Abstract
Background
Mycotoxins are metabolites produced by phytopathogenic and spoilage fungi in animal feed as a result of poor storage. The mycotoxins can also originate in the field and are excreted in milk when dairy animals consume such feeds, posing a public health risk concern.
Methods
The aim of this study was to conduct a risk assessment in the informal sub-value chains of rural and peri-urban dairy systems in Nakuru County, by determining the prevalence and quantity levels of mycotoxins in animal feeds and milk. A total of 74 animal feed samples and 120 milk samples were simultaneously collected from individual cows and actors in the informal dairy value chain. Feed samples were analyzed for Aflatoxin B1 (AFB1) and Deoxynivalenol (DON) while milk samples were analyzed for Aflatoxin M1 (AFM1) using commercial Enzyme Linked Immune Sorbent Assay (ELISA) method.
Results
Aflatoxin B1 contamination levels in 56 % (41/74) of the animal feeds exceeded the European Union (EU) limits of 5 μg/kg ranging between 0 and 147.86 μg/kg. Deoxynivalenol (DON) was identified in 63 % (27/43) of all the animal feeds ranging between 0 and 179.89 μg/kg. In the peri-urban dairy system, 48.5 % (33/68) of the milk samples were contaminated with the AFM1 concentration above the EU regulation of 0.05 μg/L ranging between 0.017 and 0.083 μg/L. All milk samples from the rural dairy system had AFM1 contamination levels below the EU limits of 0.05 μg/L ranging between 0 and 0.041 μg/L. Linear regression model showed significant association of abiotic factors; pH, water activity and moisture content of animal feed with AFB1 and DON contamination of the animal feeds.
Conclusions
The results obtained from this study indicate that the peri-urban dairy farms, where intensive management predominate face the challenge of quality feeds, and one contributing factor is the on-farm production and handling of animal feeds.
Similar content being viewed by others
Background
Mycotoxins are a diverse group of fungal secondary metabolites that are harmful to animals and humans. These toxins are produced by saprophytic fungi during storage or by pathogenic fungi during plant growth. Aflatoxin B1 and B2 are the main metabolites produced by fungi of the genus Aspergillus particularly A. flavus, A. parasiticus and A. nomius (Richard 2007; Reddy et al. 2010). Animals fed on AFB1 and B2 contaminated feeds excrete into their milk the toxic AFM1 and M2, respectively which are metabolized in the liver. AFM1 is of particular interest being the hydroxylated metabolite of the AFB1 parent compound. AFs are highly carcinogenic causing liver cancer in humans (Zinedine et al. 2007). Deoxynivalenol (DON) is associated primarily with Fusarium graminearum and F. culmorum, both of which are important plant pathogens which cause fusarium head blight in wheat and fusarium ear blight in maize (Bottalico and Perrone 2002). DON is a mycotoxin belonging to the group of trichothecenes, which contaminates grains and cereal-based food and feed (Korosteleva et al. 2009). It is associated with acute gastrointestinal adverse effects such as vomiting (emesis) both in animals and humans (Vincelli et al. 2002).
The toxins can be passed down the food chain and contaminate milk and meat posing a greater danger to the health of humans (Flores-Flores et al. 2015; Leszczynska et al. 2001). Milk and milk products are traditionally the staple food commodities for the African communities. They are among the main entry routes of AFM1 into the human dietary system in Africa (Hell and Mutegi 2011). Considering that milk and milk derivatives are consumed daily and, moreover, that they are of primary importance in the diet of children who are most vulnerable, many African countries have accepted the maximum admissible levels of 0.05 μg/L, set by the European Union (EC 2006a).
Worldwide, a high and increasing proportion of dairy cattle are kept in intensive systems making AFs be an increasing problem to dairy farmers (Unnevehr and Delia 2013). About 80 % of the milk in Kenya is produced by smallholder farmers, especially at rural and peri-urban dairy farming (Muriuki 2011). Kenyan small-scale dairy farmers practicing intensive dairy systems lack knowledge on the safe formulation of feed rations and as a result, they feed their dairy animals mostly on farm formulations from crop residues and cereals that are discarded due to mold spoilage. These farmers also feed their dairy animals on commercially prepared concentrates from uncertified agro-vet dealers. Rural and peri-urban dairy farmers lack knowledge on proper feed formulation and storage such as silage making and lack of properly constructed feed stores (Lukuyu et al. 2011).
Research studies reveal that urban dairy farmers in Kenya spend nine times more money to purchase commercial feeds than their rural counterparts (Thorpe et al. 2000) and are at a higher risk of feeding AFB1-contaminated animal feeds (Kang'ethe and Lang'a 2009). There is also no monitoring and evaluation (M&E) system and inadequate enforcement of regulation in Kenya to evaluate the standards of market animal feeds (Nyaata et al. 2000). These factors contribute to the occurrence of mycotoxins in animal feeds and result in the carry over effect of AFM1 in milk in the dairy value chain in Kenya. However, only very scarce data exists on the occurrence of mycotoxin contamination of animal feeds and AFM1 in milk on rural and intensive small-scale dairy farms. Thus, this study aimed to provide information on the occurrence of common mycotoxins in the feeds and milk at rural and peri-urban dairy sub-value chains.
Methods
Study design and site description
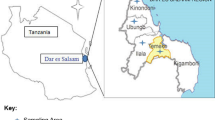
A cross-sectional study was conducted in the Nakuru County dairy value chain between March 2015 and October 2015. The study was carried out in three divisions in Nakuru County, Kenya namely; Olenguruone, Wanyororo, and Bahati. Olenguruone division represented a rural dairy system which lies at 35° 40'60"E and 0° 34'60"S in DMS (degree minute seconds). Wanyororo and Bahati divisions represented the peri-urban dairy system as they surround Nakuru town and lie at 36° 16′ 12″ E and 0° 12′ 0″ S. Nakuru County has 52,670 small-scale farms with a population density of 35,500 dairy cows, 20,500 zebu (Bos indicus) and 15,000 exotic dairy cattle (Bos taurus). Both production systems in Nakuru County have high production capacities with 110,000 l of milk per day which translates to 40,150,000 l per year (MoALF 2012). This study implemented a value chain approach by investigating occurrence of mycotoxin contamination of the animal feeds and milk at the on-farm production stage, transportation, processing and marketing outlets from which milk is channeled to urban Nakuru consumers mostly through informal market agents.
Participant selection and sample collection
The approximate sample size for dairy farms was determined from the formula n = Z2 P exp (1 –P exp)/L2, where Z is confidence level of 95 %, L is desired precision of 10 % and Pexp is expected prevalence of 50 % (Thursfield 1995). The calculated sample size was 78 farms in the rural dairy system and 42 farms in the peri-urban dairy system. A total of 97 animal feed samples were collected from randomly selected individual smallholder farms in rural and peri-urban dairy systems. A representative sample of 500 g was taken after mixing from storage bags into sterile plastic sampling bags and transported to the laboratory for analysis. Drying of samples was done by aseptically segregating 400 g of the sample and keeping it in an oven with the temperature set between 50 and 60 °C for 2 days to an average of 88 % dry matter content. The samples were then ground and stored at 20 °C under cool, dry conditions for analysis. The remainder of the sample was used for physico-chemical analysis. Milk samples from lactating cows on the same farm were collected. A total of 120 milk samples were collected from the individual lactating cows on small holder farms (n = 69), milk transporters bringing milk to cooperative dairy outlets (n = 30), cooperatives (n = 12) and milk bars (n = 19) in sterile 60 ml tubes. Samples were transported in cool boxes to the Egerton university laboratory under ice and frozen at −20 °C until analyzed within 3 months of collection.
Sample analysis
Determination of moisture content of animal feed samples
The moisture content was determined according the procedure provided by the Association of Official Analytical Chemists (AOAC) International (AOAC 2000). Samples weighing 2 g were dried in triplicates an oven at 105 °C for 3 h. Cooling of the dried samples was done in a desiccator for 10 min. Moisture content was calculated as the loss in weight expressed as a percent of the original weight of the animal feed. The amount of moisture was reported in terms of loss in weight.
Determination of water activity in animal feed samples
A durotherm (Aw Messer- Germany) was calibrated using a saturated solution of barium chloride and left to stand for 3 h until water activity reading was at 0.900 in an incubator. This was done as described by manufacturer’s instructions. Approximately 10 g of feed sample was finely chopped into small pieces and placed in triplicates in the durotherm. The water activity levels were recorded after 3 h at a temperature of 20 °C.
pH analysis of animal feed samples
The samples of animal feeds in triplicates were subjected to pH analysis of the glass electrode according to manufacturer's instructions. Approximately 20 g of air dried feed samples was transferred into 100 ml shaking bottle. 50 ml of distilled water was added and shaken for 2 h in the reciprocal shaker. The pH was determined by a precision pH meter PHS-3B (China) after a short but vigorous shaking. The pH meter was calibrated with buffers 4.0 and 7.0.
Enzyme immunoassay for aflatoxin B1 totals in animal feeds
The quantitative analysis of AFB1 in animal feed samples was performed by competitive ELISA (RIDASCREEN® Aflatoxin total, R-Biopharm) procedure as described by R-biopharm GmbH, Product code R4701. Prior to analysis of the samples, the ELISA method was validated to ensure data quality.
The sample preparation procedure was based on manufacturers of ELISA kit recommendations. The manufacturer’s recommendations were followed except 20 g of the sample and 100 ml of methanol/water (70:30 v/v) was used instead of two grams of the sample and 10 mls of methanol/water (HPLC grade methanol was purchased from Fisher Scientific, USA) to extract the AFB1. The entire extract was filtered. A Filtrate of 100 μL was diluted with 600 μL of the sample dilution buffer. A sample of 50 μL per well was employed in the assay. According to the manufacturer's instructions, the detection limit for feed samples was 1.75 μg/kg with the recovery rate of 85 %. AFB1 in animal samples was measured according to the instructions of the manufacturer using standards (0, 0.5, 1.5, 4.5, 13.5, and 40.5 μg/kg). All samples were run in duplicates. The AFB1 was measured photometrically at 450 nm (Readwell strip, ROBONIK, India).
Enzyme immunoassay for Deoxynivalenol in animal feeds
The quantitative analysis of DON in animal feed samples was performed by competitive ELISA (RIDASCREEN® DON, R-Biopharm) procedure as described by R-biopharm GmbH, Product code R5906. Prior to analysis of the samples, the ELISA method was validated to ensure data quality.
The sample preparation procedure was based on manufacturers of ELISA kit recommendations. Five grams of the ground sample was weighed and added into a suitable container with 25 ml of distilled water and shaken vigorously for 3 min. The extract was filtered through Whatman paper No. 1. A sample of 50 μL per well was employed in the assay.
According to the manufacturer's instructions, the detection limit for feed samples was 18.5 μg/kg with the recovery rate of 85 %. DON in animal samples was measured according to the instructions of the manufacturer using standards (0, 3.7, 11.1, 33.3, and 100 μg/kg). All samples were run in duplicates. The DON was measured photometrically at 450 nm (Readwell strip, ROBONIK, India).
Enzyme immunoassay for aflatoxin M1 in milk
The quantitative analysis of AFM1 in pasteurized milk samples was performed by competitive ELISA (RIDASCREEN® AFM1, R-Biopharm) procedure as described by R-biopharm GmbH, Product code R1121. Prior to analysis of the samples, the ELISA method was validated to ensure data quality.
The sample preparation procedure was based on manufacturers of ELISA kit recommendations. Milk samples (20 mL of milk) were thawed and centrifuged at 3500 g for 10 min at 10 °C before they were analyzed using an ELISA kit for M1. The creamy upper layer was completely discarded, and the lower phase was used for ELISA quantitative analysis.
According to the manufacturer's instructions, the detection limit for milk samples was 5 μg/L with a recovery rate of 95 %. AFM1 in skimmed milk samples was measured according to the instructions of the manufacturer using standards (0, 0.01, 0.02, 0.04, and 0.08 μg/L). All samples were run in duplicates. The AFM1 was measured photometrically at 450 nm using ELISA reader (Readwell strip, ROBONIK, India).
ELISA methods validation
The analytical quality of the ELISA methods was assured by the Limit of Detection (LOD) which determined experimentally by measuring the concentration of 20 blank matrix samples and then calculated by the formula: Mean concentration of blank samples + 3-fold standard deviation of the concentrations of blank samples. The Limit of Quantification (LOQ) was determined experimentally by measuring the concentration of 20 blank matrix samples and then calculated by the formula: Mean concentration of blank samples + 9-fold standard deviation of the concentrations of blank samples (R-Biopharm 2015). The validation of the ELISA methods was carried out with the determination of the recoveries and the coefficient of variation (% CV). The mean absorbance values obtained for the standards and the samples were divided by the absorbance value of the zero standards and multiplied by 100. The zero standard was thus made equal to 100 %, and the absorbance values of other standards and samples were quoted in percentages of this value. The values calculated for the standards were entered in a system of coordinates semi- logarithmically and analyzed against the mycotoxin concentration using Excel (Microsoft, Inc. USA). The mycotoxin concentration in μg/L or μg/kg corresponding to the absorbance of each sample was read from the calibration curve. Calibration curves were prepared for each mycotoxin AFM1, AFB1, DON and coefficients of determination (r2) were calculated respectively. In milk, AFM1 curve was prepared from standard solutions in range 0.005–0.08 μg/L with r2 = 0.988. In animal feeds, the AFB1 curve was obtained from the standard solutions in range 0.05–40.5 μg/kg with r2 = 0.982. In animal feeds, DON curve was prepared from standard solutions in the range of 3.7–100 μg/kg with r2 = 0.987. In milk, recovery of the method was evaluated by analyzing spiked certified extracts in triplicates at the level of 0.01 and 0.05 μg/L corresponding to the maximum value allowed by the European Commission. Recoveries for AFB1and DON were calculated by comparing the response for each mycotoxin with that of known spiked mycotoxin levels expressed as a percentage. The validation experiments were performed as described for the samples above. The validation parameters (Table 1) were calculated and expressed using European Official Decision procedure for screening methods (EC 2002) and their values were in accordance with recommendations given in Commission Decision (EC 2006a). Both the recovery and % CV (Table 2) are in compliance with Commission Regulation (EC 2006a).
Statistical analysis
One regression model was fitted to determine the overall association between the predictors and outcomes. The predictors were moisture content, water activity and pH. The outcome were the concentration of AFB1 and DON in animal feed. To improve model fit, outcome variables were natural log transformed and then the regression coefficients were back transformed to original scale to ease interpretation. Homoscedasticity was assessed by plotting standardized residuals against predicted values and by the use of Cooke-Weisberg test. Examination of residuals for normality was done using the normal probability plot and the Shapiro-Wilk test while the model fit was checked with Akaike’s Information Criteria (AIC). Data obtained from mycotoxin analysis in feeds and milk was also tested for analysis of variance (ANOVA) and means comparisons was done using Tukey’s Honestly Significant Difference (HSD) test at P ≤ 0.05. STATA statistical software Version 9 (Statacorp, College TX, 2007) was used for analyses.
Results
AFB1 contamination feeds
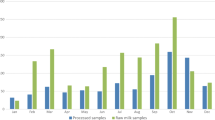
Animal feed contamination with AFB1 was more frequent in rural (60 %) than in peri-urban (53 %) dairy system as illustrated in Fig. 1, but the median concentration was higher in peri-urban (60.43 μg/kg) than in the rural (12.25 μg/kg) system from the estimates in Table 3. The analysis of variance (ANOVA) showed concentrates had significantly higher AFB1 levels compared to forage at P < 0.001 (Table 4). The lowest observed level of AFB1 contamination of 2.31 μg/kg was from a hay feed sample from rural while the highest AFB1 level of 147.86 μg/kg was observed in an on-farm formulated concentrate feed sample that contained maize germ from peri-urban as shown in Table 3. Association of water activity (aw) and moisture content of the feeds with levels AFB1 contamination of the feeds was significant at P < 0.05 and P < 0.01 respectively (Table 5).
DON contamination in animal feeds
Figure 2 illustrates that the contamination of feeds with DON was more frequent at 71 % with a higher concentration of median 60.61 μg/kg in the peri-urban than the rural dairy system at frequency of 53 % with concentration of median 21.62 μg/kg (Table 3). The analysis of variance (ANOVA) (Table 4) showed the DON contamination was significantly higher in concentrates than in forage. The lowest level of DON contamination of 4.37 μg/kg was obtained from hay sample from rural dairy system while the highest level of DON contamination of 179.89 μg/kg was observed in silage feed sample from peri-urban system as shown in Table 3. Association of water activity, pH and moisture content of feeds with DON contamination levels was significant at P < 0.01, P < 0.01 and P < 0.01 respectively (Table 5).
AFM1 contamination
In the peri-urban system, the prevalence of AFM1 contamination ranged from 68 % at production, 29 % at transporters, 40 % at cooperatives and 17 % at milk bar outlets with a median value of 0.073 μg/L at production level as shown in Fig. 3 and Table 6. All milk samples in the rural system were contaminated with AFM1 concentration levels of less than 0.05 μg/L with a median value of 0.006 μg/L at production level as shown in Fig. 4 and Table 6. A majority of the samples along the rural value chain were below the limit of quantification of 0.005 μg/L as shown in Fig. 4.
The lowest level of AFM1 contamination was 0.001 μg/L obtained from a milk sample from an individual cow at the rural dairy system. The highest AFM1 level was 0.083 μg/L from a milk sample from an individual cow at peri-urban dairy system as shown in Table 6. The reducing trend of AFM1 contamination along the value chain was observed with milk from cooperatives and milk bars having slighter range of contamination compared to milk from individual cows at production.
Discussion
Aflatoxin B1 contamination in animal feeds
The analysis of variance (ANOVA) (Table 4) showed there was significant difference in aflatoxin B1 contamination in animal feeds samples between the two dairy systems where the study was conducted. Feed samples from peri-urban had significantly higher levels of aflatoxin B1 ranging between 0 and 147.86 μg/kg compared to rural dairy system which was ranging between 2.31 and 84.41 μg/kg (Table 3). This may be attributed to prolonged storage of animal feeds (hay, concentrates and silage) under precarious conditions in small stores by peri-urban dairy farmers, who practice stall feeding due to lack of grazing fields. The unsuitable storage conditions accompanied by the tropical climate in Kenya may provide the increasing fungi occurrence and mycotoxin production. In contrast, as indicated by previous studies (Baltenweck et al. 1998), rural farmers mainly practice free range grazing on fields directly with pasture grasses or using the cut and carry system without prior feed storage while supplementing the dairy cows with minimal proportions of commercial concentrates.
The analysis of variance (ANOVA) (Table 4) higher AFB1 contamination of was observed in concentrates commonly utilized in the peri-urban ranging between 0 and 147.86 μg/kg than forages commonly used in the rural dairy system with AFB1 levels of ranging between 0 and 29.52 μg/kg (Table 3). This could be attributed to lengthy storage of on-farm formulated concentrates by peri-urban farmers because of animal feed shortages observed in the dairy system due to lack of grazing fields thus forcing them to formulate excess compounded concentrates. Prolonged storage conditions expose concentrates to the environmental conditions like humidity and temperatures that favour the growth of Aspergillus spp. (Soler et al. 2010). Besides, farmers lacked proper storage facilities for animal feeds with inadequate roofing leading to exposure of animal feeds to precipitation. These conditions contribute to mould growth leading to aflatoxin contamination.
Peri-urban farmers also used low-quality ingredients in the formulation of on-farm formulated concentrates leading to aflatoxin contamination of animal feeds. A study by (Richards and Godfrey 2003) in Nakuru County showed 42 % of urban and peri-urban farmers fed compounded concentrates to lactating cows. Other studies had shown that dairy farmers in the peri- urban areas of Kenya mostly use maize grains milled to make on-farm formulated concentrates to feed their cattle. The grains used are usually those that are contaminated with moulds at harvesting time and are separated from the healthy grains which are meant for human consumption. The mould invaded grains have been associated with aflatoxin contamination (Muture and Ogana 2005).
Extrinsic abiotic factors that affect growth of mycotoxin producing fungi measured in animal feeds included moisture content, water activity (aw) and pH. Concentrates was identified moisture content, aw and pH ranging between 11.20–71.30 %, 0.51–0.88 and 5.98–6.92 respectively that favour growth of mycotoxin producing fungi. Most storage fungi grow at aw below 0.75. The required aw for Aspergillus spp. growth is between 0.61 and 0.91 (Oviedo et al. 2011). Neutral pH ranging between 6 and 7 is also more suitable for mould growth which was exhibited in the study. From previous studies, optimum pH for aflatoxin production by Aspergillus spp. is between 3.5 and 8.0 (Oviedo et al. 2011). The toxin-producing fungi such as Aspergillums flavus and A. parasiticus species show enormous growth under environmental moisture of between 50–60 %, temperature conditions of 25 °C and 85–90 % relative humidity (Bakirci 2001).
Concentrates from both rural and peri-urban areas had high AFB1 contamination above the European Directives (Directive 2002/32/EC (EC 2002) and amending Directive 2003/100/EC (EC 2003) of 5 μg/kg. This could be attributed to lack of quality assurance system in the animal feeds value chain in Kenya. Animal feed ingredients used in formulations were not guaranteed of quality and safety while local agro-vets lack specified regulatory guidelines for animal feed distribution and proper storage.
Overall, the prevalence of AFB1 contamination of animal feeds was above the EU maximum limit of 5 μg/kg in both rural dairy system and peri-urban system with 60 and 52 % respectively (Fig. 1). This condition presented a concern in the dairy industry in this region as the risk of AFB1 toxicity in dairy cows was high in both dairy systems. This situation exposed cows to the risk of chronic intoxication with main target organ being the liver leading to hepatotoxicity, decreased weight gain, and decreased feed consumption, decreased reproductive performance and abortions (Haschek et al. 2013). The reduced performance in dairy cows would cause farmers large milk and economic losses.
DON contamination in animal feeds
The analysis of variance (ANOVA) (Table 4) showed that there was significant difference in DON contamination in animal feeds samples between the two dairy systems where the study was conducted. Feed samples from peri-urban had significantly higher levels of DON contamination ranging between 0 and 179.89 μg/kg compared to rural dairy system ranging between 0 and 89.79 μg/kg (Table 3). This could be attributed to use of low-quality raw materials in feed formulation. This finding indicated that DON contamination may have occurred in the pre-storage period and probably the feed ingredients were contaminated before storage (Haschek et al. 2013).
The analysis of variance (ANOVA) (Table 4) showed high levels of DON contamination was observed in commercial and on-farm formulated concentrates. This is attributed to the fact that local feed processors and on-farm formulations contain a great proportion of on-farm produced cereals. In corn, Fusarium moulds are associated with ear rot and stalk rot, and in small grains, they are associated with diseases such as head blight (scab). In wheat, excessive moisture at flowering and afterward is associated with increased incidence of mycotoxin formation. In corn, Fusarium diseases are more commonly associated with insect damage, warm conditions at silking, and wet conditions late in the growing season (Placinta et al. 1999). The highest DON contamination of 179 μg/kg was observed in silage feed sample from the peri-urban system. This could have been caused by the silage being exposed to oxygen, causing yeast to utilize lactic acid in silage as a substrate causing an elevation of pH above 4.5 and the silage becoming conducive for mould growth.
Silage is green forage preserved by lactic acid fermentation under anaerobic conditions. Silage with a terminal pH of less than 4.5 is ideal since it prevents fungal growth (Liu et al. 2011). Neutral pH ranging between 6 and 7 is suitable for mould growth than a low pH level and for this reason well-prepared silage is less susceptible to fungal spoilage. Silos should be properly sealed to prevent aerobic conditions that favour mould growth and further mycotoxin production.
Linear regression model showed significant association of water activity, pH and moisture content of animal feeds with DON contamination of the animal feeds (Table 5). The maximum amount of DON is produced by F. graminearum at 0.98 aw while Optimum DON production by Fusarium spp. is at pH of 7.5 (Comerio et al. 1999). The trichothecene DON persists in the animal feed at ≤0.90aw after it has already been produced (Hope et al. 2005).
All samples in this study were below the maximum limits for DON in the feed of 1250 μg/kg set in EU regulation 1881/2006 (EC 2006b). This implied that risk of DON toxicity in dairy cows was low in both dairy systems. The impact of DON on dairy cattle was not established, but clinical data shows an association between DON and poor performance in dairy herds (Côté et al. 1986). In previous studies, the DON-contaminated feed has caused a great economic loss in livestock, especially swine industry due to a well documented reduction in feed consumption and weight gain. High dose acute DON exposure resulted in emesis, abdominal distress, increased salivation and listlessness (Haschek et al. 2013).
The prevalence of AFM1 in milk along the value chain of rural and peri-urban dairy systems
The study revealed that the peri-urban dairy system is contaminated with AFM1 along the value chain ranging between 0.017 and 0.083 μg/L as shown in Table 6. The majority 48.5 % (33/68) of the milk samples from the peri-urban dairy system were above the EU regulation of 0.05 μg/L (EC 2006a) as shown in Fig. 3. The study also revealed that all milk samples in the rural dairy system were below the EU regulation of 0.05 μg/L (EC 2006a) as shown in Table 6. The cause for major differences in AFM1 contamination levels of milk samples taken from rural and peri-urban farms can be explained by the different types of feeds that were provided to cows in these dairy systems. Peri-urban farms fed their cattle mainly on AFB1 contaminated concentrates made of ingredients such as chicken feacal waste, maize germ, cotton and sunflower seed cake while most rural farms produced organic milk with lowest levels of AFM1 by feeding their cows on a basic diet of pasture that comprised the tropical grass species Pennisetum clandestinum and Pennisetum purpureum also known as Napier grass. However, the concentrations of AFM1 from the peri-urban dairy system in this study were lower compared to earlier studies in the urban Kenya reaching 0.68 μg/L (Kang'ethe and Lang'a 2009).
Milk samples from consumption nodes which comprise milk bars and processors level in the value chain had a narrower range of contamination compared to milk from farms in both systems as shown in Table 6. This could be explained by the effect of diluting due to bulking milk during transportation and at the collection centers prior to processing.
The study also showed a moderate correlation between AFB1 contamination in feed samples and AFM1 contamination in milk samples (r = 0.46 P < 0.001) collected from the same dairy farm. AFM1 is excreted in milk within twelve hours of consumption of contaminated animal feeds (Fink-Gremmels 2008; Battacone et al. 2003). The occurrence of AFM1 in milk and dairy products is a public health concern in the peri-urban dairy system which supplies milk to urban consumers. Milk is a primary part of the diet in Kenyan households and the effects of exposure to AFM1 have been associated with poor growth in neonates and children (Haschek et al. 2013).
Recent studies in Ethiopia show that 91.8 % of milk samples exceeded the maximum level set by EU regulations (Gizachew et al. 2016). Serbia (76 %) of milk samples exceeded the maximum level set by EU regulations (Škrbić et al. 2014). In Brazil, 46 % of ultra-high temperature milk samples were AFM1 positive with AFM1 (Iha et al. 2013). In Pakistan, 71 % were positive with AFM1 (Iqbal and Asi 2013). In this study, AFM1 levels in milk from both dairy systems are lower than those found in some other studies in different countries published recently. The difficulty of comparing results among different countries in the world is attributed to different investigative procedures used, sources of feed AFB1 contamination, different on-farm feeding practices, climatic situations, animal feed handling and storage conditions, the sampling time and procedures.
Milk consumption levels in Kenya are among the highest in the developing world (SDP 2004) with an average of 100 kg/year per capita. Keeping in view the high levels of AFM1 in peri-urban dairy system production node, there is dire need to improve storage and handling conditions of animal feed. This will mitigate the AFB1 levels in feed/feed ingredients and ultimately decrease the toxin in animal milk. The results showed that feeds used in peri-urban for the dairy animals are heavily contaminated with AFB1.
Conclusion
Results of this study indicate that the level of mycotoxin contamination in commercial and on-farm formulated concentrates require attention in-order to put mitigation strategies in place to reduce AFB1 exposure to dairy animals, especially in the peri-urban dairy system. The results suggest mitigation measures should focus on on-farm formulated concentrates with risk assessment evaluation of ingredients used in formulating them being recommended. DON contamination in animal feeds was minimal, but levels could vary year to year depending on feed handling conditions. Results of the survey indicate that organic milk produced in the rural dairy system is of high quality with low concentrations of AFM1.
Abbreviations
AFB1, aflatoxin B1; AFM1, aflatoxin M1; ANOVA, analysis of variance; aw, water activity; BMBF, German Ministry of Education and Research; DMS, degree minute seconds; DON, Deoxynivalenol; EC, European Commission; ELISA, Enzyme Linked Immune Sorbent Assay; EU, European Union; HPLC, High performance liquid chromatography; ReLOAD, Reduction of Post-Harvest Losses and Value Addition in East African Food Value Chains
References
AOAC. Official methods of analysis of AOAC International. 17th ed. Gaithersburg: AOAC International; 2000.
Bakirci I. A study on the occurrence of aflatoxin M 1 in milk and milk products produced in Van province of Turkey. Food Control. 2001;12(1):47–51.
Baltenweck I, Staal SJ, Owango M, Muriuki H, Lukuyu B, Gichungu G, Kenyanjui M, Njubi D, Tanner J, Thorpe W. Intensification of dairying in the greater Nairobi Milk-shed: Spatial and housing analysis. Smallholder dairy (Research and Development) Project, MoA/KARI/ILRI. Collaborative Research Report. Nairobi: ILRI (International Livestock Research Institute); 1998. http://cgspace.cgiar.org/bitstream/handle/10568/1751/Baltenweck%20et%20al-1998-Dairy%20intensification%20greater%20Nairobi%20spatial&HH.pdf?sequence=1.
Battacone G, Nudda A, Cannas A, Borlino AC, Bomboi G, Pulina G. Excretion of aflatoxin M1 in milk of dairy ewes treated with different doses of aflatoxin B1. J Dairy Sci. 2003;86(8):2667–75.
Bottalico A, Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J Plant Pathol. 2002;108:611–24.
Comerio RM, Pinto VF, Vaamonde G. Influence of water activity on deoxynivalenol accumulation in wheat. Mycotoxin Res. 1999;15(1):24–32.
Côté LM, Dahlem AM, Yoshizawa T, Swanson SP, Buck WB. Excretion of deoxynivalenol and its metabolite in milk, urine, and feces of lactating dairy cows. J Dairy Sci. 1986;69(9):2416–23.
EC Directive. Directive of The European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed 2002/32/EC. 2002.
EU Directive. Commission Directive 2003/100/EC of 31 October 2003 amending Annex I of Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed. L285/233–L285/237. Brussels: EU-commission; 2003.
European Commission. Commission Regulation 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off J Eur Union. 2006a;L70:12–34.
European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuff. 2006R1881-EN-01.09. 2014-014.001-1. 2006b.
Fink-Gremmels J. Mycotoxins in cattle feeds and carry-over to dairy milk: a review. Food Addit Contam. 2008;25(2):172–80.
Flores-Flores ME, Lizarraga E, de Cerain AL, González-Peñas E. Presence of mycotoxins in animal milk: a review. Food Control. 2015;53:163–76.
Gizachew D, Szonyi B, Tegegne A, Hanson J, Grace D. Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control. 2016;59:773–9.
Haschek WM, Rousseaux CG, Wallig MA, editors. Haschek and Rousseaux's handbook of toxicologic pathology. New York: Academic; 2013. p. 1214–6.
Hell K, Mutegi C. Aflatoxin control and prevention strategies in key crops of Sub-Saharan Africa. Afr J Microbiol Res. 2011;5(5):459–66. Kor.
Hope R, Aldred D, Magan N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett Appl Microbiol. 2005;40(4):295–300.
Iha MH, Barbosa CB, Okada IA, Trucksess MW. Aflatoxin M 1 in milk and distribution and stability of aflatoxin M 1 during production and storage of yoghurt and cheese. Food Control. 2013;29(1):1–6.
Iqbal SZ, Asi MR. Assessment of aflatoxin M 1 in milk and milk products from Punjab, Pakistan. Food Control. 2013;30(1):235–9.
Kang'ethe EK, Lang'a KA. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr Health Sci. 2009;9(4):218–26.
Korosteleva SN, Smith TK, Boermans HJ. Effects of feed naturally contaminated with Fusarium mycotoxins on metabolism and immunity of dairy cows. J Dairy Sci. 2009;92(4):1585–93.
Leszczynska J, Maslowska J, Owczarek A, Kucharska U. Determination of aflatoxins in food products by the ELISA method. Czech J Food Sci. 2001;19(1):8–12.
Liu Q, Zhang J, Shi S, Sun Q. The effects of wilting and storage temperatures on the fermentation quality and aerobic stability of stylo silage. Anim Sci J. 2011;82(4):549–53.
Lukuyu B, Franzel S, Ongadi PM, Duncan AJ. Livestock feed resources: Current production and management practices in central and northern rift valley provinces of Kenya. Livest Res Rural Dev. 2011;23(5):112.
Ministry of Livestock Development Department and Fisheries -MoALF. District Livestock Production Annual Report, Nakuru North. Nairobi, Kenya. 2012.
Muriuki HG. Dairy development in Kenya. Rome: Food and Agricultural Organization; 2011.
Muture BN, Ogana G. Aflatoxin levels in maize and maize products during the 2004 food poisoning outbreak in Eastern Province of Kenya. East Afr Med J. 2005;82(6):275–9.
Nyaata OZ, Dorward PT, Keatinge JDH, O’Neill MK. Availability and use of dry season feed resources on smallholder dairy farms in central Kenya. Agrofor Syst. 2000;50(3):315–31.
Oviedo MS, Ramirez ML, Barros GG, Chulze SN. Influence of water activity and temperature on growth and mycotoxin production by Alternaria alternata on irradiated soya beans. Int J Food Microbiol. 2011;149(2):127–32.
Placinta CM, D'mello JPF, Macdonald AMC. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Technol. 1999;78(1):21–37.
R-Biopharm AG. Good ELISA Practice Manual. 2015. http://www.r-biopharm.com/wp-content/uploads/534/2015-09_Good_ELISA_Practice_Manual_EN_Web.pdf.
Reddy KRN, Raghavender CR, Reddy BN, Salleh B. Biological control of Aspergillus flavus growth and subsequent aflatoxin B 1 production in sorghum grains. Afr J Biotechnol. 2010;9(27):4247–50.
Richard JL. Some major mycotoxins and their mycotoxicoses — An overview. Int J Food Microbiol. 2007;119(1):3–10.
Richards JI, Godfrey SH. Urban livestock keeping in sub-Saharan Africa: Report of a workshop held on 3-5 March 2003 in Nairobi, Kenya. Aylesford, Kent: Natural Resources International Ltd; 2003. p. 118.
SDP. A series of policy briefs (demand for dairy products in Kenya; employment generation in the Kenya dairy industry; competitiveness of the smallholder dairy enterprise in Kenya; public health issues in Kenyan milk markets; improved child nutrition through cattle ownership in Kenya; and uncertainty of cattle numbers in Kenya) for the dairy industry policy reform forum held at Grand Regency Hotel, Nairobi. 2004.
Škrbić B, Živančev J, Antić I, Godula M. Levels of aflatoxin M1 in different types of milk collected in Serbia: assessment of human and animal exposure. Food Control. 2014;40(1):113–9.
Soler CM, Hoogenboom G, Olatinwo R, Diarra B, Waliyar F, Traore S. Peanut contamination by Aspergillus flavus and Aflatoxin B1 in granaries of villages and markets in Mali, West Africa. J Food Agric Environ. 2010;8:195–203.
Thorpe W, Muriuki HG, Omore A, Owango MO, Staal S. Development of smallholder dairying in Eastern Africa with particular reference to Kenya. In A paper prepared for the UZ/RVAU/DIAS/DANIDA-ENRECA Project Review Workshop 10–13 January 2000. 2000.
Thursfield M. Diagnostic testing in veterinary epidemiology. 2nd ed. Cambridge: Blackwell Science Ltd; 1995. p. 483.
Unnevehr L, Grace D (Eds.) Aflatoxins: finding solutions for improved food safety (vol. 20) Intl Food Policy Res Inst; Washington DC,USA 2013.
Vincelli P, Parker G. Fumonisin, vomitoxin, and other mycotoxins in corn produced by Fusarium fungi. University of Kentucky Cooperative Extension Service; 2002. ID 121(8).
Zinedine A, González-Osnaya L, Soriano JM, Moltó JC, Idrissi L, Mañes J. The presence of aflatoxin M1 in pasteurized milk from Morocco. Int J Food Microbiol. 2007;114(1):25–9.
Funding
This research work was supported financially by German Ministry of Education and Research (BMBF) through ReLOAD Project: Reduction of Post-Harvest Losses and Value Addition in East African Food Value Chains.
Authors’ contributions
The experimental and analytical work was done by CMM, JWM, PSM, and BOB were involved in the designing of the experiment, data analysis, interpretation of the results and manuscript development. All authors read and approved the final manuscript.
Authors’ information
Ms. Caroline Makau is an MSc. student in the Department of Dairy and Food Science and Technology at Egerton University, Kenya. She also holds BSc. Food Science and Technology from the same university besides also having working experience in the food manufacturing industries in Kenya. Prof. Matofari and Dr. Muliro are senior lecturers in the Department of Dairy and Food Science and Technology in Egerton University while Prof. Bebe is a senior lecturer at the Department of Animal Science at Egerton University. All supervisors have many years of teaching experience at the University and have been involved in the supervision of many graduate students in their researches.
Competing interests
The authors of this article declare that they don’t have any financial and non-financial competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Makau, C.M., Matofari, J.W., Muliro, P.S. et al. Aflatoxin B1 and Deoxynivalenol contamination of dairy feeds and presence of Aflatoxin M1 contamination in milk from smallholder dairy systems in Nakuru, Kenya. FoodContamination 3, 6 (2016). https://doi.org/10.1186/s40550-016-0033-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40550-016-0033-7