Abstract
Backgrounds
The aim of this study is investigating the benefits and harms of neuromuscular blocking agents (NMBAs) in patients with acute respiratory distress syndrome (ARDS).
Methods
We comprehensively searched PubMed, EMBASE, and Cochrane library for randomized controlled trials comparing NMBAs to any other comparator. We pooled data using relative risk (RR) for dichotomous outcomes and weighted mean difference (WMD) for continuous outcomes, with 95% confidence intervals. We assessed the quality of included studies using the Cochrane tool and levels of evidence using the GRADE method.
Results
Finally, six RCTs (n = 1557 patients) were eligible for analysis. The results showed NMBAs use was not associated with reduced 28 days mortality (RR 0.78; 95% CI, 0.58 to 1.06; P = 0.11), 90 days mortality (RR, 0.92; 95% CI, 0.81 to 1.04; P = 0.16), and intensive care unit (ICU) mortality (RR, 0.90; 95% CI, 0.79 to 1.03; P = 0.13) in patients with ARDS. However, 21–28 days mortality was slightly lower in patients received NMBAs (RR 0.73; 95% CI, 0.54 to 0.99; P = 0.04; I2 = 53%). Besides, NMBAs use could improve the PaO2/FiO2 ratio at 48 and 72 h, decrease plateau pressure and PEEP at 72 h. Additionally, NMBAs had no significant effects on days free of ventilation at day 28 (WMD, 0.55; 95% CI, − 0.46 to 1.57; P = 0.29), days not in ICU at day 28 (WMD, 0.12; 95% CI, − 0.85 to 1.08; P = 0.82), ICU-acquired weakness (RR, 1.23; 95% CI, 0.99 to 1.93; P = 0.06). Finally, NMBAs use was associated with a lower risk of barotrauma (RR, 0.55; 95% CI, 0.35 to 0.85; P = 0.007).
Conclusion
In patients with respiratory distress syndrome, NMBAs may be beneficial in reverse refractory hypoxemia and may be associated with reduced short-term mortality and incidence of barotrauma. However, there is no significant effects of NMBAs on mid-term and long-term mortality, and further studies are required.
Similar content being viewed by others
Backgrounds
Acute respiratory distress syndrome (ARDS) is a life-threatening condition characterized by refractory acute hypoxemia [1]. It is a major cause of morbidity and mortality in intensive care unit (ICU) [2,3,4]. A number of interventions have been proposed in the past decade; however, few of them obtained strong recommendation [5, 6]. Only lung-protective mechanical ventilation strategy has been proven beneficial for prognosis of these patients [5, 7]. Neuromuscular blocking agents (NMBAs) may be a useful therapeutic strategy in patients with ARDS [8]. The ARDS et Curarisation Systematique (ACURASYS) trial conducted in 2010 found early administration of a 48-h infusion of NMBA was associated with a lower risk of death in patients with moderate-to-severe ARDS [9]. It is important to realize that patients in the control group in this study received deep sedation, and this is inconsistent with the current guidelines [10, 11]. A meta-analysis including 5 studies systematically reviewed the effects of NMBAs on ARDS. They concluded the application of NMBAs could reduce the mortality of patients with moderate-to-severe ARDS [12]. However, the results of this meta-analysis are mainly affected by the ACURASYS trial [9]. Based on the limited evidence and potential adverse events, NMBAs is only weakly recommended in the current guidelines [13,14,15]. A new multi-center randomized control study (Reevaluation of Systemic Early Neuromuscular Blockade [ROSE] trial) just published recently [16]. Thus, the main aim of this study is to investigate the effects of NMBAs in moderate-to-severe ARDS by an update meta-analysis.
Methods
This study was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (An additional file shows the detailed information on PRISMA checklist [see Additional file 1: Figure S1]). The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42019137195).
Literature search strategies
PubMed, EMBASE and Cochrane library were searched from their inception to Jun 2019. There was no language limitation. Additional file 1: Table S1 shows the detailed literature search strategies. The reference lists of related articles were searched for additional studies. In addition, we searched Clinical.gov for ongoing studies and unpublished data. Two authors (ZJZ, LBJ) independently performed literature search, any disagreement was resolved by discussion or consultation with a third author (MZ).
Study selection and data extraction
Two authors (ZJZ, LBJ) did study selection and data extraction. And disagreement was resolved by discussion or consultation with another author (SZ). Firstly, we excluded duplicated articles. Then, we excluded clearly non-relevant articles by screening titles and abstracts. Finally, we included eligible studies by reading the full-text of remaining studies. The following data were extracted: name of first author, publication year, country, sample size, characteristics of included patients, intervention strategies, control strategies, endpoints and other items necessary for quality evaluation. If necessary, we would contact the author of original articles for additional data.
Inclusion criteria
Patients: adult acute respiratory distress syndrome defined by each study.
Intervention: neuromuscular blocking agents regardless of drug type, dose, or use duration.
Control: none or placebo.
Endpoints: the primary endpoints included 21 to 28 days mortality (short-term mortality), ICU mortality (mid-term mortality) and 90 days mortality (long-term mortality). The secondary endpoints included respiratory parameter such as PaO2/FIO2, plateau pressure (Pplat), and positive end-expiratory pressure (PEEP) at 24 h, 48 h, 72 h; days free of ventilation at day 28 (DFV); days not in ICU at day 28; incidence of biotrauma and ICU-acquired weakness.
Study quality evaluation
Two authors (SZ and XF) evaluated the qualities of all eligible studies using the Cochrane Risk of Bias Tool. The following domains were evaluated: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each domain was classified as low risk of bias, unclear risk of bias and high risk of bias.
Statistical analysis
Relative risks (RRs) and weighted mean differences (WMDs) with 95% confidence intervals (CIs) were used to estimate the pooled effect of dichotomous variables and continuous variables respectively. Heterogeneity between studies was assessed using the Q statistic and I2 statistic. P < 0.10 or I2 ≥ 50% indicated there was significant heterogeneity between studies and random effect model was used, otherwise, the fixed effect model would be used. If there was no significant heterogeneity, we would perform additional sensitivity analyses using random effects models to test the robustness of the results. A different number of studies were included in the various primary and secondary end-points analysis, detailed citations for included studies were shown in the different results. Publication bias would be assessed using Funnel plot and Egger test, if the number of included studies was over 10.
In order to minimize the risks of random errors resulting from sparse data during repetitive test, we performed trial sequence analysis (TSA) and calculated the optimal information size for the primary endpoints. In addition, we constructed the adjusted boundary line for favoring the NMBAs or controls to decide whether the meta-analysis could be terminated early. The optimal information size was calculated using α = 0.05 (two-sided), β = 0.20 (power 80%), the anticipated relative risk reduction, and the incidence in control arm.
A p value less than 0.05 was considered statistically significant. All analyses were performed in RevMan 5.3 (Cochrane Collaboration, Oxford) software. TSA was perfomed using Trial Sequential Analysis v.0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, available from www.ctu.dk/tsa).
Grade
GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) was used to evaluate the level of evidence. All patient center endpoints (21–28 days mortality, ICU mortality and 90 days mortality, DFV at day 28, days not in ICU at day 28; incidence of biotrauma and ICU-acquired weakness) were graded as high, moderate, low, and very low. This process was performed on GRADEpro GDT (https://gradepro.org/).
Results
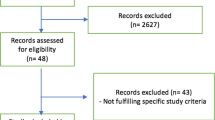
A total of six studies containing 1557 patients were included in the analysis [9, 16,17,18,19,20]. Figure 1 shows the detailed information of literature selection. All the data were obtained from published papers, including a meta-analysis [21] or by contacting the author of original articles. Four studies [9, 17,18,19] performed in France, one study [20] performed in China, and one study [16] performed in United States of America. Vecuronium was used in one study [20], and cisatracurium was used in the remaining studies [9, 16,17,18,19]. Table 1, Additional file 1: Tables S2, S3 and S4 show the detailed characteristics of included studies.
Risk of bias
Risk of bias of all included trials were assessed according to the Cochrane Collaboration tool. Most studies were judged at high risk of bias or unclear risk of bias in the domain of blinding. Detailed information about risk of bias of included studies are presented in the Additional file 1: Figures S2, S3, and Table S5.
Publication bias
As only six studies were included in this meta-analysis, we did not evaluate the publication bias [22].
The primary endpoint
Effect of NMBAs on mortality
21–28 days mortality.
Six studies [9, 16,17,18,19,20] were eligible for 21–28 days mortality. Mortality at 21-day was reported only in one study [20], which was at high risk of bias. There was significant heterogeneity between studies and random-effect model was used. The rate of 21–28 days mortality was slightly lower in patients received NMBAs with moderate significant heterogeneity (RR 0.73; 95% CI, 0.54 to 0.99; P = 0.04; I2 = 53%; Fig. 2a). But there was no statistically significant effects of NMBAs on 28 days mortality, by excluding the trial which reported mortality at day 21 (RR, 0.78; 95% CI, 0.58 to 1.06; P = 0.11; I2 = 50%; Additional file 1: Figure S4).
ICU mortality.
Five studies [9, 16,17,18,19] were eligible for ICU mortality. There was no significant heterogeneity between studies for ICU mortality, and fixed effect model was used. There was no significant effects of NMBAs on ICU mortality (RR, 0.90; 95% CI, 0.79 to 1.03; p = 0.13; I2 = 43%; Fig. 2b). Sensitivity analysis showed similar results by performed with random effect model (Table 2).
90 days mortality.
Five studies [9, 16,17,18,19] were eligible for 90 days mortality. There was no significant heterogeneity between studies for 90 days mortality, and fixed effect model was used. NMBAs use could not significantly reduce the 90 days mortality (RR, 0.92; 95% CI, 0.81 to 1.04; P = 0.16; I2 = 49%; Fig. 2c). Sensitivity analysis performed with random effect model showed similar results (Table 2).
The TSA showed the cumulative Z-curve neither crossed the monitoring boundary curve and nor reached the required information size, indicating further studies are required. Detailed information about TSA are presented in Additional file 1: Figures S5, S6 and S7.
The secondary endpoints
PaO2/FiO2
No statistically significant difference was found at 24 h between two groups (WMD, 17.66; 95% CI, − 0.36 to 35.68; P = 0.05; I2 = 71%; 5 trials; Fig. 3a) [9, 16,17,18,19]. The pooled analysis showed better PaO2/FiO2 in the NMBAs group at 48 h (WMD, 29.47; 95% CI, 1.38 to 57.55; P = 0.04; I2 = 69%; 4 trials; Fig. 3b) [16,17,18,19] with significant heterogeneity, and 72 h (WMD, 12.39; 95% CI, 4.80 to 19.99; P = 0.001; I2 = 37%; 4 trials; Fig. 3c) with no significant heterogeneity [9, 16,17,18]. Lyu [20] reported the results of PaO2/FiO2 at 48 h separately in patients with moderate and severe ARDS. We contacted the corresponding author for additional data, but received no reply. Therefore, we performed a sensitivity analysis by including moderate or severe ARDS or both, separately. The results showed better PaO2/FiO2 in the NMBAs group at 48 h when included severe ARDS alone, or both moderate and severe ARDS. No statistically significant was found if only moderate ARDS was included alone (Table 2).
Plateau pressure (Pplat)
There was no statistical significant effects of NMBAs on Pplat at 24 h (WMD, − 0.10; 95% CI, − 1.20 to 1.00; P = 0.86; I2 = 56%; 5 trials; Fig. 4a) [9, 16,17,18,19] and 48 h (WMD, − 0.08; 95% CI, − 0.76 to 0.59; P = 0.81; I2 = 0%; 4 trials; Fig. 4b) [16,17,18,19]. Sensitivity analysis of Pplat at 48 h showed similar results by performed with random effect model (Table 2). NMBAs use could decrease the Pplat (WMD, − 0.81; 95% CI, − 1.38 to − 0.25; P = 0.005; I2 = 25%; 4 trials; Fig. 4c) at 72 h [9, 16,17,18], but this difference did not achieve statistical significance in random effect model (Table 2).
Positive end-expiratory pressure (PEEP)
The difference in PEEP between two groups did not achieve statistical significance at 24 h (WMD, − 0.23; 95% CI, − 0.90 to 0.45; P = 0.51; I2 = 56%; 5 trials; Fig. 5a) [9, 16,17,18,19] and 48 h (WMD, − 0.39; 95% CI, − 0.87 to 0.09; P = 0.11; I2 = 0%; 4 trials; Fig. 5b) [16,17,18,19]. But this difference was statistically significant (WMD, − 0.43; 95% CI, − 0.83 to − 0.03; P = 0.03; I2 = 0%; 4 trials; Fig. 5c) at 72 h [9, 16,17,18]. Sensitive analysis by changing the model showed similar results (Table 2).
Days free of ventilation at day 28 (DFV)
Five studies [9, 16,17,18,19] reported days free of ventilation at day 28. There was no difference of the DFV at day 28 (WMD, 0.55; 95% CI, − 0.46 to 1.57; P = 0.29; I2 = 13%; Additional file 1: Figure S8), either in fixed or random effect model (Table 2).
Days not in ICU at day 28
Three studies [9, 16, 19] reported days not in ICU at day 28. There was no statistically significant effects of NMBAs on days not in ICU at day 28 (WMD, 0.12; 95% CI, − 0.85 to 1.08; P = 0.82; I2 = 13%; Additional file 1: Figure S9), either using fixed or random effect model (Table 2).
Barotrauma
Five studies [9, 16,17,18,19] reported the incidence of barotrauma. NMBAs use could reduce the risk of barotrauma (RR, 0.55; 95% CI, 0.35 to 0.85; P = 0.007; I2 = 0%; Additional file 1: Figure S10) with no significant heterogeneity. Fixed and random effect model showed similar results (Table 2).
Effect of NMBAs on ICU-acquired weakness
Four studies [9, 16,17,18] reported the incidence of ICU-acquired weakness. The diagnosis of ICU-acquired weakness was made by Medical Research Council (MRC) scale in two trials [9, 16], and was not specially defined in two studies [17, 18]. We did not found that NMBAs use was associated with increased risk of ICU-acquired weakness (RR, 1.23; 95% CI, 0.99 to 1.53; P = 0.06; I2 = 0%; Additional file 1: Figure S11) with no significant heterogeneity. Detailed information about four trials reported ICU-acquired weakness were given in the Additional file 1: Table S6. Post hoc sensitive analyses showed similar results (Table 2).
Evidence level
A summary of the evidence level according the GRADE was presented in Additional file 1: Figure S12.
Discussion
Our meta-analysis found that use of NMBAs in patients with ARDS might have benefits on short-term mortality, but had no significant effect on mid-term and long-term mortality. In addition, we found use of NMBAs could improve the PaO2/FiO2 ratio at 48 and 72 h, reduce the Pplat and PEEP at 72 h and was associated with less risk of barotrauma. Finally, our results showed use of NMBAs did not affect the days free of ventilation, the days not in ICU at day 28 and the risk of ICU- acquired weakness.
NMBAs may have beneficial effects on patients with ARDS through a variety of mechanisms. Such as decrease the oxygen consumption of respiratory and other muscles, reducing cardiac output, increasing the mixed venous partial pressure of oxygen, and increasing the partial pressure of arterial oxygen. By paralyzing respiratory muscles, neuromuscular blocking agents may indirectly minimize various manifestations of ventilator-induced lung injury [23]. The most used NMBAs in patients with ARDS is cisatracurium. Comparison with cisatracurium, vecuronium has different pharmacological properties [24]. Lyu [20] evaluated the effects of vecuronium in patients with moderate or severe ARDS and they found vecuronium is associated with better prognosis. However, low methodological quality may bias their results, and they did not compare the different effects between cisatracurium and vecuronium. Sottile et al. [25] compared the effects of cisatracurium with vecuronium in patients with or at risk for ARDS. They found there was no difference of mortality and hospital length of stay between two groups. Nevertheless, patients in the cisatracurium group experienced a shorter duration of mechanical ventilation and ICU length of stay. This may be because the metabolism and elimination of cisatracurium is independent of organ function and vecuronium is associated with a higher risk of ICU-acquired weakness [8]. In a United States national survey, 94% of respondents used either bolus or infusion neuromuscular blockade in patients with ARDS and 62.1% of respondents used NMBAs as tier 1 rescue strategy [26]. Due to limited evidence, recent guidelines only suggested use of NMBAs in patients with a PaO2/FiO2 less than 150 with weak recommendation [15, 27].
Three meta-analysis published separately in 2012, 2013 and 2018 reported NMBAs were associated with improved oxygenation and a lower risk of mortality and barotrauma [12, 21, 28]. However, these pooled results were affected mainly by the ACURASYS trial [9]. Our meta-analysis updated the results with the latest ROSE trial [16], which included more patients than all previous published studies. However, our results are different from the results of the previous meta-analysis. There are several possible explanations for this result. The most important factor may be the difference in sedation levels. Patients in the control group received light sedation according to the current guidelines [10, 11, 29, 30], in the ROSE trial, however, those patients received deep sedation in other previous published studies. It has been reported that deep sedation use in critically ill patients is independently associated with delayed extubation and increased mortality [31]. Moreover, Akoumianaki et al. [32] proposed a new mechanism for ventilator dyssynchrony in patients with ARDS in 2013, called reverse triggering. And deep sedation level may increase the incidence of reverse triggering, and the latter is associated with poor prognosis in patients with ARDS [33]. In addition, different median time from ARDS diagnosis to randomization, percentage of prone positioning, higher PEEP and other treatment strategies also need to be taken into consideration.
Our study showed that patients in the NMBAs group had a significant higher PaO2/FiO2 at 48 h and 72 h, and reduced Pplat and PEEP at 72 h. Although the difference of these parameters did not reach statistical significance before 48 h, but the trend toward improved results in patients who received NMBAs from 48 h to 72 h is clearly. These results indicate NMBAs therapy attenuate early hypoxemia in adult patients with ARDS. Additionally, NMBAs related complications has also been a focus of concern, especially the ICU-obtained weakness [15]. In the present study, we did not find short-term use of NMBAs could increase the incidence of ICU-obtained weakness. However, the diagnosis of ICU-obtained weakness is inconsistent and subjective and many other factors can affect the incidence of ICU-acquired weakness [34, 35]. Besides, the ROSE trial found use of cisatracurium is associated with a higher risk of serious cardiovascular events [16]. The authors speculated this may be associated with the use of deep sedation [16]. In addition, accumulation of laudanosine may increase the incidence of bradycardia and hypotension [36].
Study strengths and limitations
The strength of our study is that the newest published multiple center RCT was included. Several limitations of our meta-analysis should be concerned. Although there was no significantly statistic heterogeneity between studies in most analytic models, it is important to note the unneglectable clinical heterogeneity. Different ARDS definition criteria, type of neuromuscular blocking agents, dosage regimens, mechanical ventilation strategies, and various adjunct treatments may bias the results. In addition, only six studies were eligible, and we cannot perform subgroup analysis according different important variables. Furthermore, the small sample sizes of four studies make our results are mainly depend on the ACURASYS [9] trial and ROSE trial [16].
Implications for clinical practice and further researches
In the present study, we found NMBAs use is beneficial for reverse of hypoxemia and may be associated with decreased shorter-term mortality (21~28 days mortality). But they had no significantly statistical effects on long-term mortality. Along with the higher risk of serious cardiovascular events among patients received NMBAs, we do not suggest routinely use of NMBAs in all patients with ARDS. Severe ARDS patients with patient–ventilator dyssynchronies, or who are vulnerable to ventilator-induced injury may benefit from NMBAs use. Thus, we think NMBAs could be used for improvement of oxygenation in patients with severe ARDS. Additionally, further studies should focus on the following major topics. Firstly, in recent years, different subgroups or phenotypes of ARDS have been pay attention to [37]. Patients who are response to NMBAs therapy should be identified in further studies. Then, the optimal dose, time and duration of NMBAs remain unclear. The ACURASYS [9] and ROSE [16] studies used high dosages of cisatracurium (37.5 mg/h). Clinical assessment should be used in combination with Train-of-four (TOF) to titrate the optimal NMBAs’ dose [15]. The median time from ARDS diagnosis to NMBAs use is different between the ACURASYS trial [9] and ROSE trial [16] (16 h vs 7.6 h). So far, there have been no studies focusing on effects of different initiation time. Although all prospective studies limit NMBAs use within 48 h of ARDS set, there is significant heterogeneity between centers in clinical practices.
Conclusions
In patients with respiratory distress syndrome, NMBAs may be beneficial in reverse refractory hypoxemia and may be associated with reduced short-term mortality and incidence of barotrauma. However, there is no significant effects of NMBAs on moderate and long-term mortality, and further studies are required according to insufficient evidence based on current research.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article and its supplementary information files.
Abbreviations
- ARDS:
-
acute respiratory distress syndrome
- CI:
-
confidence interval
- cm:
-
centimeter
- d:
-
day
- DFV:
-
days free of ventilation at day 28
- FIO2 :
-
action of inspiration O2
- GRADE:
-
Grades of Recommendation, Assessment, Development, and Evaluation
- h:
-
hour
- ICU:
-
intensive care unit
- IV:
-
Inverse Variance
- Kg:
-
kilogram
- mg:
-
milligram
- M-H:
-
Mantel-Haenszel
- min:
-
minute
- mL:
-
milliliter
- N:
-
number
- NA:
-
not available
- NMBAs:
-
neuromuscular blocking agents
- PaO2 :
-
partial pressure of oxygen
- PEEP:
-
positive end-expiratory pressure
- Pplat:
-
plateau pressure
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RR:
-
relative risk
- TSA:
-
trial sequence analysis
- WMD:
-
weighted mean difference
- μg:
-
microgram
References
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–23.
Summers C, Singh NR, Worpole L, Simmonds R, Babar J, Condliffe AM, Gunning KE, Johnston AJ, Chilvers ER. Incidence and recognition of acute respiratory distress syndrome in a UK intensive care unit. Thorax. 2016;71:1050–1.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93.
Salim A, Martin M, Constantinou C, Sangthong B, Brown C, Kasotakis G, Demetriades D, Belzberg H. Acute respiratory distress syndrome in the trauma intensive care unit: morbid but not mortal. Arch Surg. 2006;141:655–8.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710.
Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–30.
Guo L, Wang W, Zhao N, Guo L, Chi C, Hou W, Wu A, Tong H, Wang Y, Wang C, Li E. Mechanical ventilation strategies for intensive care unit patients without acute lung injury or acute respiratory distress syndrome: a systematic review and network meta-analysis. Crit Care. 2016;20:226.
Torbic H, Duggal A. Neuromuscular blocking agents for acute respiratory distress syndrome. J Crit Care. 2019;49:179–84.
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16.
Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, et al. Executive summary: clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:1532–48.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77.
Tao W, Yang LQ, Gao J, Shao J. Neuromuscular blocking agents for adult patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. J Trauma Acute Care Surg. 2018;85:1102–9.
Cho YJ, Moon JY, Shin ES, Kim JH, Jung H, Park SY, Kim HC, Sim YS, Rhee CK, Lim J, et al. Clinical practice guideline of acute respiratory distress syndrome. Tuberc Respir Dis (Seoul). 2016;79:214–33.
Claesson J, Freundlich M, Gunnarsson I, Laake JH, Moller MH, Vandvik PO, Varpula T, Aasmundstad TA. Scandinavian clinical practice guideline on fluid and drug therapy in adults with acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2016;60:697–709.
Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, McGee W, McManus C, Meade M, Nix S, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44:2079–103.
National Heart L, Blood Institute PCTN, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008.
Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, Papazian L. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–9.
Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, Perrin G, Gainnier M, Bongrand P, Papazian L. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–57.
Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, Perrin G, Rambaud R, Adda M, Hraiech S, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med. 2017;43:408–18.
Lyu G, Wang X, Jiang W, Cai T, Zhang Y. Clinical study of early use of neuromuscular blocking agents in patients with severe sepsis and acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:325–9.
Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, Meade MO. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:R43.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93.
Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363:1176–80.
Price D, Kenyon NJ, Stollenwerk N. A fresh look at paralytics in the critically ill: real promise and real concern. Ann Intensive Care. 2012;2:43.
Sottile PD, Kiser TH, Burnham EL, Ho PM, Allen RR, Vandivier RW, Moss M. Colorado pulmonary outcomes research G: an observational study of the efficacy of Cisatracurium compared with Vecuronium in patients with or at risk for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:897–904.
Alhurani RE, Oeckler RA, Franco PM, Jenkins SM, Gajic O, Pannu SR. Refractory hypoxemia and use of rescue strategies. A U.S. National Survey of adult Intensivists. Ann Am Thorac Soc. 2016;13:1105–14.
Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, Forel JM, Guerin C, Jaber S, Mekontso-Dessap A, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69.
Neto AS, Pereira VG, Esposito DC, Damasceno MC, Schultz MJ. Neuromuscular blocking agents in patients with acute respiratory distress syndrome: a summary of the current evidence from three randomized controlled trials. Ann Intensive Care. 2012;2:33.
Shehabi Y, Bellomo R, Kadiman S, Ti LK, Howe B, Reade MC, Khoo TM, Alias A, Wong YL, Mukhopadhyay A, et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. Crit Care Med. 2018;46:850–9.
Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Seppelt IM, Webb S, Weisbrodt L. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–31.
Stephens RJ, Dettmer MR, Roberts BW, Ablordeppey E, Fowler SA, Kollef MH, Fuller BM. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care Med. 2018;46:471–9.
Akoumianaki E, Lyazidi A, Rey N, Matamis D, Perez-Martinez N, Giraud R, Mancebo J, Brochard L, Richard JM. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest. 2013;143:927–38.
Slutsky AS, Villar J. Early paralytic agents for ARDS? Yes, no, and sometimes. N Engl J Med. 2019;380:2061–3.
Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005;11:126–32.
Hraiech S, Dizier S, Papazian L. The use of paralytics in patients with acute respiratory distress syndrome. Clin Chest Med. 2014;35:753–63.
Fodale V, Santamaria LB. Laudanosine, an atracurium and cisatracurium metabolite. Eur J Anaesthesiol. 2002;19:466–73.
Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25:12–20.
Acknowledgements
We would like to thank all the authors of the primary research material. We would like to thank in particular the authors of the ROSE trial, Profs Derek C. Angus (University of Pittsburgh, 3550 Terrace St., Pittsburgh, PA 15261), Marc Moss (COLORADO Clinical Center, University of Colorado Hospital), B. Taylor Thompson, Christine A. Ulysse and Douglas Hayden (Clinical Coordinating Center, Massachusetts General Hospital Biostatistics Center), who provided additional data or clarification of their work.
Funding
None.
Author information
Authors and Affiliations
Contributions
LBJ, MZ and ZJZ conceived and designed the study. ZJZ, LBJ and MZ did the literature search. ZJZ, LBJ and SZ did the study selection and data extraction. CG provided additional patient data for the analysis. SZ and XF did quality evaluation. ZZJ, LBJ, SZ, JBD did analyze and interpreted the data. ZZJ, LBJ and SZ drafted the manuscript. MZ, XF and CG revised the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content and approved the final version. LBJ and MZ are the guarantors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
An approval by an ethics committee was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Study search strategy (from the inception to June 30, 2019). Table S2. Baseline characteristics of included trials. Table S3. Baseline respiratory parameters of included trials (mean ± SD). Table S4. Primary cause of ARDS, n (%). Table S5. Methodologic quality of included trials. Table S6. Effect of NMBAs on ICU-acquired weakness. Figure S1. PRISMA checklist. Figure S2. Risk of bias summary (each risk of bias item for each included study). Figure S3. Risk of bias graph (each risk of bias item presented as percentages across all included studies). Figure S4. Forest plot for the mortality of 28 days estimated with random effect model. Figure S5. Trial sequential analysis of the NMBAs on 28 days mortality. Figure S6. Trial sequential analysis of the NMBAs on ICU mortality. Figure S7. Trial sequential analysis of the NMBAs on 90 days mortality. Figure S8. Forest plot of DFV at day 28 estimated with fixed effect model. Figure S9. Forest plot of days not in ICU at day 28 estimated with fixed effect model. Figure S10. Forest plot of barotrauma estimated with fixed effect model. Figure S11. Forest plot of ICU-acquired weakness estimated with fixed effect model. Figure S12. GRADE summary of findings.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zheng, Z., Jiang, L., Zhang, S. et al. Neuromuscular blocking agents for acute respiratory distress syndrome: an updated meta-analysis of randomized controlled trials. Respir Res 21, 23 (2020). https://doi.org/10.1186/s12931-020-1287-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-020-1287-4