MW, DeRosa F, Anderson DG. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials. 2021;275: 120966. https://doi.org/10.1016/j.biomaterials.2021.120966.
Radchatawedchakoon W, Krajarng A, Niyomtham N, Watanapokasin R, Yingyongnarongkul BE. High transfection efficiency of cationic lipids with asymmetric acyl-cholesteryl hydrophobic tails. Chemistry. 2011;17(11):3287–95. https://doi.org/10.1002/chem.201001622.
Puchkov PA, Maslov MA. Lipophilic Polyamines as promising components of Liposomal gene delivery systems. Pharmaceutics. 2021;13(6):920. https://doi.org/10.3390/pharmaceutics13060920.
Kauffman AC, Piotrowski-Daspit AS, Nakazawa KH, Jiang Y, Datye A, Saltzman WM. Tunability of Biodegradable Poly(amine- co-ester) Polymers for Customized Nucleic Acid Delivery and Other Biomedical Applications. Biomacromol. 2018;19(9):3861–73. https://doi.org/10.1021/acs.biomac.8b00997.
Cui J, Piotrowski-Daspit AS, Zhang J, Shao M, Bracaglia LG, Utsumi T, Seo YE, DiRito J, Song E, Wu C. Poly(amine-co-ester) nanoparticles for effective Nogo-B knockdown in the liver. J Control Release. 2019;304:259–67. https://doi.org/10.1016/j.jconrel.2019.04.044.
Chen Z, Lichtor PA, Berliner AP, Chen JC, Liu DR. Evolution of sequence-defined highly functionalized nucleic acid polymers. Nat Chem. 2018;10(4):420–7. https://doi.org/10.1038/s41557-018-0008-9.
Kim J, Vaughan HJ, Zamboni CG, Sunshine JC, Green JJ. High-throughput evaluation of polymeric nanoparticles for tissue-targeted gene expression using barcoded plasmid DNA. J Control Release. 2021;337:105–16. https://doi.org/10.1016/j.jconrel.2021.05.047.
Wang Y, Luo J, Truebenbach I, Reinhard S, Klein PM, Höhn M, Kern S, Morys S, Loy DM, Wagner E. Double Click-Functionalized siRNA Polyplexes for Gene Silencing in Epidermal Growth Factor Receptor-Positive Tumor Cells. ACS Biomater Sci Eng. 2020;6(2):1074–89. https://doi.org/10.1021/acsbiomaterials.9b01904.
Benner NL, McClellan RL, Turlington CR, Haabeth OAW, Waymouth RM, Wender PA. Oligo(serine ester) Charge-Altering Releasable Transporters: Organocatalytic Ring-Opening Polymerization and their Use for in Vitro and in Vivo mRNA Delivery. J Am Chem Soc. 2019;141(21):8416–21. https://doi.org/10.1021/jacs.9b03154.
Curtin CM, Tierney EG, McSorley K, Cryan SA, Duffy GP, O’Brien FJ. Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv Healthc Mater. 2015;4(2):223–7. https://doi.org/10.1002/adhm.201400397.
Zhang R, Jing W, Chen C, Zhang S, Abdalla M, Sun P, Wang G, You W, Yang Z, Zhang J. Inhaled mRNA Nanoformulation with Biogenic Ribosomal Protein Reverses Established Pulmonary Fibrosis in a Bleomycin-Induced Murine Model. Adv Mater 2022:e2107506. https://doi.org/10.1002/adma.202107506
Zhang X, Qin B, Wang M, Feng J, Zhang C, Zhu C, He S, Liu H, Wang Y, Averick SE. Dual pH-Responsive and Tumor-Targeted Nanoparticle-Mediated Anti-Angiogenesis siRNA Delivery for Tumor Treatment. Int J Nanomedicine. 2022;17:953–67. https://doi.org/10.2147/ijn.S340926.
Liu C, Tang C, Yin C. Co-delivery of doxorubicin and siRNA by all-trans retinoic acid conjugated chitosan-based nanocarriers for multiple synergistic antitumor efficacy. Carbohydr Polym. 2022;283: 119097. https://doi.org/10.1016/j.carbpol.2022.119097.
Leng Q, Chen L, Lv Y. RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics. 2020;10(7):3190–205. https://doi.org/10.7150/thno.42640.
Badieyan ZS, Berezhanskyy T, Utzinger M, Aneja MK, Emrich D, Erben R, Schüler C, Altpeter P, Ferizi M, Hasenpusch G. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J Control Release. 2016;239:137–48. https://doi.org/10.1016/j.jconrel.2016.08.037.
Sargazi S, Siddiqui B, Qindeel M, Rahdar A, Bilal M, Behzadmehr R, Mirinejad S, Pandey S. Chitosan nanocarriers for microRNA delivery and detection: A preliminary review with emphasis on cancer. Carbohydr Polym. 2022;290: 119489. https://doi.org/10.1016/j.carbpol.2022.119489.
Shanmuganathan R, Edison T, LewisOscar F, Kumar P, Shanmugam S, Pugazhendhi A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int J Biol Macromol. 2019;130:727–36. https://doi.org/10.1016/j.ijbiomac.2019.02.060.
Negm NA, Hefni HHH, Abd-Elaal AAA, Badr EA, Abou Kana MTH. Advancement on modification of chitosan biopolymer and its potential applications. Int J Biol Macromol. 2020;152:681–702. https://doi.org/10.1016/j.ijbiomac.2020.02.196.
Kolonko AK, Bangel-Ruland N, Goycoolea FM, Weber WM. Chitosan Nanocomplexes for the delivery of ENaC Antisense Oligonucleotides to airway Epithelial cells. Biomolecules. 2020;10(4):553. https://doi.org/10.3390/biom10040553.
Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17(3):160–72. https://doi.org/10.1038/nrn.2015.29.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902.
Tang M, Chen Y, Li B, Sugimoto H, Yang S, Yang C, LeBleu VS, McAndrews KM, Kalluri R. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. Faseb j. 2021;35(5): e21557. https://doi.org/10.1096/fj.202002777RR.
Yang J, Luo S, Zhang J, Yu T, Fu Z, Zheng Y, Xu X, Liu C, Fan M, Zhang Z. Exosome-mediated delivery of antisense oligonucleotides targeting alpha-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol Dis. 2021;148: 105218. https://doi.org/10.1016/j.nbd.2020.105218.
Grossen P, Portmann M, Koller E, Duschmalé M, Minz T, Sewing S, Pandya NJ, van Geijtenbeek SK, Ducret A, Kusznir EA. Evaluation of bovine milk extracellular vesicles for the delivery of locked nucleic acid antisense oligonucleotides. Eur J Pharm Biopharm. 2021;158:198–210. https://doi.org/10.1016/j.ejpb.2020.11.012.
Atkin-Smith GK, Poon IKH. Disassembly of the Dying: Mechanisms and Functions. Trends Cell Biol. 2017;27(2):151–62. https://doi.org/10.1016/j.tcb.2016.08.011.
Wang Y, Pang J, Wang Q, Yan L, Wang L, Xing Z, Wang C, Zhang J, Dong L. Delivering Antisense Oligonucleotides across the Blood-Brain Barrier by Tumor Cell-Derived Small Apoptotic Bodies. Adv Sci (Weinh). 2021;8(13):2004929. https://doi.org/10.1002/advs.202004929.
Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol Pharm. 2015;12(10):3650–7. https://doi.org/10.1021/acs.molpharmaceut.5b00364.
Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell Mol Bioeng. 2016;9(3):315–24. https://doi.org/10.1007/s12195-016-0457-4.
Liu L, Bai X, Martikainen MV, Kårlund A, Roponen M, Xu W, Hu G, Tasciotti E, Lehto VP. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat Commun. 2021;12(1):5726. https://doi.org/10.1038/s41467-021-26052-x.
Parodi A, Molinaro R, Sushnitha M, Evangelopoulos M, Martinez JO, Arrighetti N, Corbo C, Tasciotti E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–68. https://doi.org/10.1016/j.biomaterials.2017.09.020.
Somiya M, Kuroda S. Development of a virus-mimicking nanocarrier for drug delivery systems: The bio-nanocapsule. Adv Drug Deliv Rev. 2015;95:77–89. https://doi.org/10.1016/j.addr.2015.10.003.
Kim MG, Park JY, Shim G, Choi HG, Oh YK. Biomimetic DNA nanoballs for oligonucleotide delivery. Biomaterials. 2015;62:155–63. https://doi.org/10.1016/j.biomaterials.2015.04.037.
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res. 2016;33(10):2373–87. https://doi.org/10.1007/s11095-016-1958-5.
Neha D, Momin M, Khan T, Gharat S, Ningthoujam RS, Omri A. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin Drug Deliv. 2021;18(9):1261–90. https://doi.org/10.1080/17425247.2021.1912008.
Alphandéry E. Natural Metallic Nanoparticles for Application in Nano-Oncology. Int J Mol Sci 2020, 21(12). https://doi.org/10.3390/ijms21124412
Lee SWL, Paoletti C, Campisi M, Osaki T, Adriani G, Kamm RD, Mattu C, Chiono V. MicroRNA delivery through nanoparticles. J Control Release. 2019;313:80–95. https://doi.org/10.1016/j.jconrel.2019.10.007.
Graczyk A, Pawlowska R, Chworos A. Gold Nanoparticles as Carriers for Functional RNA Nanostructures. Bioconjug Chem. 2021;32(8):1667–74. https://doi.org/10.1021/acs.bioconjchem.1c00211.
Gong N, Teng X, Li J, Liang XJ. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. ACS Appl Mater Interfaces. 2019;11(1):37–42. https://doi.org/10.1021/acsami.8b18288.
Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–3. https://doi.org/10.1126/science.364652.
Na K, Sethuraman VT, Bae YH. Stimuli-sensitive polymeric micelles as anticancer drug carriers. Anticancer Agents Med Chem. 2006;6(6):525–35. https://doi.org/10.2174/187152006778699068.
Huang Z, Xu H, Meyers AD, Musani AI, Wang L, Tagg R, Barqawi AB, Chen YK. Photodynamic therapy for treatment of solid tumors–potential and technical challenges. Technol Cancer Res Treat. 2008;7(4):309–20. https://doi.org/10.1177/153303460800700405.
Cheng X, Gao J, Ding Y, Lu Y, Wei Q, Cui D, Fan J, Li X, Zhu E, Lu Y. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv Sci (Weinh). 2021;8(16): e2100876. https://doi.org/10.1002/advs.202100876.
Sun W, Zhao X, Fan J, Du J, Peng X. Boron Dipyrromethene Nano-Photosensitizers for Anticancer Phototherapies. Small. 2019;15(32): e1804927. https://doi.org/10.1002/smll.201804927.
Matsushita-Ishiodori Y, Ohtsuki T. Photoinduced RNA interference. Acc Chem Res. 2012;45(7):1039–47. https://doi.org/10.1021/ar200227n.
Chen L, Li G, Wang X, Li J, Zhang Y. Spherical Nucleic Acids for Near-Infrared Light-Responsive Self-Delivery of Small-Interfering RNA and Antisense Oligonucleotide. ACS Nano. 2021;15(7):11929–39. https://doi.org/10.1021/acsnano.1c03072.
Fliervoet LAL, Zhang H, van Groesen E, Fortuin K, Duin N, Remaut K, Schiffelers RM, Hennink WE, Vermonden T. Local release of siRNA using polyplex-loaded thermosensitive hydrogels. Nanoscale. 2020;12(18):10347–60. https://doi.org/10.1039/d0nr03147j.
Shi Z, Li SK, Charoenputtakun P, Liu CY, Jasinski D, Guo P. RNA nanoparticle distribution and clearance in the eye after subconjunctival injection with and without thermosensitive hydrogels. J Control Release. 2018;270:14–22. https://doi.org/10.1016/j.jconrel.2017.11.028.
Wang C, Wang X, Du L, Dong Y, Hu B, Zhou J, Shi Y, Bai S, Huang Y, Cao H. Harnessing pH-Sensitive Polycation Vehicles for the Efficient siRNA Delivery. ACS Appl Mater Interfaces. 2021;13(2):2218–29. https://doi.org/10.1021/acsami.0c17866.
Wang P, Yin T, Li J, Zheng B, Wang X, Wang Y, Zheng J, Zheng R, Shuai X. Ultrasound-responsive microbubbles for sonography-guided siRNA delivery. Nanomedicine. 2016;12(4):1139–49. https://doi.org/10.1016/j.nano.2015.12.361.
Du XJ, Wang ZY, Wang YC. Redox-sensitive dendrimersomes assembled from amphiphilic Janus dendrimers for siRNA delivery. Biomater Sci. 2018;6(8):2122–9. https://doi.org/10.1039/c8bm00491a.
Dalmina M, Pittella F, Sierra JA, Souza GRR, Silva AH, Pasa AA, Creczynski-Pasa TB. Magnetically responsive hybrid nanoparticles for in vitro siRNA delivery to breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2019;99:1182–90. https://doi.org/10.1016/j.msec.2019.02.026.
Wang Y, Yu RZ, Henry S, Geary RS. Pharmacokinetics and Clinical Pharmacology Considerations of GalNAc(3)-Conjugated Antisense Oligonucleotides. Expert Opin Drug Metab Toxicol. 2019;15(6):475–85. https://doi.org/10.1080/17425255.2019.1621838.
Craig K, Abrams M, Amiji M. Recent preclinical and clinical advances in oligonucleotide conjugates. Expert Opin Drug Deliv. 2018;15(6):629–40. https://doi.org/10.1080/17425247.2018.1473375.
Yu RZ, Gunawan R, Post N, Zanardi T, Hall S, Burkey J, Kim TW, Graham MJ, Prakash TP, Seth PP. Disposition and Pharmacokinetics of a GalNAc3-Conjugated Antisense Oligonucleotide Targeting Human Lipoprotein (a) in Monkeys. Nucleic Acid Ther. 2016;26(6):372–80. https://doi.org/10.1089/nat.2016.0623.
Kay E, Stulz R, Becquart C, Lovric J, Tängemo C, Thomen A, Baždarević D, Najafinobar N, Dahlén A, Pielach A. NanoSIMS Imaging Reveals the Impact of Ligand-ASO Conjugate Stability on ASO Subcellular Distribution. Pharmaceutics 2022, 14(2). https://doi.org/10.3390/pharmaceutics14020463
Echevarría L, Goyenvalle A. Preclinical Evaluation of the Renal Toxicity of Oligonucleotide Therapeutics in Mice. Methods in molecular biology (Clifton, NJ). 2022;2434:371–84. https://doi.org/10.1007/978-1-0716-2010-6_26.
Migliorati JM, Liu S, Liu A, Gogate A, Nair S. Bahal R Rasmussen TP Manautou JE Zhong X-b. Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs Drug Metabolism and Disposition. 2022;50(6):888–97. https://doi.org/10.1124/dmd.121.000417.
Nishi R, Ohyagi M, Nagata T, Mabuchi Y, Yokota T. Regulation of activated microglia and macrophages by systemically administered DNA/RNA heteroduplex oligonucleotides. Mol Ther. 2022;30(6):2210–23. https://doi.org/10.1016/j.ymthe.2022.02.019.
Packard BZ, Wrightson JA Jr, Komoriya A. An Oligonucleotide Delivery Platform to Enable Assessment of Intracellular Transcripts in Live Cells by Flow Cytometry. Cytometry A. 2020;97(9):945–54. https://doi.org/10.1002/cyto.a.24174.
Liang XH, Nichols JG, De Hoyos CL, Sun H, Zhang L, Crooke ST. Golgi-58K can re-localize to late endosomes upon cellular uptake of PS-ASOs and facilitates endosomal release of ASOs. Nucleic Acids Res. 2021;49(14):8277–93. https://doi.org/10.1093/nar/gkab599.
Crooke ST, Baker BF, Kwoh TJ, Cheng W, Schulz DJ, Xia S, Salgado N, Bui HH, Hart CE, Burel SA. Integrated Safety Assessment of 2’-O-Methoxyethyl Chimeric Antisense Oligonucleotides in NonHuman Primates and Healthy Human Volunteers. Mol Ther. 2016;24(10):1771–82. https://doi.org/10.1038/mt.2016.136.
Chi X, Gatti P, Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discovery Today. 2017;22(5):823–33. https://doi.org/10.1016/j.drudis.2017.01.013.
Huang L, Low A, Damle SS, Keenan MM, Kuntz S, Murray SF, Monia BP, Guo S. Antisense suppression of the nonsense mediated decay factor Upf3b as a potential treatment for diseases caused by nonsense mutations. Genome Biol. 2018;19(1):4. https://doi.org/10.1186/s13059-017-1386-9.
Bennett CF. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu Rev Med. 2019;70:307–21. https://doi.org/10.1146/annurev-med-041217-010829.
Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. 2020;382(3):244–55. https://doi.org/10.1056/NEJMoa1905239.
Sreeharsha N, Chitrapriya N, Jang YJ, Kenchappa V. Evaluation of nanoparticle drug-delivery systems used in preclinical studies. Ther Deliv. 2021;12(4):325–36. https://doi.org/10.4155/tde-2020-0116.
Bae YH, Park K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv Drug Deliv Rev. 2020;158:4–16. https://doi.org/10.1016/j.addr.2020.06.018.
Harloff-Helleberg S, Nielsen LH, Nielsen HM. Animal models for evaluation of oral delivery of biopharmaceuticals. J Control Release. 2017;268:57–71. https://doi.org/10.1016/j.jconrel.2017.09.025.
Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philosophy. Ethics, and Humanities in Medicine. 2009;4(1):2. https://doi.org/10.1186/1747-5341-4-2.
Xu S, Yang K, Li R, Zhang L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int J Mol Sci 2020, 21(18). https://doi.org/10.3390/ijms21186582
Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951–67. https://doi.org/10.1038/s41551-021-00698-w.
Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine (Lond). 2019;14(1):93–126. https://doi.org/10.2217/nnm-2018-0120.
Acknowledgements
Not applicable.
Funding
The work was supported by the Research funds of Natural Science Foundation of Hunan Province (No. 2020JJ9009, S.L.) and the Hunan Provincial Science and Technology Plan (No. 2016TP2002).
Author information
Authors and Affiliations
Contributions
S.H. and S.L. planned the manuscript. S.H. wrote and coordinated the draft. X.H., Y.L., J.W., and D.X. reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, S., Hao, XY., Li, YJ. et al. Nonviral delivery systems for antisense oligonucleotide therapeutics. Biomater Res 26, 49 (2022). https://doi.org/10.1186/s40824-022-00292-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40824-022-00292-4