Abstract
Aflatoxin contamination of animal feed threatens livestock production and can harm human health when aflatoxin B1 (AFB1) is carried over as aflatoxin M1 (AFM1) into milk for human consumption; therefore, aflatoxins in cattle concentrate feeds sold in Dar es salaam, Tanzania were determined in this study. Aflatoxins in cattle concentrate feeds were determined using Enzyme-linked Immuno-sorbent Assay (ELISA) and High-Performance Liquid Chromatography with a Fluorescent Detector (HPLC-FLD) and potential carry-over was determined using carry-over equations. Aflatoxins were found in 78% of the concentrate feed samples in the range LOD to 161.32 µg/kg. The mean total aflatoxins (TAFs) was 25.89 ± 3.3 µg/kg, higher than WHO/US-FDA limit of 20 µg/kg in feed although the difference was insignificant (P = 0.81). AFB1 mean was 18.87 ± 2.45 µg/kg and significantly exceeded the WHO/US-FDA limit of 5 µg/kg of AFB1 in dairy feed (P = 3.05 × 10–10). Aflatoxins B2, G1 and G2, were also detected ranging from ND – 75.06 µg/kg. The calculated AFM1 in milk was in the range 0.001 – 0.363 µg/L in low milkers, 0.002–0.666 µg/L in medium milkers and 0.002–0.806 µg/L in high milkers. Carry-over was estimated to range from 1.2 to 1.7%. This study revealed that concentrate feed sold in Dar es salaam was highly contaminated with aflatoxins; noteworthy aflatoxin B1 exceeded the WHO/US-FDA limit implying potential carry-over into milk which could expose milk consumers to aflatoxins, hence livestock feed chain participants need to be sensitized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High quality animal feed is essential for livestock productivity and profitability which eventually contributes to human food and nutrition security [1]. Compared to forage, concentrate feeds provide more energy and nutrients such as protein, vitamins, minerals, amino acids, enzymes and organic acids, therefore feeding livestock on concentrates has been proposed among possible solutions to supplement the growing livestock feed demand [1, 2] and to provide the nutritional requirements for improved livestock productivity. Globally, cereals and cereal based products such as maize (corn), wheat, barley, sorghum, and oats grains are constituents of livestock feed and in the developing world, maize (corn) is a major component of human food and animal feed [3].
The production of cereals and cereal-based products is threatened by mycotoxin contamination world-wide [3, 4] but this problem is more pronounced in tropical countries due to hot and humid climates [5]. Mycotoxins which threaten human and animal safety include: aflatoxins (AFs), fumonisins (FMs), ochratoxins (OTs), trichothecenes (TRCs), and zearalenone (ZEN) [3, 6] however aflatoxins are considered the most toxic[6]. Aflatoxins which are naturally occurring mycotoxins produced by Aspergillus flavus and Aspergillus parasticus have been detected in livestock feed and human food worldwide and have often resulted in deleterious consequences on health and the economy [4, 7,8,9,10].
Consumption of aflatoxin contaminated feed has been reported to predispose livestock to infectious diseases, increased mortality and lower productivity [3, 5, 11]. Furthermore, the potential for carry-over of aflatoxins into milk and meat products threatens the quality and safety of human food [12, 13]. Human long-term exposure to aflatoxins is reported to result in immune suppression, stunted growth and hepatocellular carcinoma [14, 15]. In Tanzania, attention to aflatoxin contamination was intensified by an aflatoxicosis outbreak reported in central zone (Dodoma and Manyara regions) in year 2016 which affected 68 people resulting in 20 deaths [16].
Studies on aflatoxin contamination of maize and animal feed in East Africa revealed presence of aflatoxins in concentrations higher than East African Community (EAC) standard of 10 µg/kg in maize for human consumption and 20 µg/kg in livestock feed from major agroecological zones in Kenya [17, 18], in Uganda [7, 19], in Tanzania [20, 21]. While studies on aflatoxin contamination in Rwanda, Burundi and South Sudan are still few, aflatoxin concentrations higher than the EAC limit have been reported in maize, other grains and animal feed [22,23,24,25] demonstrating that aflatoxin contamination is a threat to food and feed security in East Africa.
Investigations on aflatoxin contamination of maize and other cereals indicate that it is still a serious problem in Tanzania with detections higher than the Tanzania Bureau of standards (TBS) acceptable levels of 5 μg/kg [26,27,28] as a result of poor post-harvest practices and limited awareness [29], therefore, animal feed is at risk of contamination. Aflatoxin B1 in animal feed was reported in maize bran (76 µg/kg) and sunflower cake (63 µg/kg) at levels higher than the international allowable standard of 20 µg/kg for animal feeds [30].
When Aflatoxin B1 in feed is consumed by cows, it is converted into aflatoxin M1 in their milk [12] which can threaten the health of milk consumers. Aflatoxin carry-over in milk, cheese and meat products was reported by several authors [31,32,33,34], yet consumption of aflatoxin contaminated feed can threaten human food safety. Moreover, more than one type of aflatoxin can be present in a feed sample, thus a need for a more comprehensive analysis of occurrence of aflatoxins in cattle feeds in order to determine their prevalence and the potential risk of carry-over of aflatoxin B1 into milk.
In mammals, aflatoxin B1 is metabolised to aflatoxin M1, which is excreted in milk. Different mathematical formulae have been proposed for calculation of carry-over of aflatoxin B1 into aflatoxin M1 in milk [12, 35]; although no single carry-over equation is considered superior, the carry-over rate is dependent on aflatoxin B1 intake, type of feed, milk yield and stage of lactation, the animal species, breed and general condition of the animal among other factors [34,35,36]; for example a carry-over of aflatoxin B1 into milk was reported to reach 6% depending on the intake of aflatoxin B1 and milk yield [12, 32]. While treatments for reduction of aflatoxins carry-over using calcium bentonite and activated charcoal treatment have been proposed [32] the effect of aflatoxin carry-over from contaminated feed remains a threat to the safety of human food derived from animal products.
In Eastern African countries, maize bran remains a relatively cheap ingredient for the formulation of livestock feed [30] its reported contamination with aflatoxins increases the potential of feed contamination. A high demand for animal products including milk in urban areas has led to an increase in urban farming [37] resulting in increased demand for animal feed. Supplementing forage with feed concentrates containing maize bran is affordable and increasingly popular yet potentially harmful. In this study, aflatoxins occurrence in cattle concentrate feed sold in Dar Es Salaam was investigated to determine its prevalence and to estimate the carry-over into milk.
2 Materials and methods
2.1 Study area
Cattle concentrate feeds were collected from four main districts of Dar es Salaam namely: Ilala (6.9276° S, 39.1336° E), Temeke (6.9488° S, 39.4450° E), Kinondoni (6.7053° S, 39.1127° E) and Ubungo (6.7925° S, 39.2087° E), as shown in Fig. 1. Dar es Salaam, the most populated city in Tanzania, lies16 m above sea level; receives about 1114 mm/43.9 inch of precipitation annually and has an average annual temperature of about 27 °C [38].
2.2 Determination of sample size
The sample sizes for cattle feeds from each district were calculated using the method by Daniel and Cross [39] with a sample size calculator at 95% confidence level, 5% precision rate.
where: N = Population; Z = standard normal distribution = 1.96 at 95% confidence interval; σ = Population standard deviation; and d is the error component of interval estimated = 5%
2.3 Sample collection
One hundred and three (103) livestock feed-processors and feed-dealers were identified in the districts of Temeke, Ubongo, Kinondoni and Ilala through a survey. The concentrate feed samples, were collected from feed processors and agro-vet stores in each district from June to September 2021; a sample of 500 g was collected from each sampling point following the sampling plan from CXS 193–1995(Rev.2019)General Standard for Contaminants and Toxins in Food and Feed, 1995) [40] and EAS 900:2017, Cereals and Pulses – Sampling published by East African Community.
Briefly, in a consignment of < 20 bags of concentrate feed, a 25 g sample was drawn from every bag, in a consignment of > 20 and < 100 bags, a 20 g sample was drawn from 25 bags which were randomly selected for sampling while in a consignment of > 100 and < 1000 bags, a 10 g sample was drawn from 55 bags which were randomly selected. In total 81 samples (Table 1) were collected from the key actors i.e. Temeke 20, Ubungo 20, Kinondoni 14 and Ilala 27 by excluding twenty (20) actors who produced or sold other types of livestock feed since the focus of this study was cattle feed. The concentrate feed samples were collected in paper bags to prevent fungal growth during shipment and were kept at 20° C till further analysis. All equipment used during analysis were cleaned to minimize sample contamination as recommended in Codex- CXS 193-1995, last amendment-2019.
2.4 Sample analysis using enzyme-linked immunosorbent assay (ELISA)
Sample preparation and analysis followed the methods described in Richard et al. [41]. Briefly, a test sample was prepared by transferring 20 g of the finely ground and homogenized concentrate sample into an Erlenmeyer flask, followed by addition of NaCl (4 g). These were mixed with extraction solvent (100 ml) made up of analytical grade methanol and water (70:30), shaken thoroughly for 3 min, and the mixture filtered using a filter paper (Whatman No. 01). The filtrate (50 µL) was transferred to the premixing wells where it was mixed with an enzyme labelled aflatoxin conjugate (50 µL).
The resultant solution (100 µL) was transferred into the anti-aflatoxin microtiter plate where it was incubated for ten minutes at room temperature in the dark. After incubation, the excess liquid was poured off and the plate washed three times with phosphate buffer solution, any unbound enzyme conjugate and aflatoxin molecules was removed in this washing step. Developing solution (100 mL, chromogen substrate) was added onto the plate, followed by addition of 25 mL chromogen substrate. The enzyme converted the colourless chromogen into a blue product, and 50 mL of stop solution was added and mixed thoroughly with rotary motion for 30 s, leading to colour change from blue to yellow. The absorbance of the sample was measured with microplate reader at 450 nm.
2.5 Sample analysis using high performance liquid chromatography with a fluorescence detector (HPLC-FLD)
HPLC-FLD was used for confirmation and quantification of Aflatoxins following the ISO 16050 Foodstuffs method. A ground and homogenised sample (25 g), was transferred into the Erlenmeyer flask where an extraction solvent (100 mL) made up of methanol: water (70:30) was added. The flask containing the mixture was wrapped and covered with aluminium foil, and shaken using a gyratory shaker for 30 min at 250 rpm. The mixture was then filtered using filter paper (Whatman no 1) to obtain a filtrate/ extract. The extract (4 mL) was diluted with distilled water (8 mL), mixed in a Teflon tube, and vortexed for 1 min after which it was subjected to a clean-up using an immunoaffinity column. The bonded aflatoxin was eluted from the column using 0.5 ml, of methanol (HPLC grade) three times into the sample vial which was later vortexed for 30 s. From the vortexed vial, 0.3 ml of eluate was pipetted and mixed with 0.6 ml of distilled water and 0.1 ml acetonitrile.
The eluate sample (10 µL) was injected into an HPLC (1200 series Agilent Technology) for aflatoxin detection. Isocratic mobile phase (water: methanol: acetonitrile 60: 30: 10) was used for separation of aflatoxin on a C-18 (ZORBAX RX-C18, 4.6 × 250 mm) column at a temperature of 30 °C and flow rate of 1.2 mL/min. Detection of aflatoxins was achieved with fluorescence detector (FLD) at an emission wavelength 465 nm and an excitation wavelength of 360 nm. Separated peaks were recognized and computed by comparing with the standards.
Validation of the analytical methods was carried out according to international conference on Harmonization guidelines by using the mixed standard solutions and certified reference materials (CRM). A standard solution (B1, G2, G1, G2) of the concentration: 1 ng/mL, 5 ng/mL, 10 ng/mL and 15 ng/mL were prepared in order to prepare calibration curve as described in ISO 16050 Foodstuffs method.
2.6 Statistical analysis
A one sample T-test [42] was used to compare aflatoxin concentration calculated from concentrate feed sample with the recommended standards for total aflatoxin and AFB1. Descriptive statistics were used to calculate the mean and total aflatoxins. Correlational statistics were used to compare ELISA and HPLC-FLD. Regression statistics was used to calculate the correlation factor during HPLC-FLD method validation. One-way ANOVA used to establish and compare level of contamination among clusters.
2.7 Prediction of carry-over of Aflatoxin B1 into Aflatoxin M1 in milk
Carry-over was was determined by the method of Guo, et al. [43] as shown in equation (1).
Where: M milk = quantity of daily milk yield (kg); Mfeed = Quantity of feed (kg) contaminated with AFB1 per day; CAFM1 = Concentrations of AFM1 in milk per day(µg/kg) and CAFB1 = Concentration of AFB1 in feed (µg/kg).
The concentration of aflatoxin M1 was estimated using the method by Van Eijkeren et al. [35] as shown in equation ii, because it considers parameters that have been reported to affect carry-over rates including: aflatoxin contamination of feed, milk yield, the breed of cow, the source of contamination and the composition of total feed.
Where: Cmilk = is the concentration of aflatoxin M1 in milk; D is the daily intake of AFB1(µg/day); M is daily milk production (kg/day) α = 0.032 and β = 17 are constants.
The concentration of AFB1 in concentrate feed was determined using HPLC as previously described and the total dietary intake was determined based on the amount of concentrate feed consumed. In Dar es salaam, the common breeds of dairy cattle are cross breeds of Friesian, Ayrshire and Jersey. For healthy feeding, a dairy cow is recommended supplementary feeding with 1 kg of concentrates for every 2 L above 5 L obtained (https://ishamba.com/documents/2/DAIRYCOWPRODUCTION.pdf). Therefore, the concentrate feed taken to be to be 2.3 kg for low milkers (up10 kg of milk/cow/day) 5 kg for medium milkers (up to15 kg of milk/cow/day) and 7 kg for high milkers (up to 20 kg of milk/cow/day).
3 Results and discussion
3.1 HPLC- FLD method validation
Aflatoxins G1, G2, and B2 standards in the concentration range 0.25–15 µg/kg were used for calibration and to determine linearity. The coefficients of correlations (r2) were all greater than 0.999. The percentage recovery for individual standards concentration in the concentration range of 0.25 to 15 µg/kg ranged from 70 to 100%; when a certified reference material (CRM) of concentration = 100 µg/kg was used, the recovery ranged from 80 to 110%. Limit of quantification and limit of detection evaluated and the results indicated in Table 2. This complies with the requirements of methods of analysis of aflatoxin contamination in animal feeds as recommended in CXS 193–1995. Selectivity was demonstrated by absence of peaks in the chromatographic windows of the results for in a blank and the four peaks for aflatoxin G1, aflatoxin G2, aflatoxin B1 and aflatoxin B2, were well isolated with good resolution and narrow symmetric peak within 20 min as shown in Fig. 2.
3.2 Determination of aflatoxins in cattle concentrate feeds
Based on the ELISA kit, sixty-three 63/81 (78%) samples were found to be contaminated by aflatoxins. In Temeke cluster 18/20 (90%) samples tested positive for aflatoxin while 11/14 (79%) from Kinondoni; 21/27 (78%) from Ilala and 13/20 (65%) from Ubungo were contaminated with aflatoxins. ELISA method for detection of aflatoxin has been used by different researchers [44, 45] because it is fast, cheap and can analyse 96 sample simultaneously without a need extensive clean up [46] and good data was obtained in our study.
3.3 Quantification of aflatoxins in cattle concentrate feed using HPLC-FLD
The aflatoxin concentration in concentrate feed samples are shown in Table 3. The total aflatoxins (TAFs) observed from this study was LOD to 161.32 ppb, (mean 25.89 ± 3.3 ppb), and 37 (46%) of the samples were contaminated above the WHO/TBS recommended standard for total aflatoxin in feed (15 ppb). The mean TAFs contamination was significantly higher than the WHO/TBS recommendation (p = 0.002). Samples contaminated below the recommended standards were 26 (32%) and those found with aflatoxins below detection limit (LOD) were 18 (22%). Occurrence of aflatoxin above TBS/EAC limit indicated that the concentrate feed was not suitable for animal consumption in Tanzania.
The animal feed value chain is susceptible to aflatoxin contamination; limited surveys of aflatoxin contamination have also reported aflatoxin contamination in chicken commercial feed samples in Morogoro [47], in sunflower cake feed in Singida [44], in United Kingdom [48], in Nigeria [49], and in Kenya [50]. Our findings agree with other authors on the urgent need for control of aflatoxin contamination in animal feed to avoid negative consequences of the livestock value chain.
This study also revealed the predominance of AFB1 contamination (range LOD to 133.17 ppb, mean 18.79 ± 2.45 ppb) with 36 (44%)) of the samples contaminated above the recommended limit, while, 28 (35%) samples were AFB1 contaminated below the recommended WHO/TBS standards; only 19 samples (23%) were contaminated below LOD. The level of AFB1 contamination was significantly higher than recommended WHO/TBS recommended limit of 10 ppb (p = 0.001). AFB1, is regarded as a hepatocellular cancer-causing agent, [51] hence its presence in cattle feed concentrate is of animal and human health concern. Due to the potential for aflatoxin carry-over, human health may also be at risk.
Other major types of aflatoxin determined were AFB2 (range LOD to 8.21 ppb, mean 1.82 ± 0.27 ppb), AFG1 (range LOD to 75.06 ppb, mean 7.75 ± 2.32 ppb) and AFG2 (range LOD to 7.87 ppb, mean 1.30 ± 0.350 ppb). Among these aflatoxins the level of contamination was AFB1 > AFG1 > AFB2 > AFG2 but the level of contamination among these types of aflatoxin was different (p = 1 × 10–13) indicating high variations. Comparison of contamination among the different types of aflatoxins indicated that contamination from AFB2 and AFG2 were not significant different (p = 0.83), but all others were significantly different (p < 0.05) as indicated in Table 4.
Aflatoxin can reach to human being through consumption of aflatoxin contaminated cattle’s product such as milk or milk product and meat resulting into undesirable short- and long-term health effects [5, 16]. Aflatoxin contamination of feed also has a negative impact on the economy due to increased veterinary care cost and reduced livestock production [52].
3.4 Estimation of carry-over of aflatoxin B1 into aflatoxin M1 in milk
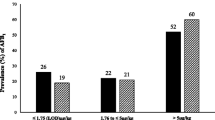
The calculated amount of aflatoxin M1 in milk using the method by Van Eijkeren et al. [35] was in the range 0.001–0.363 µg/L in low milkers (LM), 0.002–0.666 µg/L in medium milkers (MM) and 0.002–0.806 µg/L in high milkers (HM). The calculated concentration of aflatoxin M1 in milk exceeded the acceptable 0.05 µg/kg as set by the EU and TBS in 39/81 (~ 47%) of the HM, 37/81 (~ 46%) in MM and in 32/81 (~ 40%) of the LM. These findings are slightly higher than that reported by Kitigwa et al. [21] where the prevalence of aflatoxin M1 (AFM1) in raw cow milk was found at 30.7%, out of which 27.9% exceeded the 0.05 μg/kg limit for raw cow milk. The high values obtained by calculation could be an overestimation due to the assumptions made using the ideal the amounts of concentrate feed supposed to be consumed by the dairy cattle i.e. low milkers = 2.3 kg, medium milkers = 5 kg and high milkers = 7 kg ((https://ishamba.com/documents/2/DAIRYCOWPRODUCTION.pdf);however, concentrate feed is usually used to supplement foraging and thus the actual amount of concentrate feed and hence the aflatoxin M1 carried over in milk could be less than what was calculated.
In all the cases where the concentration of aflatoxin B1 was higher than 10 µg/kg which is the maximum acceptable value according to the Tanzania Bureau of standards (TBS), the calculated concentration of aflatoxin M1in milk was higher than the recommended 0.05 μg/kg in milk, this could be explained by bioconcentration [12, 35]). Furthermore, in high milkers a concentration of AFB1 was ≥ 8.85 µg/kg, leading to a calculated concentration of AFM1 in milk higher than the recommendation. Generally high milkers tend to be fed more concentrate and their dietary intake tends to be higher than low and medium milkers. Carry-over from feed to aflatoxin M1 in milk was calculated and found to be at 1.2% in low milkers, 1.5% in medium milkers and 1.7% in high milkers as shown in Table 5. This carry-over percentage is comparable with other studies of low yielding dairy cattle (< 30 kg milk/day) milked twice a day [36] and lower than 2.5–6.5% reported for high yielding (> 30 kg milk/day) holstein dairy cows [34, 36] as expected [12]; carry-over was however higher than the 0.1% reported for Indonesian crossbred Friesian Holstein (PFH) fed on AFB1-naturally contaminated feed and bentonite in the diet [53] demonstrating the contribution of dietary intake of AFB1 to carry over int AFM1 in milk.
4 Conclusion
This study revealed high level of aflatoxins contamination in concentrate feed collected from Dar es salaam-Tanzania; this high prevalence was attributed to low level of awareness and poor post-harvest handling practices related. The calculated concentration of AFM1 into milk was estimated to be higher than the acceptable concentration in cases where the cattle are fed 40–47% of the concentrate feed implying potential exposure of humans who consume milk and milk products. In order to avoid undesirable effects related to exposure, concentrate feed manufacturers, sellers and all other value chain actors need to be sensitized on good management practices. Further studies can involve a profile of the actual diet fed to dairy cattle in Dar es salaam and experimental determination of the AFM1 in the milk collected.
Data availability
All data that support the findings of this study are available on request from the corresponding author, using email gbirungi@must.ac.ug. Data on participants identity can only be shared in coded formats which protect their identities.
References
Balehegn M, Duncan A, Tolera A, Ayantunde AA, Issa S, Karimou M, Zampaligré N, André K, Gnanda I, Varijakshapanicker P, Kebreab E, Dubeux J, Boote K, Minta M, Feyissa F, Adesogan AT. Improving adoption of technologies and interventions for increasing supply of quality livestock feed in low- and middle-income countries. Glob Food Sec. 2020 Sep;26:100372. PMID: 33324534; PMCID: PMC7726233. https://doi.org/10.1016/j.gfs.2020.100372.
García SC, Pedernera M, Fulkerson WJ, Horadagoda A, Nandra K. Feeding concentrates based on individual cow requirements improves the yield of milk solids in dairy cows grazing restricted pasture. Aust J Exp Agric. 2007;47(5):502–8. https://doi.org/10.1071/EA05349.
Pereira CS, Cunha SC, Fernandes JO. Prevalent mycotoxins in animal feed: occurrence and analytical methods. Toxins. 2019. https://doi.org/10.3390/toxins11050290.
Magnoli AP, Poloni V, Cavaglieri L. Impact of mycotoxin contamination in the animal feed industry. Curr Opin Food Sci. 2019;29:99–108. https://doi.org/10.1016/j.cofs.2019.08.009.
Nakavuma JL, Kirabo A, Bogere P, Nabulime MM, Kaaya AN, Gnonlonfin B. Awareness of mycotoxins and occurrence of aflatoxins in poultry feeds and feed ingredients in selected regions of Uganda. Int J Food Contam. 2020. https://doi.org/10.1186/s40550-020-00079-2.
Nazhand A, Durazzo A, Lucarini M, Souto EB, Santini A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. 2020. Foods. https://doi.org/10.3390/foods9050644.
Omara T, et al. The scourge of aflatoxins in Kenya: a 60-year review (1960 to 2020). J Food Qual. 2021. https://doi.org/10.1155/2021/8899839.
Zhao L, et al. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J Anim Sci Biotechnol. 2021. https://doi.org/10.1186/s40104-021-00603-0.
Bervis N, et al. Field monitoring of aflatoxins in feed and milk of high-yielding dairy cows under two feeding systems. Toxins (Basel). 2021. https://doi.org/10.3390/TOXINS13030201.
Meijer N, Kleter G, de Nijs M, Rau ML, Derkx R, van der Fels-Klerx HJ. The aflatoxin situation in Africa: systematic literature review. Compr Rev Food Sci Food Saf. 2021;20(3):2286–304. https://doi.org/10.1111/1541-4337.12731.
Popescu RG, Rădulescu AL, Georgescu SE, Dinischiotu A. Aflatoxins in feed: types, metabolism, health consequences in swine and mitigation strategies. Toxins. 2022. https://doi.org/10.3390/toxins14120853.
Zentai A, Jóźwiak Á, Süth M, Farkas Z. Carry-over of aflatoxin B1 from feed to cow milk—a review. Toxins (Basel). 2023;15(3):195. https://doi.org/10.3390/toxins15030195.
Farkas Z, et al. A systematic review of the efficacy of interventions to control aflatoxins in the dairy production chain—feed production and animal feeding interventions. Toxins. 2022. https://doi.org/10.3390/toxins14020115.
Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Perspect. 2003;111(2):217–20. https://doi.org/10.1289/ehp.5753.
Jiang Y, Jolly PE, Ellis WO, Wang JS, Phillips TD, Williams JH. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int Immunol. 2005;17(6):807–14. https://doi.org/10.1093/intimm/dxh262.
Kamala A, et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018;11(3):311–20. https://doi.org/10.3920/WMJ2018.2344.
Kenji MG, Mvula MA. Aflatoxins in Kenyan malted maize. Scientific Reports of the Faculty of Agriculture, Okoyama University. 2000;89:5–7.
Collins S. Aflatoxins in Kenya: an overview the aflacontrol project: reducing the spread of aflatoxins in Kenya and Mali what are aflatoxins and why are they dangerous? 2010. http://programs.ifpri.org/afla/afla.asp.
Kitya D, Bbosa GS, Mulogo E. Aflatoxin levels in common foods of South Western Uganda: a risk factor to hepatocellular carcinoma. Eur J Cancer Care (Engl). 2010;19(4):516–21. https://doi.org/10.1111/j.1365-2354.2009.01087.x.
Sasamalo MM, et al. Aflatoxins contamination of maize at harvest and during storage in Dodoma, Tanzania. Inter J Innovative Res Dev. 2018;7(6). https://doi.org/10.24940/ijird/2018/v7/i6/JUN18005.
Kitigwa JS, Kimaro EG, Nagagi Y, Kussaga JB, Suleiman RA, Matemu A. Occurrence and associated risk factors of aflatoxin contamination in animal feeds and raw milk of three agroecological zones in Tanzania. World Mycotoxin J. 2023;16(2):149–63.
Elzupir AO, Younis YMH, Fadul MH, Elhussein AM. Determination of aflatoxins in animal feed in Khartoum State, Sudan. J Anim Vet Adv. 2009;8(5):1000–3. https://doi.org/10.3923/javaa.2009.1000.1003.
Boevre DM, Saeger DS, Harvey J, Marguerite N. Evaluation of mycotoxin content in soybean (Glycine max L.) grown in Rwanda. Afr J Food Agric Nutr Dev. 2018;18(3):13808–24. https://doi.org/10.18697/ajfand.83.17710.
Udomkun P, et al. Occurrence of aflatoxin in agricultural produce from local markets in Burundi and Eastern Democratic Republic of Congo. Food Sci Nutr. 2018. https://doi.org/10.1002/fsn3.787.
Umereweneza D, Kamizikunze T, Muhizi T. Assessment of mycotoxins types in some foodstuff consumed in Rwanda. Food Control. 2018;85:432–6. https://doi.org/10.1016/j.foodcont.2017.10.015.
Boni SB, et al. Aflatoxin contamination in Tanzania: quantifying the problem in maize and groundnuts from rural households. World Mycotoxin J. 2021;14(4):553–64. https://doi.org/10.3920/wmj2020.2646.
Massomo SMS. Aspergillus flavus and aflatoxin contamination in the maize value chain and what needs to be done in Tanzania. Sci Afr. 2020. https://doi.org/10.1016/j.sciaf.2020.e00606.
Ndwata AH, Rashid SA, Chaula DN. Aflatoxins B1 contamination levels in maize and awareness of aflatoxins among main maize stakeholders in Chemba and Kondoa Districts, Tanzania. Afr J Microbiol Res. 2022;16(6):223–37. https://doi.org/10.5897/ajmr2022.9637.
Kimario ME, Moshi AP, Ndossi HP, Kiwango PA, Shirima GG, Kussaga JB. Smallholder farmers’ storage practices and awareness on aflatoxin contamination of cereals and oilseeds in Chamwino, Dodoma, Tanzania. J Cereals Oilseeds. 2022;13(1):13–23. https://doi.org/10.5897/JCO2020.0220.
Mwakosya AW, Limbu SM, Majaliwa N, Zou X, Shi J, Kibazohi O. Aflatoxin B1 variations in animal feeds along the supply chain in Tanzania and its possible reduction by heat treatment. Food Agric Immunol. 2022;33(1):192–206. https://doi.org/10.1080/09540105.2022.2045908.
Costamagna D, et al. Quantification of aflatoxin M1 carry-over rate from feed to soft cheese. Toxicol Rep. 2019;6:782–7. https://doi.org/10.1016/j.toxrep.2019.07.004.
Mugerwa S, Kabirizi J, Zziwa E. Effect of supplementing lactating goats fed on aflatoxin contaminated feed with calcium bentonite and activated charcoal on aflatoxin M1 concentration, excretion and carryover in milk. Uganda J Agric Sci. 2016;16(1):83. https://doi.org/10.4314/ujas.v16i1.6.
Pleadin J, et al. Pathways of mycotoxin occurrence in meat products: a review. Processes. 2021. https://doi.org/10.3390/pr9122122.
Churchill KA. The carry-over of aflatoxins in dairy feed to milk of modern Holstein dairy cows. Ph.D. Thesis. Cornell University; Ithaca, NY, USA: Dec 30, 2017.
van Eijkeren JCH, Bakker MI, Zeilmaker MJ. A simple steady-state model for carry-over of aflatoxins from feed to cow’s milk. Food Addit Contam. 2006;23(8):833–8. https://doi.org/10.1080/02652030600779890.
Britzi M, et al. Carry-over of aflatoxin B1 to aflatoxin M1 in high yielding Israeli cows in mid- and late-lactation. Toxins (Basel). 2013;5(1):173–83. https://doi.org/10.3390/toxins5010173.
Mlozi Malongo RS. Urban agriculture: Ethnicity, Cattle Raising and Some Environmental Implications in the City of Dar es Salaam, Tanzania. African Studies Review, 1997;40(3):1–28. https://www.africabib.org/htp.php?RID=180664808
https://climateknowledgeportal.worldbank.org/country/tanzania/climate-data-historical. Accessed 04 Mar 2024.
Daniel WW, Cross CL. Biostatistics: a foundation of analysis in health sciences. 2013. 10th Edition, John Wiley & Sons, Hoboken.
GENERAL STANDARD FOR CONTAMINANTS AND TOXINS IN FOOD AND FEED CXS 193-1995 Adopted in 1995 Revised in 1997, 2006, 2008, 2009 Amended in 2010, 2012, 2013, 2014, 2015, 2016, 2017, 2018, 2019, 2021, 2022, 2023
Richard JL, Bennett GA, Rosst PF, Nelson PE. Analysis of naturally occurring mycotoxins in feedstuffs and food. J Anim Sci. 1993;71(9):2563–74. PMID: 8407669. https://doi.org/10.2527/1993.7192563x.
Skoog Douglas A, West Donald M, Holler F James, Crouch Stanley R. Fundamentals of analytical chemistry. Ed. 9th Singapore: Cengage Learning, 2014. ISBN 1285607198, 9781285607191
Guo W, Fan Z, Fan K, Meng J, Nie D, Tangni EK, Li Z, Zhao Z and Han Z. In vivo kinetics and biotransformation of aflatoxin B1 in dairy cows based on the establishment of a reliable UHPLC-MS/MS method. Front Chem. 2021;9:809480. https://doi.org/10.3389/fchem.2021.809480.
Mohammed S, Munissi JJE, Nyandoro SS. Aflatoxin M1 in raw milk and aflatoxin B1 in feed from household cows in Singida, Tanzania. Food Addit Contam Part B Surveill. 2016;9(2):85–90. https://doi.org/10.1080/19393210.2015.1137361.
Seetha A, et al. Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 2017;33(4):323–31. https://doi.org/10.1007/s12550-017-0286-x.
Yao H, Hruska Z, Mavungu JDD. Developments in detection and determination of aflatoxins. World Mycotoxin J. 2015;8(2):181–91. https://doi.org/10.3920/WMJ2014.1797.
Kajuna FF, Temba BA, Mosha RD. Surveillance of Aflatoxin B1 contamination in chicken commercial feed in Morogoro, Tanzania. Livestock Res Rural Dev. 2013; 25.
D’Mello JPF. Antinutritional factors and mycotoxin. Farm animal metabolism and nutrition. 2000; pp. 383–403. ISBN: 978-0-85199-378-2
Ejioffor Tochukwu E, Mgbeahuruike Anthony C, Nwoko Emanuela I, Okoroafor Obianuju N, Aronu Chinwe J, Chukwuemeka Nwaigwe O, Felix Adiong A, Atawal Adong F, Idolor Onwumere OS. Aflatoxigenic fungi in Nigerian poultry feeds: effects on broiler performance. Int J Livestock Prod. 2018;9(11):308–17.
Kang'ethe EK, Lang'a KA. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr Health Sci. 2000;9(4):218–26. PMID: 21503172; PMCID: PMC3074399.
Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiol Biomark Prev. 2009;18(3):846–53. PMID: 19273485; PMCID: PMC3535829. https://doi.org/10.1158/1055-9965.EPI-08-0697.
Alvarado AM, Zamora-Sanabria R, Granados-Chinchilla F. A focus on aflatoxins in feedstuffs: levels of contamination, prevalence, control strategies, and impacts on animal health. Aflatoxin Control Anal Dectect Health Risk. 2017. https://doi.org/10.5772/intechopen.69468.
Sumantri I, Murti TW, van der Poel AFB, Boehm J, Agus A. Carry-over of aflatoxin B1-feed into aflatoxin M1-milk in dairy cows treated with natural sources of aflatoxin and bentonite. J Indonesian Trop Anim Agri. 2012;37(4):271–7. https://doi.org/10.14710/jitaa.37.4.271-277
Funding
The authors acknowledge: Inter-University Council for East Africa (IUCEA), KFW donors for supporting the Master’s scholarship programme of Sahan Sylivester Jonathan; Dr. Athuman Yusuph Ngenya the Director General of Tanzania Bureau of Standard (TBS) and, Mr. A. Makaka of the food laboratory TBS for support in sample analysis.
Author information
Authors and Affiliations
Contributions
This work was generated from a master of science research work done by SSJ under the supervision of IN and GB as Postgraduate supervisors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from Mbarara University of Science and Technology Research and Ethics Committee.
Consent for publication
All authors listed consent to publication of the findings.
Competing interests
Authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jonathan, S.S., Nalumansi, I. & Birungi, G. Aflatoxins in cattle concentrate feed and potential carry-over of aflatoxin B1 into milk in Dar es Salaam, Tanzania. Discov Agric 2, 15 (2024). https://doi.org/10.1007/s44279-024-00018-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00018-1