Abstract
Background
Neuromuscular blockade agents (NMBAs) can be used to facilitate mechanical ventilation in critically ill patients. Accumulating evidence has shown that NMBAs may be associated with intensive care unit (ICU)-acquired weakness and poor outcomes. However, the long-term impact of NMBAs on mortality is still unclear.
Methods
We conducted a retrospective analysis using the 2015–2019 critical care databases at Taichung Veterans General Hospital, a referral center in central Taiwan, as well as the Taiwan nationwide death registry profile.
Results
A total of 5709 ventilated patients were eligible for further analysis, with 63.8% of them were male. The mean age of enrolled subjects was 67.8 ± 15.8 years, and the one-year mortality was 48.3% (2755/5709). Compared with the survivors, the non-survivors had a higher age (70.4 ± 14.9 vs 65.4 ± 16.3, p < 0.001), Acute Physiology and Chronic Health Evaluation II score (28.0 ± 6.2 vs 24.7 ± 6.5, p < 0.001), a longer duration of ventilator use (12.6 ± 10.6 days vs 7.8 ± 8.5 days, p < 0.001), and were more likely to receive NMBAs for longer than 48 h (11.1% vs 7.8%, p < 0.001). After adjusting for age, sex, and relevant covariates, the use of NMBAs for longer than 48 h was found to be independently associated with an increased risk of mortality (adjusted HR: 1.261; 95% CI: 1.07–1.486). The analysis of effect modification revealed that this association was tended to be strong in patients with a Charlson Comorbidity Index of 3 or higher.
Conclusions
Our study demonstrated that prolonged use of NMBAs was associated with an increased risk of long-term mortality in critically ill patients requiring mechanical ventilation. Further studies are needed to validate our findings.
Similar content being viewed by others
Background
Mechanical ventilation (MV) is a relevant organ support system and is increasingly used in intensive care units (ICUs) worldwide [1,2,3]. Asynchrony is common in critically ill patients requiring MV and consists of reverse triggering [4] with breath stacking. This can potentially cause diaphragmatic injury, especially in patients with acute respiratory distress syndrome (ARDS) [5]. The use of neuromuscular blockade agents (NMBAs) has been found to reduce asynchrony and short-term mortality in patients with moderate-to-severe ARDS [6, 7].
However, increasing evidence has identified that the use of NMBAs may lead to deleterious impacts, including disuse atrophy of the diaphragm and ICU-acquired weakness [8,9,10]. The conflicting evidence regarding the role of NMBAs highlights the crucial need to investigate the long-term impact of NMBAs on mortality in critically ill ventilated patients.
In the present study, we linked data from the Taiwanese National Health Insurance Research Database (NHIRD) and the critical care database at Taichung Veterans General Hospital (TCVGH) to examine the relationship between the use of NMBA and long-term mortality in critically ill ventilated patients. We hypothesized that the infusion of NMBA would associate with a higher one-year mortality rates in this group of patients.
Part of the results from the current study have been accepted as an abstract for a poster presentation at the 2023 ATS International Conference (May 19–24, 2023, Washington, DC).
Methods
Subjects and data collection
We conducted a retrospective cohort study at TCVGH, a tertiary care teaching hospital located in central Taiwan with approximately 1500 beds. We collected data on consecutive adult patients who were admitted to the medical ICUs and received MV between 2015 and 2020. Patients who used NMBAs continuously for more than 8 h within the first 72 h of ICU admission were included in the analysis. We considered the recent clinical practice guidelines and selected a time frame of 48 h for patient grouping [11,12,13].
Demographic characteristics of the patients included in the study, such as age, sex, Charlson Comorbidity Index (CCI), etiology for ICU admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) scores, laboratory data, and ventilatory parameters of mechanical ventilation, were obtained from the TCVGH clinical data warehouse. We obtained the discharged patients’ date of death from the nationwide death registration profile of NHIRD in Taiwan.
We excluded patients who died within 72 h after ICU admission to ensure that we had complete data for the subjects enrolled in this study. In patients with more than two ICU admissions, we defined the first ICU admission as the index ICU admission.
Outcome
The primary outcome of interest was the time to one-year all-cause mortality following admission to the ICU. Given that the National Health Insurance (NHI), a compulsory program, has been implemented in Taiwan since 1995, with nearly 99.9% coverage of the population in 2019 [14], the date of death among enrolled patients in this study should be accurate.
Statistical analyses
Data for categorical variables are presented as numbers (percentages), while data for continuous variables are shown as means ± standard deviation. Kaplan–Meier analysis was conducted to analyze the association between long-term mortality and the use of NMBA. We conducted a Cox proportional hazards model to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for one-year all-cause mortality after adjustment for potential confounders such as age, gender, CCI, etc. Statistical analyses were two-sided, and the level of significance was set at 0.05.
We also utilized the Wald test to examine the modification effect by covariates, to assess whether the magnitude of association between the use of NMBAs and long-term mortality differed based on clinical variables such as age, sex, body mass index (BMI), CCI, APACHE II, FIO2 (fraction of inspired oxygen), and PEEP (positive end-expiratory pressure).
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of TCVGH with a waiver of informed consent since it was a retrospective analysis of anonymous data (IRB number: SE20249B).
Results
Patient enrollment and characteristics
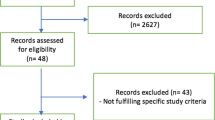
Figure 1 shows the process of patient enrollment. A total of 27,619 patients who were admitted to the ICUs from January 2015 to December 2019 were included. Given that we focused on critically ill patients receiving mechanical ventilation for more than 2 days in medical ICUs, we excluded the patients who were admitted to the surgical ICU (n = 1634), underwent cardiovascular surgery (n = 5560), and those who died within 3 days after admission (n = 511). A total of 5709 patients were eligible for further analysis, with their baseline characteristics summarized in Table 1.
The mean age of the enrolled patients was 67.8 ± 15.8 years, and 63.8% of them were men. The most common etiology for ICU admission was acute respiratory failure (n = 2469, 43.2%), followed by pneumonia (n = 658, 11.5%) and sepsis other than pneumonia (n = 562, 9.8%). Regarding the severity of the patients, the mean APACHE II score was 26.4 ± 6.6, and the mean SOFA score was 8.5 ± 3.6 on the first day of ICU admission. The overall in-hospital mortality rate was 27.6%, with 90-day and 1-year mortality rates of 34.2% and 48.3%, respectively. The 1-year mortality rate of patients who received NMBAs infusion for more than 48 h was 57.4%.
We further divided critically ill ventilated patients based on their 1-year mortality. As compared to the survivors, non-survivors were older and more frequently male. They were also more commonly admitted due to acute respiratory failure or pneumonia. The non-survivor also had a higher CCI, APACHE II score, and day-1/3/7 SOFA scores, as well as a lower BMI than those in the survivor group.
The use of NMBAs and ventilatory parameters among enrolled patients
The mean ventilator days of the included patients was 10.1 ± 9.8 days, while the mean FIO2 at ICU admission was 50.7 ± 21.0%, and the mean PEEP was 7.9 ± 4.6 (cmH2O). Non-survivors had a higher FIO2 (48.9 ± 20.2% vs 52.4 ± 22.0%, p < 0.001) and a higher PEEP (7.6 ± 4.2 cmH2O vs 8.2 ± 4.9 cmH2O, p < 0.001) at ICU admission. On the other hand, the survivors were more likely to have shorter ventilator days (7.8 ± 8.5 vs 12.6 ± 10.6 days, p < 0.001).
In terms of the use of NMBAs, 13.4% (n = 765) of the enrolled patients underwent NMBA treatment for equal or less than 48 h, while 9.37% (n = 535) of them received infusions for more than 48 h. In contrast to non-survivors, a higher number of survivors did not receive NMBAs infusion during ICU admission.
Association between the use of NMBAs and long-term mortality in critically ill ventilated patients
We conducted Kaplan–Meier analyses to demonstrate the association between mortality and the use of NMBAs, categorized as none, use for equal or less than 48 h, and use for longer than 48 h (Fig. 2). The multivariable Cox proportional hazard regression model found that age, gender (male), high CCI, high APACHE II, lower platelet count, high blood lactate level, and high demand for FIO2 (≥ 50%) were independently associated with mortality. Conversely, a high BMI was found to be a protective factor for long-term mortality in these patients (Table 2). We found that the use of NMBA for more than 48 h was independently associated with a higher 1-year mortality (HR: 1.261; 95% CI: 1.07–1.486) after adjusting for relevant covariates, including age, sex, BMI, comorbidities, APACHE II score, blood platelet and lactate level, etiologies of ICU admission, high demand for FIO2 (≥ 50%), PaO2 and high PEEP (≥ 8 cmH2O). The use of NMBAs for equal or less than 48 h appeared to be associated with long-term mortality, but this correlation became insignificant after adjusting for covariates.
To further investigate the potentially distinct magnitudes of the mortality impact of NMBAs among different patient subgroups, we conducted an analysis of effect modification. We found that the association between the use of NMBAs and mortality appeared to be stronger in patients with a CCI equal to or higher than 3 (Table 3).
Discussion
In the current study, we found that the use of NMBAs for more than 48 h may be associated with a higher 1-year mortality rate in critically ill medical patients requiring mechanical ventilation. Moreover, we noted that the mortality impact of NMBAs tends to be stronger in critically ill patients with multiple comorbidities. These findings provide clinical evidence regarding the prolonged detrimental impact of using NMBAs in critically ill ventilated medical patients.
Notably, the clinical evidence of the role of NMBAs varies depending on the study period. Two landmark randomized controlled trials, ACURASY [6] and ROSE [15] trials, were conducted to clarify the mortality benefit of using NMBAs on patients with ARDS. The ACURASYS trial found that in patients with ARDS and a PaO2:FIO2 ratio less than 150, early administration of NMBAs for 48 h improved the 90-day mortality. The ROSE trial, in contrast, found no difference in hospital mortality among ARDS patients with or without NMBAs. The conflicting results from these two extensive randomized controlled trials may be attributed to differences in patient enrollment and treatment approaches, including variations in PEEP level and depth of sedation. Therefore, the current international guidelines [11,12,13] suggested use of NMBAs in selected conditions. For example, they may be used in moderate-to-severe ARDS patients who required continuous deep sedation or to manage overt shivering in patients needing therapeutic hypothermia. The real-world finding from our study further provides clinical evidence of prolonged impact of the use of NMBAs.
The long-term outcome among patients receiving mechanical ventilation in ICU had been a topic of interest for years. The patients who required mechanical ventilation had a higher ICU mortality up to 17–28% [16, 17]. What is more, the use of mechanical ventilation also impacts long-term function and mortality, and those patients who required prolonged ventilation had considerably high long-term mortality [18,19,20]. Similarly, in the current study, additional 20.7% of the included patient died within 1 year after discharge, and patients who received NMBAs infusion for more than 48 h had an even higher 1-year mortality rate. In the case of ARDS, studies have shown a 1-year mortality rate range from 11% to 58% [21,22,23]. One Canadian multihospital cohort reported a 3-year mortality rate of 49.3% [24]. Another multihospital study from Baltimore found that 34% of the ARDS survivors died within 5 years after discharge [25]. Worsened long-term quality of life and reduced physical function were found among ARDS survivors as well [26]. Several studies had been carried out to examine the impact of NMBAs on long-term outcome of ARDS patients. Three meta-analyses [27,28,29] including previously published RCTs (randomized controlled trials), included the 2 large RCTs (ACURASYS and ROSE) mentioned above, had not found that NMBA infusion could improve 90-day mortality. Li Bassi, G. et al. conducted propensity score analysis using the dataset from a multinational multicenter cohort to investigate the effect of NMBA infusion in COVID-19 (coronavirus disease 2019) ARDS patients [30]. They reported that no association between 90-day mortality and with NMBA use (median duration: 6 days) was found after adjusting for covariates.
The long-lasting impact of NMBAs use may be associated with a number of potential mechanisms, including ICU-acquired weakness and diaphragm disuse atrophy. Prior studies have found that the presence of ICU-acquired weakness in ICU patients is associated with both short-term [31] and long-term mortality up to 5 years [25, 32]. A number of studies have shown the association between the use of NMBAs and the risk of developing ICU-acquired weakness. Price DR et al., in their analysis of 2,254 critically ill patients from 18 studies, found that the use of NMBAs modestly associated with the development of neuromuscular dysfunction in critical illness (odds ratio 1.25; 95% CI 1.06–1.48) [32]. Another meta-analysis conducted by T Yang et al. showed that the use of NMBAs had a significant association with ICU-acquired weakness [33]. Diaphragm atrophy had been reported within 24 h of mechanical ventilation and may related to worse outcomes, including prolonged ICU admission and a lower probability of weaning from the ventilator [34, 35]. However, there was a scarcity of real-world clinical data regarding the impact of NMBA on diaphragm function. Animal models found varied effects on diaphragm function with two NMBAs: while rocuronium exacerbated diaphragm weakness, cisatracurium did not demonstrate the same effects [36]. To sum up, further studies are needed to determine whether the use of NMBAs could potentially contribute to the development of ICU-acquired weakness and diaphragm dysfunction, and subsequently affect long-term outcomes.
In our study, we found that the use of NMBA for longer than 48 h was associated with increased 1-year mortality in critically ill ventilated patients. Furthermore, the analysis of effect modification showed that the strength of association between the use of NMBAs and poor long-term outcomes appears to be higher among patients with more comorbidities (CCI higher or equal to 3). Previously, multiple comorbidities had been considered as a risk factor for ICU-acquired weakness and increased mortality in ventilated patients [19, 37]. Similar to our finding, Pfoh ER et al. conducted a multihospital prospective cohort study and found that comorbidities were associated with a greater long-term physical decline in 193 ARDS survivors [38]. Clinicians should exercise caution when using NMBAs for an extended period in patients with multiple comorbidities, considering the existing data.
The current study has several limitations. First, this study was conducted at a single center, so the findings may not be applicable to other healthcare systems. However, the data were obtained from daily critical care, and the drawback of generalization should be partly reduced. Second, we excluded the patients who admitted to surgical ICU. Inevitably this might further mitigate the generalizability. But by excluding the patients who required post-operative care, we could possibly enhance the homogeneity of studied population. Third, some unmeasured cofounders, such as use of corticosteroid, measure of ICU-acquired weakness and rehabilitation, may exist in this study. Fourth, the dose of the NMBA was not recorded in the databases used in the current study, it is plausible that the dose of NMBAs may affects the outcomes as well. Fifth, the assessment related to diaphragmatic functions was not performed in the facility. Thus, we could not quantify the impacts of NMBAs on diaphragm from the current data.
Conclusion
In the present study, we found that the administration of NMBAs for more than 48 h was associated with an increase in 1-year mortality among mechanically ventilated medical patients. Our findings highlight the risks of prolonged use of NMBAs in critical care patients. Further studies are warranted to clarify the possible mechanism and verified our findings.
Availability of data and materials
The data underlying this article will be shared on request to the corresponding author.
Abbreviations
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- ARDS:
-
Acute respiratory distress syndrome
- BMI:
-
Body mass index
- CCI:
-
Charlson Comorbidity Index
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- FIO2 :
-
Fraction of inspired oxygen
- HR:
-
Hazard ratio
- ICU:
-
Intensive care unit
- NHI:
-
National Health Insurance
- NHIRD:
-
National Health Insurance Research Database
- NMBA:
-
Neuromuscular blocking agent
- PaO2 :
-
Partial pressure of arterial oxygen
- PaO22FIO2 ratio:
-
Ratio of partial pressure of arterial oxygen to inspired oxygen fraction
- RCT:
-
Randomized controlled trial
- SOFA:
-
Sequential Organ Failure Assessment
- TCVGH:
-
Taichung Veterans General Hospital
References
Zilberberg MD, Nathanson BH, Ways J, Shorr AF. Characteristics, hospital course, and outcomes of patients requiring prolonged acute versus short-term mechanical ventilation in the United States, 2014–2018. Crit Care Med. 2020;48:1587–94.
Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–53.
Marmor M, Liu S, Long J, Chertow GM, Rogers AJ. Prolonged hospitalization following acute respiratory failure. Chest. 2021;159:1867–74.
Rodrigues A, Telias I, Damiani LF, Brochard L. Reverse triggering during controlled ventilation: from physiology to clinical management. Am J Respir Crit Care Med. 2023;207:533–43.
de Haro C, Ochagavia A, López-Aguilar J, Fernandez-Gonzalo S, Navarra-Ventura G, Magrans R, et al. Patient-ventilator asynchronies during mechanical ventilation: current knowledge and research priorities. Intensive Care Med Exp. 2019;7:43.
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16.
Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363:1176–80.
Wang W, Xu C, Ma X, Zhang X, Xie P. Intensive care unit-acquired weakness: a review of recent progress with a look toward the future. Front Med (Lausanne). 2020;7: 559789.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35.
Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191:1126–38.
Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44:2079–103.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Alhazzani W, Belley-Cote E, Møller MH, Angus DC, Papazian L, Arabi YM, et al. Neuromuscular blockade in patients with ARDS: a rapid practice guideline. Intensive Care Med. 2020;46:1977–86.
Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175:1527–9.
Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008.
Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8:905–13.
Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–30.
Wilson ME, Barwise A, Heise KJ, Loftsgard TO, Dziadzko M, Cheville A, et al. Long-term return to functional baseline after mechanical ventilation in the ICU. Crit Care Med. 2018;46:562–9.
Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194:831–44.
Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:544–53.
Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40:388–96.
Oh TK, Song IA. Trends in mortality, treatment, and costs of management of acute respiratory distress syndrome in South Korea: analysis of data between 2010 and 2019. Yonsei Med J. 2022;63:452–60.
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304.
Parhar KKS, Zjadewicz K, Soo A, Sutton A, Zjadewicz M, Doig L, et al. Epidemiology, mechanical power, and 3-year outcomes in acute respiratory distress syndrome patients using standardized screening. An observational cohort study. Ann Am Thorac Soc. 2019;16:1263–72.
Dinglas VD, Aronson Friedman L, Colantuoni E, Mendez-Tellez PA, Shanholtz CB, Ciesla ND, et al. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45:446–53.
Fazzini B, Battaglini D, Carenzo L, Pelosi P, Cecconi M, Puthucheary Z. Physical and psychological impairment in survivors of acute respiratory distress syndrome: a systematic review and meta-analysis. Br J Anaesth. 2022;129:801–14.
Torbic H, Krishnan S, Harnegie MP, Duggal A. Neuromuscular blocking agents for ARDS: a systematic review and meta-analysis. Respir Care. 2021;66:120–8.
Tarazan N, Alshehri M, Sharif S, Al Duhailib Z, Møller MH, Belley-Cote E, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: updated systematic review and meta-analysis of randomized trials. Intensive Care Med Exp. 2020;8:61.
Shao S, Kang H, Tong Z. Early neuromuscular blocking agents for adults with acute respiratory distress syndrome: a systematic review, meta-analysis and meta-regression. BMJ Open. 2020;10: e037737.
Li Bassi G, Gibbons K, Suen JY, Dalton HJ, White N, Corley A, et al. Early short course of neuromuscular blocking agents in patients with COVID-19 ARDS: a propensity score analysis. Crit Care. 2022;26:141.
Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37:3047–53.
Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44:2070–8.
Yang T, Li Z, Jiang L, Wang Y, Xi X. Risk factors for intensive care unit-acquired weakness: a systematic review and meta-analysis. Acta Neurol Scand. 2018;138:104–14.
Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel PM, Jorens PG. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care. 2015;19:422.
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197:204–13.
Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43:1441–52.
Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46:637–53.
Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Mendez-Tellez PA, Shanholtz C, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–66.
Acknowledgements
We appreciate the staff of Artificial Intelligence Studio at Taichung Veterans General Hospital for their cooperation in this study.
Funding
The current study was supported by Ministry of Science and Technology Taiwan (MOST 109‑2321‑B‑075A‑001). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Study design and concept: C-L, W-CC, T-YY, C-LW and M-CC. Study coordination: C-LW and M-CC. Acquisition of data: C-L, W-CC, K-CP, T-YY, C-LW and M-CC. Statistical analysis: K-CP, WCC and M-CC. Interpretation of data: C-L, W-CC and M-CC. Drafting the manuscript: C-L, W-CC and M-CC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of TCVGH with a waiver of informed consent since it was a retrospective analysis of anonymous data (IRB number: SE20249B).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, C., Chao, WC., Pai, KC. et al. Prolonged use of neuromuscular blocking agents is associated with increased long-term mortality in mechanically ventilated medical ICU patients: a retrospective cohort study. j intensive care 11, 55 (2023). https://doi.org/10.1186/s40560-023-00696-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-023-00696-x