Abstract
Background
Fresh in-hull pistachio fruits are very perishable and hence have a limited storage life, with marketers and customers wishing for a longer shelf life. The present research was performed to explore the combined effects of different packaging conditions (ambient atmospheric condition, and passive- and active-modified atmosphere packaging) as well as chitosan coating (0, 1, and 1.5%w/v) on postharvest quality of fresh in-hull pistachios stored at 5 °C and 85–90% relative humidity for 45 days. The efficacy of treatments in prolongation of pistachio fruit shelf life was evaluated by determining weight loss, kernel firmness, acid (AV) and peroxide values (PV), mold and yeast count, aflatoxin content, and hull color parameters (L*, a*, b*, C*, h°, and BI) at 0, 15, 30 and 45 days of storage. In addition, O2 and CO2 concentrations in MAP headspace were monitored.
Results
By applying high molecular weight chitosan coatings and MAP conditions, PV and AV values, microbial growth, aflatoxin B1 production, and weight loss values were inhibited, as well as kernel firmness reduction and hull color deterioration during storage were controlled. Active-MAP treatments in most cases performed better than passive-MAP treatments in this regard (P < 0.05). Combining chitosan coating with MAP demonstrated additive synergistic effects. At the end of storage, 1.5% chitosan-coated treatment under active-MAP maintained firmness (87% of the control treatment), hull lightness (76% of the control treatment), and microbial count (similar to the control treatment). It also maintained minimum weight loss (6.39%), acid (0.91 meq O2 kg−1 oil) and peroxide (0.49 goleic acid/100 goil) values compared to other treatments, proving that chitosan coating combined with MAP was more effective in preserving pistachio quality parameters. The equilibrium O2 and CO2 levels of passive-MAP treatments were maintained at higher values than those of active-MAP treatments which indicated that passive-MAP treatments had higher respiration rates than those of active-MAP treatments during storage.
Conclusions
The results revealed that applying the combination technique of MAP and chitosan coating effectively preserved the quality attributes and lengthened the shelf life of fresh in-hull pistachios. Therefore, it could potentially be commercialized as a new approach for future industry application.
Graphical Abstract

Similar content being viewed by others
Introduction
The pistachio (Pistacia vera L.) is a member of the cashew family, Anacardiaceae [165]. While classified as nuts in commercial horticulture and for culinary purposes, pistachios are botanically classified as drupes. A drupe is an indehiscent fruit in which a fertilized embryo (kernel or seed) within a hardened endocarp (shell) is surrounded by a fleshy mesocarp (hull) and an exocarp (external skin). Pistachio nut is Iran’s largest export after crude oil and its derivatives. Iran, with the production of 551,307 metric tons of pistachios in 2018, has been the largest producer of pistachios in the world [42]. Pistachios are often consumed as dried or roasted. Recent studies indicated that sun drying resulted in a 60% and 38% loss of anthocyanins and vitamin E, respectively. In addition, phenolics, flavonoids, and stilbenes were significantly decreased during the sun drying [10]. Because of their high essential bioactive constituents and micro-nutrients, there is also a growing market for fresh pistachios. Fresh pistachios have a short shelf life because metabolic activities, respiration, and ethylene production continue after harvest and produce senescence and changes in pigments, aroma, texture, fatty acids, amino acids, carbohydrates, bioactive compounds, secondary metabolites, and other quality components [105]. Due to all these reasons, the postharvest preservation of fresh pistachio fruit is challenging and an effective approach should be applied to prolong its storage life. One of the useful techniques to extend the shelf life of fresh fruits is to pack them under modified atmosphere condition. Modified Atmosphere Packaging (MAP) changes the gas composition inside the package through respiration (passive-MAP) or by the addition and removal of gases from food packages (active-MAP) to manipulate the quantities of O2 and CO2. The reduced O2 and/or increased CO2 levels can minimize metabolic activity, respiration rate, ethylene production, and microbial growth of fruits and vegetables. MAP can also delay maturation, and slow compositional and textural changes associated with ripening and senescence, resulting in postharvest life extension [31]. Several studies have been performed to evaluate the effectiveness of MAP conditions in maintaining the quality and extending the storage life of fresh pistachios [77, 108, 121, 136,137,138], almond [7, 15], walnut [1, 44, 83, 160], pine nut [80], and hazelnut [90, 102].
Another recent technique to increase the shelf life of fresh fruits and vegetables is the use of edible coatings [16, 87]. Chitosan is one of the most efficient materials used as a coating on fruits and vegetables. Chitosan (poly b-(1,4)n-acetyl-d-glucosamine) polymer is industrially generated by chemical deacetylation of the chitin found in arthropod exoskeletons [169]. This biopolymer can also be derived directly from the cell wall of certain plant-pathogenic fungi. Chitosan coating has been successfully used in agricultural and food applications, mainly due to its antimicrobial and structural properties which allow it to be used as an edible coating. A chitosan coating has the potential to create a semi-permeable barrier that controls gas exchange and prevents water loss, thus preserving tissue firmness and reducing microbial decay of harvested fruits and vegetables over long periods of time [35, 36, 151]. Chitosan coating has been reported to minimize decay and control postharvest changes in fresh areca nut [139, 173], walnut [126], pistachio nut [12, 100], and chestnut [45].
Therefore, considering the importance of fresh pistachio exports in terms of economy and currency, implementing projects to improve the shelf life of fresh pistachio fruits is very important. Despite the economic and nutritional importance of pistachios, little is known about the quality changes of fresh pistachios during postharvest storage. The literature review also revealed that there is no published work on the study of chitosan coating in combination with modified atmosphere packaging on the shelf life of fresh in-hull pistachios. Therefore, the objective of this research is to assess the changes in quality-related properties of fresh in-hull Badami’s pistachio fruits coated with different chitosan concentrations (0, 1, and 1.5% w/v) under passive-MAP (P-MAP), active-MAP (A-MAP), and ambient atmospheric condition (AAC) during cold storage to obtain the fruits with the highest quality characteristics for export purposes.
Materials and methods
Preparation of samples
Pistachio fruit (Pistacia vera L.) cv. Badami was harvested from a commercial pistachio orchard in Faizabad, Khorasan Razavi, Iran in September 2019, when 70–80% of the fruit had changed color and the hull was easier to remove. Fruits were transported to the packaging laboratory of Department of Food Science and Technology, Ferdowsi university of Mashhad, within 3 h under 5 °C. Fresh pistachios were selected for uniformity in shape, size, and color. Fruits with any symptoms of mechanical injuries, fungal infestation, sunburn, cracks, and also blank nuts were discarded.
Preparation of chitosan-based edible coating
Acetic acid, high molecular weight chitosan, and Tween-80 were supplied from Sigma Chemical Co (St. Louis, MO, USA). The method for preparing the coating solutions was described by Ali et al. [4]. Chitosan solutions of 1 and 1.5% (w/v) were prepared by dissolving 1.0 or 1.5 g of chitosan in 100 ml distilled water containing 0.5 ml (v/v) of glacial acetic acid. The solutions were continually heated and stirred for 24 h. The pH of the solutions was adjusted to 5.6 by 1 N NaOH, and 0.1 ml of Tween-80 was also added as an emulsifier to boost wet-ability. As a control, an acidic solution (pH 5.6) containing Tween-80, without chitosan, was used.
Treatment of pistachio fruits
Fruits were divided into two groups: coated (1 and 1.5% w/v chitosan) and uncoated. The fruits were then dipped into different concentrations of chitosan (0, 1, and 1.5% w/v) for 1 min. The excess solution was drained off and then the fruit was air-dried. Both coated and uncoated fresh pistachios were divided into three groups (ambient atmospheric condition (AAC), passive-MAP (P-MAP), and active-MAP (A-MAP)). P-MAP: ambient air composition (21% O2, < 0.1% CO2, and 78% N2) and A-MAP: (10% O2 and 90% N2). The P-MAP and A-MAP samples, each weighing 0.2 kg, were packed into linear low-density polyethylene (LLDPE) bags (thickness: 120 μm; area: 0.098 m2; PO2: 3314 ml μm m−2 h−1 atm−1; PCO2: 9024 ml μm m−2 h−1 atm−1) and heat-sealed. A-MAP Henkelman machine (Gustav Muller and Co., Bad Homburg, Germany) was used to perform MAP packaging and the initial internal gas compositions were set for the groups to create passive and active MAPs. The AAC samples were kept in the refrigerator without packaging. All samples were stored at 5 °C, RH (85–90%), and at 15-day interval over a period of 45 days, the experiments were carried out. At each sampling time, three samples of each treatment were removed from storage and used for measuring purposes.
Weight loss
Weight loss was determined by weighing whole pistachio fruits before and after storage time using a laboratory level weighing balance with 0.0001 g accuracy (RADWAG, AS 220.R2, Poland). Values were expressed as the percentage of loss of weight proportional to the initial weight.
Evaluation of gas composition inside package
The headspace gas composition (%O2 and %CO2) of MAP treatments were determined at selected time intervals (from the beginning to the 5th day, 3 times a day; from the 5th day to the 10th day, once a day; and from the 10th day onwards, once every 5 days) during the storage time prior to the opening of packages, through a silicone septum using a needle connected to a calibrated O2/CO2 gas analyzer (WITT, GmbH & Co KG D-38,454, Germany) in order to determine the effects of both chitosan coating and MAP on the fruit respiration, shortly after removal from cold storage. After approximately 30 s, screen displays of the O2 and CO2 percentages were provided. Each package was used only for a single determination of the headspace gas composition to prevent changes in the headspace gas composition due to gas sampling. The amounts of each gas were expressed as a v/v percentage.
Firmness
Pistachio kernel firmness was measured using a texture analyzer (TA.XT Plus, Stable Micro Systems Ltd., Godalming, UK) connected to the Stable Micro Systems software (version 6,1,14,0, Godalming, Surrey, UK). The penetration speed was 5.00 mm/min and the applied probe was 2 mm in diameter. The maximum force (Fmax) needed to penetrate into the central part of pistachio nut over 5.00 mm was used as a firmness measurement. The average firmness of 9 nuts per treatment was expressed as Newton (N) [136].
Properties of pistachio oils
According to Chatrabnous et al. [24], 3 g of ground kernels was extracted using pure hexane (100 ml) at 4 °C in dark for 48 h. The samples were then centrifuged at 4000 × g for 20 min. The supernatant was filtered through a filter paper and the solvent was removed by applying a rotary evaporation device. The peroxide value was determined using the iodometric titration method [18] and measured in terms of meq of oxygen per kg of extracted oil. The acid value was evaluated by titration method based on the percentage of oleic acid [8].
Microbial analysis
Mold and yeast count, reported as logarithm colony forming units (CFU) per gram, were determined according to the method of Sabaghi et al. [126]. Ten grams of pistachio fruits was crushed and homogenized in 90 mL of sterile NaCl solution (8.5 g/L) for 2 min with a stomacher (Stomacher 400, Seward Laboratory Blender, BA7021, London, UK). Mold and yeast counts were obtained by pour-plate inoculation of 1 ml of suspension on yeast extract glucose chloramphenicol (YGC) agar (Merck, Darmstadt, Germany) and incubating plates at 25 °C for 5 days.
Aflatoxin production measurement
A ground-up pistachio nut sample of approximately 50 g was homogenized with 5 g of sodium chloride, 100 ml n-hexane, and 200 ml of methanol–water (8:2) mixture for 3 min and extracted according to ISIRI method 6872 [66]. An aliquot of each suspension was passed through the immunoaffinity column (Rhone Diagnostics Technologies, Glasgow, UK). A Kobra cell (Rhone Diagnostics Technologies) was used to derivatize the samples. Aflatoxins were quantified using an HPLC system (HPLC 1525, Milford, MA, USA) equipped with an L-2485 fluorescence detector (Hitachi, VWR) and InertSustainSwift C18 (150 mm × 4.6 mm id) with the particle size of 5.0 μm (GL Sciences, Tokyo, Japan). The column temperature was adjusted to 30 °C and an auto-sampler was used to make sample injections (20 μl) at room temperature. The mobile phase consisted of a mixture of water, methanol and acetonitrile (6:3:1, v/v) with 305 mg KBr and 250 μl of nitric acid (2500 ml mobile phase). The excitation and emission wavelengths were 365 nm and 440 nm, respectively. The flow rate was 1 ml/min.
Color evaluation
The pistachio hull color parameters (20 fruits per replicate per treatment) were analyzed using a computer vision system which includes a lighting system, a color digital camera (Canon Powershot G1X, Tokyo, Japan), and ImageJ software (version 1.49). ImageJ software is capable of processing the color image to LAB values. In food research, color is commonly depicted using the L* a* b* color space. L* value measures the level of black to white (0–100), a* red to green (+ = red and − = green), and b* yellow to blue (+ = yellow and − = blue). Sample images were captured, and then color measurements were computed within the L* a* b* color system [120]. Measurements were carried out in triplicate.
The recorded values of a* and b* were converted into chroma (C*) and hue (h◦) according to Eqs. (2) and (3), respectively. Chroma is the saturation of color that ranges from dull (low value) to vivid color (high value) and hue angle is known as a color wheel with red–purple at an angle of 0, yellow at 90, bluish-green at 180, and blue at 270 [104]. C* (chroma), hue angle, and BI (browning index) were calculated using AOAC [8] methods during storage. The values for the above were determined using the following equations [104]:
where \(\mathrm{x}=\frac{a-1.75L}{5.645L+ a - 3.012\mathrm{b}}\)
Statistical analysis
In this study, the effects of chitosan concentrations (in 3 levels), and modified atmosphere packaging conditions (in 3 groups) were studied on the quality of fresh in-hull Badami's pistachio fruit for 45 days. The experiments were carried out in a completely randomized, factorial design with a confidence level of 95%. Each treatment was repeated three times. The data obtained for the samples were statistically analyzed using one-way analysis of variance (ANOVA). The statistical significance of differences between mean values was calculated at P < 0.05 with the Duncan’s New Multiple Range Test in the general model of the SPSS statistical package (IBM SPSS Statistics 22.0).
Results and discussion
Weight loss
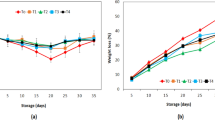
The weight loss of fresh produce is an important parameter, since it contributes to economic losses [157]. The average weight loss of fresh pistachios at different chitosan coating concentrations, packaging types, and storage times at 5 °C is shown in Fig. 1. The type of treatment and storage time had significant effects on fresh pistachios’ weight loss (P < 0.05). As depicted in Fig. 1, the weight loss of pistachios increased during storage time for all conditions tested due to transpiration and respiration [153]. The highest weight loss at the end of storage time was recorded in uncoated treatment under AAC (20.68%) (Fig. 1). According to Fig. 1, chitosan coating decreased the weight loss values of pistachio fruits. Of course, this reduction was significant in ACC treatments (P < 0.05), but not significant in A-MAP and P-MAP treatments (P > 0.05). This observation also reported for orange [38], banana [145], apple [116], pear [167], sweet potatoes [158], carrot [115], strawberries and red raspberries [59], papaya [143], litchi [36, 142], and mango [27]. The lower weight loss values in chitosan-coated samples might be due to the gas diffusion barrier properties of stomata, the organelles which control the transpiration process and gas exchange between the fruit and the atmosphere [78, 103, 171]. In addition, the higher chitosan coating concentration potentially resulted in a higher thickness coating, further reducing weight loss as previously shown by some other researchers [4, 63].
Weight loss of fresh in-hull pistachios coated with different chitosan concentrations (0, 1, and 1.5% w/v) and stored in AAC, P-MAP, and A-MAP at 5 °C. Values represent the means of weight loss measurements in three replicate bags. Vertical bars represent standard errors. Different letters indicate a significant difference
According to our data, applying the MAP technique also reduced the weight loss of fresh pistachios during low temperature storage (Fig. 1). This observation is consistent with the study findings of other researchers on de‐hulled fresh pistachio nuts [137], fresh in-hull pistachio nuts [50, 136], unripe hazelnuts in husk [90], and green walnut fruit [83]. In fact, packaging film by producing a saturated atmosphere of moisture, minimized the difference in water vapor pressure between the fruit surrounding and the fruit itself, thereby preventing transpiration [20, 73, 134, 152]. Furthermore, MAP by lowering the oxygen level surrounding the fruit, decreased weight loss due to the decline of respiration rate [89]. For this reason, A-MAP performed better than P-MAP in reducing weight loss (Fig. 2). Actually, in the respiratory process, the commodity generates CO2, water, and heat. The produced water usually remains within the tissue, although some water accumulation was also observed in fresh pistachio packages due to respiration. In addition, CO2 escapes and accounts for part of the weight loss of harvested organs as a result of the mass balance between O2 intake and CO2 release [11]. This rate of carbon depletion is directly proportional to the respiration rate and is typically a minor part (3 to 5%) of the overall weight loss [19] except for cases where moisture loss rates are low. Heat generation within the tissue can contribute to additional loss of weight. This heat is dissipated by direct heat transfer to the environment and by water evaporation. The heat of respiration increases the temperature of tissue, which in turn creates a water vapor pressure deficit, thus increasing evaporation.
Carbon dioxide (closed symbols) and oxygen (open symbols) concentrations at the headspace of fresh in-hull pistachios packed in different modified atmospheres (P-MAP and A-MAP) and coated with different chitosan concentrations (circle: 0%, square: 1%, and triangle: 1.5% w/v) at 5 °C. Values represent the means of measurements in three replicate bags. Vertical bars represent standard errors
Our findings revealed that the reduction in weight loss of fresh pistachios during cold storage was more dramatically attributed to packaging type than chitosan coating (Fig. 1). In addition, as can be seen, 1.5% chitosan-coated treatment under A-MAP had the least weight loss (6.39%) at the end of storage time (Fig. 1); however, there was no significant difference between 1% and 1.5% chitosan-coated treatments under A-MAP and P-MAP conditions (P > 0.05). It was concluded that chitosan coating in accompaniment with MAP is an effective method for managing weight loss in fresh pistachios due to their synergistic effect. This is in accordance with the research results of combining the coating and MAP technique for reducing weight loss in cucumber [88], button mushroom [69], eggplant [51], pear [167], and litchi [33]. In addition, it was found that weight losses greater than 8% considerably diminished the postharvest quality of fresh pistachios. Indeed, packaged fruits did not exhibit extreme symptoms of shriveling relative to non-packaged fruits, suggesting that the packaged fruits maintained freshness during storage. According to Kays [76], water loss of more than 4–6% leads to noticeable wilting or wrinkling of the surface of most commodities.
Headspace gas composition
The O2 and CO2 gas composition inside the packaging were investigated for 45 days. In this study, a sharp drop in O2 and a rise in CO2 concentrations were found in headspace gas composition of both the coated and uncoated fresh pistachios under P-MAP and A-MAP during storage at 5 °C (Fig. 2). Significant differences were also found in O2 and CO2 concentrations between all treatments during storage (P < 0.05).
The oxygen consumption and carbon dioxide production of fresh products creates a modified atmosphere in the packaging system, which is directly related to tissue respiration and the properties of packaging material. After an interaction between the respiration and gas exchanges in and out of the bags, these gases gradually reached equilibrium at about 97, 118 and 137 h for P-MAP treatments (0, 1, and 1.5% chitosan coated, respectively), and 68, 90 and 111 h for A-MAP treatments (0, 1 and, 1.5% chitosan coated, respectively) (Fig. 2). The O2 and CO2 levels of P-MAP treatments were maintained at 3.2–7.3% and 9–10.1%, respectively, which were considerably higher than those of A-MAP treatments (1.5–3.6% and 4.3–5.5%, respectively). These findings indicated that P-MAP treatments had higher respiration rates than those of A-MAP treatments during storage. Similar results were also published for de-hulled fresh pistachios [138], fresh in-hull walnut [44, 83, 160], cluster bean [156], cucumber [89], sweet cherry [164], table grapes [58], mango [118], and potato [14]. According to our data, chitosan-coated treatments showed higher O2 compositions inside the packaging than did stand-alone MAP treatments, indicating decreased respiration rates (Fig. 2). Chitosan-coated table grapes [123], Thompson and Autumn seedless grapes [125] also showed reduction in respiration rates. Chitosan coating reduced fruit respiration rate possibly by modifying the endogenous O2 and CO2 levels due to its semi-permeable filmogenic property [143] and/or good oxygen barrier property [106]. The modification of endogenous O2 and CO2 levels was shown in chitosan-coated papaya [4, 142], and citrus fruit [9]. In this study, off-flavor and off-odor were only developed in uncoated treatment under A-MAP when the concentration of O2 reached a value of 1.5% on day 15 (Fig. 2). However, the combination treatment of A-MAP and chitosan coating had an inhibitory effect on anaerobic conditions, as also observed previously by Waghmare and Annapure [157]. According our data, the optimum O2 and CO2 concentrations were created in 1.5% chitosan-coated treatment under A-MAP (Fig. 2). It could be concluded that the combination treatment is more useful in slowing down the respiration rate of fresh in-hull pistachios. The reduced respiration rate might be correlated with a delayed senescence and a decreased susceptibility to decay [124]. Other researchers have also shown that O2 and CO2 concentrations in the packages of lotus root [168], litchi [33], and cucumber [88] were greatly influenced by chitosan coating and MAP treatment, which are two primary factors in stabilization of the respiratory drift [112].
Firmness
Texture is a critical factor in the quality of fruits and represents metabolic changes and water content variations and is directly related to increase the storability potential and the resistance to mechanical injury and decay [122]. The firmness of freshly harvested kernels was about 38.2 N, which diminished with storage time and was significantly affected by the packaging conditions tested and the chitosan coating applications (P < 0.05) (Fig. 3).
Firmness of fresh in-hull pistachios coated with different chitosan concentrations (0, 1, and 1.5% w/v) and stored in AAC, P-MAP, and A-MAP at 5 °C. Values represent the means of firmness measurements in three replicate bags. Vertical bars represent standard errors. Different letters indicate a significant difference
According to Fig. 3, chitosan coating had a beneficial effect on fruit firmness, such that all chitosan-coated treatments resulted in fruits with higher kernel firmness than that of uncoated fruits at the end of storage time. Indeed, chitosan-coated treatments maintained the initial kernel firmness better than uncoated samples. In addition, significant differences were observed between 1 and 1.5% chitosan-coated treatments, and higher values for kernel firmness being found in samples coated with 1.5% chitosan during storage (Fig. 3). Actually, the reduced transpiration and improved water retention provided turgor to the fruit cells after applying the chitosan coating. Another reason for delaying softening might be the decrease in respiration rates of chitosan-coated fruits [99]. The beneficial effect of chitosan coating on firmness has also been reported for citrus fruit [9, 23, 26], strawberry [63], apple [116], avocado [17, 150], mushroom [70], papaya [4], cucumber [88], cantaloupe [93], red bell peppers [114], and grapes [128].
According to our findings, MAP also had a major effect on pistachio fruit firmness (Fig. 3). MAP-stored pistachios maintained their initial firmness better than those stored in AAC, in agreement with Zagory and Kader [172]. Inconsistently, uncoated treatment under A-MAP had the lowest firmness at the end of storage time (Fig. 3). It might be related to extensive fermentation leading to tissue decay and softening. A number of studies reported the inhibitory effect of MAP on the activities of cell wall degradation-related enzymes [34, 129]. In addition, humidity around the fruit under MAP affected weight loss and consequently fruit firmness [75]. It must be noted that the lower firmness reduction in A-MAP than P-MAP treatments could be attributed to the lower respiration rates. The effect of MAP conditions to limit firmness loss has been reported for many fruits, such as de‐hulled fresh pistachios [137], fresh in-hull walnut [160], fresh hazelnuts [102], litchi [33, 141], table grapes [92], cucumber [89], and papaya [54].
In our study, the least change in firmness was observed in 1.5% chitosan-coated treatment under A-MAP on day 45 (Fig. 3), probably due to the synergistic effect of MAP and coating. For comparison, uncoated treatment under AAC required 27.9 N to penetrate whereas 1.5% chitosan-coated treatment under A-MAP required 33.83 N on the 45th day of storage. Therefore, chitosan coating combined with MAP helped to preserve the firmness of fresh pistachios in the best possible way. The coating and MAP combination has also retained the firmness of button mushrooms [69], eggplant [51], carrot [155], and pears [167].
Peroxide value
Oxidative degradation is a major economic problem for the food industry in general and the nut industry in particular. Oxidative rancidity in nuts not only impairs their sensory quality and contributes to nutritional depletion, but can also lead to health issues as a number of lipid oxidation products are toxic both in vitro and in vivo [74]. Since more than 89% of pistachio fatty acids are unsaturated [95], oxidation in pistachios needs to be continuously monitored.
In our research, the oxidation of pistachio kernels was measured by calculating the peroxide value (PV) which represents the primary oxidation products for evaluating the kernel quality. As can be seen in Fig. 4, packaging conditions, coating concentrations and storage time, in most cases, had significant effects on PV (P < 0.05). In this study, the initial PV of kernel oil was as low as 0.15 meq O2 kg−1 oil which increased with storage time (Fig. 4). After the 45-day storage, the PV reached to 1.92, 1.41, and 1.06 meq O2 kg−1 oil for uncoated samples stored in AAC, P-MAP, and A-MAP conditions, respectively (Fig. 4). Geng et al. also reported that PV of three kinds of fresh walnuts increased steadily during refrigeration [49]. As can be seen, the PV of uncoated MAP treatments was lower than that of uncoated treatment under AAC during storage, and A-MAP treatment did better than P-MAP treatment in this regard (Fig. 4) (P < 0.05). [162] also reported that active-modified atmosphere packaging effectively prevented the rise in PV for walnut kernel oil during 120-day storage. Some other researchers also stated that the presence of higher levels of oxygen in packaging atmosphere of fresh raw pistachios [108], walnut [170, 174], almond [48, 98, 109], and hazelnuts [52] increased the peroxide value. The inhibiting effect of MAP on PV increase could be due to the high phenol content and antioxidant activity under MAP conditions, which in turn could minimize the decay of fruit [160].
Acid and peroxide values of fresh in-hull pistachios coated with different chitosan concentrations (0, 1, and 1.5% w/v) and stored in AAC, P-MAP, and A-MAP at 5 °C. Values represent the means of measurements in three replicate bags. Vertical bars represent standard errors. Different letters indicate a significant difference
According to Fig. 4, the higher peroxide values were always related to the chitosan-free treatments during storage time. In addition, 1.5% chitosan-coated treatments better prevented the increase in PV than did those coated with 1% chitosan. In fact, chitosan coating traps free radicals from the initial oxidation reactions and inhibits the development of these reactions. Chitosan also decreases the oxidative reactions rate by trapping metal ions. Furthermore, chitosan coating on kernels acts as a barrier and inhibits the entry of oxygen and peroxidants [146]. Sabaghi et al., Vanaei et al. and Maghsoudlou, and Maghsoudlou also indicated that chitosan coating prevented the PV increase in nuts [85, 126, 154].
In our study, the lowest PV was found in 1.5% chitosan-coated treatment under A-MAP (0.91 meq O2 kg−1 oil) at the end of storage time. As a result, the combination of chitosan coating and MAP demonstrated an additive inhibiting effect on the increase of PV. The Australian Macadamia Society indicates a maximum PV of 5.0 meq O2 kg−1 oil for raw and roasted kernels, while The Southern African Macadamia Growers’ Association recommends a maximum PV value of 3.0 meq O2 kg−1 oil [96]. In addition, according to established criteria, a PV greater than 1.0 meq O2 kg−1 oil is correlated with the onset of oxidative rancidity [46, 163]. Since high peroxide values can result in acute and long-term adverse health effects [57], the low levels of PV observed in our study indicate that they were not major health issues.
Acid value
Acid value (AV) reflects the amount of free fatty acids in pistachios kernel oil. The increase in AV during storage is attributed to the enzymatic lipid hydrolysis by lipase which separates fatty acids from lipid substrate and creates free fatty acids. Free fatty acids may negatively affect the flavor; they can also be exposed to lipid oxidation reactions [79]. In our study, the initial AV of kernel oils was 0.13 g oleic acid/100 g oil which was significantly influenced by storage time, packaging conditions tested and chitosan coating concentrations (P < 0.05). As can be seen in Fig. 4, the initial AV increased to 1.63, 0.91, and 1.02 g oleic acid/100 g oil for uncoated samples stored in AAC, P-MAP, and A-MAP conditions after 45-day storage, respectively. These values revealed that MAP condition had a relative inhibitory effect on the rise in AV. In addition, uncoated treatment under P-MAP inhibited the increase of AV in pistachio oil better than uncoated treatment under A-MAP, possibly due to the decreased tissue firmness created by anaerobic respiratory conditions in uncoated treatment under A-MAP which enhanced the exposure of lipase and lipid substrate. The inhibitory effect of MAP on AV has also been reported for fresh pistachios [108], walnut [24, 44, 84, 159, 160, 162, 166, 170, 174], hazelnut [52, 97], Torreya grandis nut [144], and wild almond [109]. According to our data, chitosan coating also prevented the increase of AV in kernel oils; and those samples coated with higher concentration of chitosan (1.5%) performed better in controlling AV increase (Fig. 4). In fact, chitosan coating slowed down the decay of inner kernels by maintaining the quality of the hull; thus, it preserved the oil in kernels from rancidity [161]. As can be seen in Fig. 4, the lowest AV was shown in 1.5% chitosan-coated treatment under A-MAP on the 45th day of storage (0.49 g oleic acid/100 g oil), proving that chitosan coating combined with MAP was more effective in preserving pistachios kernel oil quality. It has been mentioned in many references that the maximum permissible level of AV is 5 g oleic acid/100 g oil [13, 40, 94, 101]. As the AV levels in our study did not surpass this value, all fresh pistachio treatments were acceptable in terms of AV.
Mold and yeast growth
While mycotoxin contamination has long been a safety problem for the nut industry, microbial food safety has recently received some attention. Since 2000, several high profile food borne outbreaks and recalls have included contaminated nuts and nut products [60]. In the case of fresh pistachios, fruits with damaged hulls or inadequate protection by hulls are most susceptible to microbial contamination. Sometimes the hull is attached to the shell and both split together. This rupture of the hull, referred to as ‘early splitting’, exposes the kernel to microbial contamination. The proportion of early split pistachio nuts is normally 1–5%, but in some situations, it may be as high as 30% [130].
The counts of mold and yeast throughout the cold storage of pistachio fruit treatments are shown in Fig. 5. At harvest, pistachio fruits had 1.4 log CFU g−1 mold and yeast count which increased with increasing storage time. As can be seen, uncoated treatments under MAP retarded the mold and yeast growth compared to uncoated treatment under AAC during storage; but A-MAP treatment performed better than P-MAP treatment in reducing the growth (P < 0.05) (Fig. 5). Similarly, MAP reported to control microbial growth in fresh pistachios [108, 136, 137] and Brazil nuts [133]. The decreased decay occurrence in MAP treatments is due to the ability of low O2 and/or high CO2 levels to deter microbial decay [29]. The bacteriostatic effect of CO2 is not well known, a plausible mechanism is by decreasing the availability of O2, and the ability of CO2 to acidify and reduce pH [30]. It is also claimed that CO2 has a direct antimicrobial effect resulting in an increased lag phase and generation time during the logarithmic growth phase of the microorganisms involved [113], with inhibition being dependent on the concentration of gas and temperature.
Based on our data, chitosan coating also tend to reduce the growth of mold and yeast in fresh pistachios during the cold storage (Fig. 5). The mold and yeast growth inhibition significantly enhanced by increasing chitosan concentration (P < 0.05). As is clear from the results, the effect of chitosan coating on the reduction of mold and yeast growth was more prominent than that of MAP condition (Fig. 5). Similarly, Sabaghi et al., Maghsoudlou et al., Hernandez-Munoz et al. Reddy et al., and Romanazi et al. reported that chitosan coating decreased mold and yeast growth on fresh walnut kernel, dried pistachios, strawberry, grapes, and tomato, respectively [63, 86, 119, 124, 126]. The inhibitory effect of chitosan on microbial growth is due to the positive charge of chitosan [62, 81], metal chelating, binding with DNA [117], and oxygen barrier properties of the coating [131]. Chitosan is also known to influence the morphogenesis of cell wall interfering directly on the activities of enzymes responsible for fungal growth [39]. Chitosan has been found to be more effective in inhibiting spore germination and germ tube elongation than mycelia growth [127, 151]. The membrane disruptive properties of chitosan have also been demonstrated by Galván Márquez et al. on yeasts [91]. It has been shown that chitosan induces fruit defense responses [175]. However, other antifungal edible coatings have also been developed by Sayanjali et al. and Hashemi and Raeise for fresh pistachios and wild almond, respectively [61, 131].
According to Fig. 5, uncoated treatment under AAC showed the highest mold and yeast count after 45 days of cold storage (6.9 log CFU g−1), while 1.5% chitosan-coated treatment under A-MAP showed the lowest (1.5 log CFU g−1). Jacxsens, Devlieghere, and Debereve stated that the critical limit for mold and yeast in fruits is 105 CFU/g [68]. Our results proved that the mold and yeast growth of fresh pistachio treatments during storage, from the lowest to the highest, was in this order: chitosan-coated treatments under MAP conditions < stand-alone chitosan treatments < stand-alone MAP treatments < uncoated treatment under AAC (Fig. 5). Simões et al. and Campaniello et al. also reported that the combination of edible coatings containing chitosan and modified atmospheres preserved the microbial quality of carrot sticks and sliced strawberries, respectively [21, 140]. Moreover, as microbial growth might be responsible for some extension of softening process, the combined inhibitory effect of chitosan and MAP could also account for much decreased softening found in MAP packages treated with chitosan in this study.
Aflatoxin production
The aflatoxins are a group of toxic and carcinogenic polyketide secondary metabolites, which are generated by the strains of Aspergillus flavus, Aspergillus parasiticus, Aspergillus nomius, and Aspergillus pseudotamarii [67]. The key factor threatening pistachios exports is aflatoxin production by molds [3]. The hull, as the outermost layer of pistachio fruit, is covered with a cuticle that creates a defensive barrier against pathogen invasion. Wound pathogens, such as A. flavus, have no ability to penetrate the cuticular layer. They make entrance into the host plant by cuticle breaks caused by abrasions or insects [110].
The aflatoxin B1 production results of all treatments during cold storage are tabulated in Table 1. A low standard deviation was obtained between replicates, demonstrating the good reproducibility achieved. According to Table 1, the varying levels of aflatoxin B1 were only detected in uncoated treatments during storage. It should be noted that the production of aflatoxins B2, G1, and G2 has not been detected for all treatments throughout the storage. As can be seen in Table 1, aflatoxin B1 production of fresh pistachios increased with increasing storage time. But, applying the MAP technique significantly reduced it (P < 0.05). This reduction was more noticeable for A-MAP than P-MAP treatment after 45 days of storage. It could be attributed to the reduced oxygen level in A-MAP than P-MAP treatment, because oxygen is essential for the production of aflatoxin in aflatoxigenic fungi [111]. Other researchers have also declared that aflatoxin production is an aerobic process that may be inhibited at low O2 concentrations or blocked at high CO2 levels [53, 147, 148]. Similar to our findings, MAP has been documented to minimize aflatoxin B1 levels in pistachios [72], peanut [107, 159, 162], and Brazil nuts [133].
Based on our results, chitosan coating appeared to be more effective in reducing aflatoxin B1 production than MAP during storage, because aflatoxin B1 levels of all coated treatments even those under AAC were lower than the device detection limit (Table 1). Chitosan’s inhibitory effect on aflatoxin production is possibly attributed to its ability to chelate metals (such as zinc, magnesium, iron, and molybdenum) and also activating defense mechanisms through inducing phenolic compounds production. Most inhibitors act on the biosynthesis of aflatoxin either by altering the fungal physiological environment, by disrupting the signal transduction and genetic expression regulation, or by blocking the activity of compounds or enzymes needed by the fungus [64]. Chitosan has also been shown to inhibit the aflatoxin production of A. parasiticus and A. flavus in peanut [41]. Several researchers reported other coatings and packaging to prevent aflatoxin production in peanut, Brazil nut, cashew, walnut, almond, hazelnut, and pistachio nut [65, 82, 132, 149].
According to our data, the highest and lowest detected levels of aflatoxin B1 were found in uncoated treatment under AAC (0.63 ± 0.12 ppb) and uncoated treatment under A-MAP (0.19 ± 0.08 ppb) on the 45th day of storage, respectively. The perception that mycotoxins may have serious effects on humans and animals has prompted many countries in recent decades to establish maximum tolerated level on mycotoxins in foodstuffs and feedstuffs to protect the humans health, as well as the economical interests of producers and traders. Currently, the global range of limitations for aflatoxin B1 is 1–20 ng/g [43]. The U.S. Food and Drug Administration also set threshold levels for total aflatoxins in pistachio nuts at 20 µg/kg [2]. The allowed maximum legal limits for pistachio nuts are 4 µg/kg for total aflatoxin and 2 µg/kg for aflatoxin B1 in European Union [135]. Thus, aflatoxin B1 levels in our study were all below the level which is recommended as safe (Table 1).
Hull color evaluation
The hull color is known to be a primary factor in the determination of overall pistachio fruit quality. If it is not attractive, a potential consumer would never experience the other main attributes such as taste, texture, and odor. Changes in the hull color of harvested ripe pistachio fruits occur gradually during storage. Table 1 summarizes the changes in-hull color properties of fresh pistachios under various conditions during the storage time. The evaluation of hull color revealed that, all the samples showed decreasing L* values during storage (Table 1). Our results indicated that coating treatments impart significant changes in the hull lightness of fruits. Uncoated samples were significantly darker than coated samples throughout the storage period (P < 0.05). In addition, the chitosan concentration of coating resulted in significant differences in L* values. According to Table 1, there was an increase in a* values of fruit hulls during 45-day storage for all treatments. This rise in redness was possibly attributed to the promotion of enzymatic processes responsible for a decline in the quality of fruits, which included in browning and other reactions [27]. It is necessary to mention that, coated samples obtained lower a* values than uncoated samples, but the differences were not statistically significant (P > 0.05). The results also revealed that the use of chitosan coating provided the samples with significantly lower b* values than those of uncoated samples during storage (P < 0.05). It is worth expressing that there was an increase in C* values during storage time and the values were significantly higher for uncoated than coated fruits (P < 0.05). The h˚ values of fruit hulls did not change significantly during storage time for all treatments (P > 0.05). Although the BI values of fruit hulls increased in all treatments during storage, chitosan coating significantly controlled the upward trend (P < 0.05). As shown in Table 1, chitosan’s ability to reduce the darkening of hull was enhanced by increasing chitosan coating concentration. The highest BI value occurred in uncoated treatment under AAC on day 45, as it was predictable by its lowest L* value (Table 1). Pistachio hull darkening during storage is related to browning reactions due to the oxidation of phenols [100] which are abundant in the hull [47]. The control of moisture loss by chitosan coating and its ability to act as oxygen barrier, essential for the occurrence of browning reactions, contribute to reduce external color changes in fresh pistachio hull. Hernandez-Munoz et al. also observed that strawberries coated with 1.5% chitosan showed lower weight loss and decreased darkening than did those treated with 1% chitosan [63]. Chitosan coating also helped to postpone the fruit color change of litchi [33, 71], papaya [55], sweet potatoes [158], mushrooms [37], orange [23], cantaloupe [25], bell pepper [5], and guavas [32].
According to our data, packaging both coated and uncoated pistachio fruits under MAP significantly improved the hull color quality of fresh pistachio as compared to AAC (P < 0.05). However, A-MAP was more effective than P-MAP in controlling the deterioration of hull color parameters (Table 1). In other words, those samples under A-MAP (excluding uncoated treatment which experienced anaerobic respiration), revealed higher L* but lower a*, b*, C*, and BI values than those of P-MAP samples on the 45th day of storage. We assume that the high BI value in uncoated treatment under A-MAP was attributed to an increase in the activity of polyphenol oxidase because of the stress caused by high levels of carbon dioxide [22, 56, 102]. Amanatidou et al. also confirmed that surface browning in fresh-cut carrots could occur under low levels of O2 in MAP during storage [6]. Some other researchers have reported similar results for maintaining the desired color under MAP conditions for pistachio fruits [108, 136, 138], walnut [28], and peanuts kernels [107]. Our results revealed that 1.5% chitosan-coated treatment under A-MAP showed the highest L* but the lowest a*, b*, C*, and BI values on the 45th day of storage (Table 1), demonstrating the best retention of pistachio hull's initial red/purple color. The changes of C*, h0, and BI at day 45 of storage compared to day 0 were 41.09%, 1.38%, and 120.70%. In conclusion, the integrated treatment of chitosan and MAP showed a synergistic effect on the inhibition of pistachio hull discoloration and it was the most effective way to inhibit fresh pistachio’s surface browning. These findings are consistent with those of other researchers who studied the ability of edible coatings in combination with MAP to prevent the browning of eggplants [51], button mushrooms [69], and lotus root [168].
Conclusion
The inappropriate packaging and storage conditions impair the quality and act as obstacles for the exports. For the first time, the combined effects of MAP (A-MAP and P-MAP) and chitosan coating on the shelf life of fresh in-hull pistachio fruits during cold storage were examined. All treatments delayed weight loss, mold and yeast growth, aflatoxin B1 production, firmness loss, hull color changes, peroxide and acid values compared to uncoated treatment under ambient atmospheric condition (AAC). Taking into account the overall findings achieved, the most recommended treatment for preserving the postharvest quality and safety of the fresh in-hull pistachio fruits was found to be 1.5% chitosan-coated treatment under A-MAP (10% O2 and 90% N2). As fresh pistachio fruit is one of the most popular agricultural products, this accomplishment is of considerable practical importance for producers seeking to export the product to other countries and consumers who like consuming fresh in-hull pistachios with no lack of quality.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AAC:
-
Ambient atmospheric condition
- A-MAP:
-
Active-MAP
- BI:
-
Browning index
- CFU:
-
Colony-forming units
- LLDPE:
-
Linear low-density polyethylene
- MAP:
-
Modified atmosphere packaging
- P-MAP:
-
Passive-MAP
- YGC:
-
Yeast extract glucose chloramphenicol
References
Adiletta G, Magri A, Albanese D, Liguori L, Sodo M, Di Matteo M, Petriccione M. Overall quality and oxidative damage in packaged freshly shelled walnut kernels during cold storage. J Food Meas Charact. 2020. https://doi.org/10.1007/s11694-020-00589-9.
Administration, U.S.F. and D. Guidance for industry: action levels for poisonous or deleterious substances in human food and animal feed. Washington: USFDA; 2000.
Akhoundi A, Chizari M, Pezeshkirad GHR, Nourouzi O. An investigation of the effective factors involved in knowledge of pistachio farmers in controlling aflatoxin in Yazd province. Ahmadi: Iran. Iranian Journal Of Agricultural Sciences; 2007.
Ali A, Muhammad MTM, Sijam K, Siddiqui Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011;124:620–6.
Ali A, Noh NM, Mustafa MA. Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag Shelf Life. 2015;3:56–61.
Amanatidou A, Slump RA, Gorris LGM, Smid EJ. High oxygen and high carbon dioxide modified atmospheres for shelf-life extension of minimally processed carrots. J Food Sci. 2000;65:61–6.
Amara MB, Chiara ML, Colelli G, Abda JB. Modified atmosphere packaging and quality determination of fresh cut’Mazetto’almond kernels, in: XXX International Horticultural Congress IHC2018: International Symposium on Strategies and Technologies to Maintain Quality. 2018;349–354.
AOAC. Official methods of analysis, in: Association of Official Analytical Chemists. AOAC Press, Arlington; 1990.
Arnon H, Zaitsev Y, Porat R, Poverenov E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol Technol. 2014;87:21–6.
Ballistreri G, Arena E, Fallico B. Influence of ripeness and drying process on the polyphenols and tocopherols of Pistacia vera L. Molecules. 2009;14:4358–69.
Bartz JA, Brecht JK. Postharvest physiology and pathology of vegetables. Series: food science and technology. 2nd ed. Boca Raton: CRC Press; 2002.
Barzaman M, Mirdehghan SH. Combined preharvest application of polyamines and chitosan postharvest treatments on qualitative parameters of fresh Pistachio cv. Akbari Iran J Hortic Sci Technol. 2018;19:353–64.
Bello MO, Akindele TL, Adeoye DO, Oladimeji AO. Physicochemical properties and fatty acids profile of seed oil of Telfairia occidentalis Hook. F Int J Basic Appl Sci. 2011;11:9–14.
Beltrán D, Selma MV, Tudela JA, Gil MI. Effect of different sanitizers on microbial and sensory quality of fresh-cut potato strips stored under modified atmosphere or vacuum packaging. Postharvest Biol Technol. 2005;37:37–46.
Ben Abda J, Ben Amara M, Bahri N, Abdelli S. Effect of storage temperature and modified atmosphere packaging on quality of fresh green’Mazetto’almonds, In: VIII international postharvest symposium: enhancing supply chain and consumer benefits-ethical and technological issues 1194. 2016; 921–926.
Benítez S, Achaerandio I, Pujolà M, Sepulcre F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT-Food Sci Technol. 2015;61:184–93.
Bill M, Sivakumar D, Korsten L, Thompson AK. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014;64:159–67.
BS, 1987. British standard Institution. No. 684.
Burton WG. Post-harvest physiology of food crops. London: Longman Group Ltd.; 1982.
Caleb OJ, Mahajan PV, Al-Said FA-J, Opara UL. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences—a review. Food Bioprocess Technol. 2013;6:303–29.
Campaniello D, Bevilacqua A, Sinigaglia M, Corbo MR. Chitosan: antimicrobial activity and potential applications for preserving minimally processed strawberries. Food Microbiol. 2008;25:992–1000.
Catalano AE, Schilirò A, Todaro A, Palmeri R, Spagna G. Enzymatic degradations on fresh-cut eggplants differently packaged, In: International Conference on Quality Management of Fresh Cut Produce 746. 2007; 469–474.
Cháfer M, Sánchez-González L, González-Martínez C, Chiralt A. Fungal decay and shelf life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J Food Sci. 2012;77:E182–7.
Chatrabnous N, Yazdani N, Tavallali V, Vahdati K. Preserving quality of fresh walnuts using plant extracts. LWT. 2018;91:1–7.
Chen W, Jin TZ, Gurtler JB, Geveke DJ, Fan X. Inactivation of Salmonella on whole cantaloupe by application of an antimicrobial coating containing chitosan and allyl isothiocyanate. Int J Food Microbiol. 2012;155:165–70.
Chien P-J, Sheu F, Lin H-R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007;100:1160–4.
Chien P-J, Sheu F, Yang F-H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J Food Eng. 2007;78:225–9.
Christopoulos MV, Tsantili E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci Hortic. 2011;131:49–57.
Crisosto CH, Garner D, Crisosto G. High carbon dioxide atmospheres affect stored’Thompson seedless’ table grapes. HortScience. 2002;37:1074–8.
Daniels JA, Krishnamurthi R, Rizvi SSH. A review of effects of carbon dioxide on microbial growth and food quality. J Food Prot. 1985;48:532–7.
Daş E, Gürakan GC, Bayındırlı A. Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella Enteritidis on cherry tomatoes. Food Microbiol. 2006;23:430–8.
de Aquino AB, Blank AF, de Aquino Santana LCL. Impact of edible chitosan–cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015;171:108–16.
De Reuck K, Sivakumar D, Korsten L. Effect of integrated application of chitosan coating and modified atmosphere packaging on overall quality retention in litchi cultivars. J Sci Food Agric. 2009;89:915–20.
Deng Y, Wu Y, Li Y. Effects of high O 2 levels on post-harvest quality and shelf life of table grapes during long-term storage. Eur Food Res Technol. 2005;221:392–7.
Devlieghere F, Vermeulen A, Debevere J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004;21:703–14.
Dong H, Cheng L, Tan J, Zheng K, Jiang Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J Food Eng. 2004;64:355–8.
Eissa HAA. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J Food Qual. 2007;30:623–45.
El-Eleryan EE. Effect of chitosan and green tea on the quality of Washington Navel orange during cold storage. Am J Plant Physiol. 2015;10:43–54.
El Ghaouth A, Arul J, Asselin A, Benhamou N. Antifungal activity of chitosan on post-harvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol Res. 1992;96:769–79.
Essei E, Amadi C. Physicochemical characterisation of butternut (Jugulans cinerea) oil. Glob J Pure Appl Sci. 2009;15:3.
Fajardo JE, Waniska RD, Cuero RG, Pettit RE. Phenolic compounds in peanut seeds: enhanced elicitation by chitosan and effects on growth and aflatoxin B1 production by Aspergillus flavus. Food Biotechnol. 1995;9:59–78.
FAO, 2018. The production of pistachios in the World [WWW Document]. FAO. URL https://knoema.com/data/agriculture-indicators-production+pistachios
FAO. Worldwide regulations for mycotoxins in food and feed in 2003; 2004.
Feng WY, Jiang LQ, Ma HL, Zhu X. Effect of modified atmosphere packaging with different thicknesses of PE films on postharvest physiology and preservation of green walnut fruit. Food Sci. 2013;34:295–300.
Fernandes L, Pereira EL, do Céu Fidalgo M, Gomes A, Ramalhosa E. Physicochemical properties and microbial control of chestnuts (Castanea sativa) coated with whey protein isolate, chitosan and alginate during storage. Sci Hortic. 2020;263:109105.
Fourie PC, Basson DS. Predicting occurrence of rancidity in stored nuts by means of chemical analyses. Leb Technol. 1989;22:251–3.
Garavand F, Madadlou A, Moini S. Determination of phenolic profile and antioxidant activity of pistachio hull using high-performance liquid chromatography–diode array detector–electro-spray ionization–mass spectrometry as affected by ultrasound and microwave. Int J Food Prop. 2017;20:19–29.
Garcıa-Pascual P, Mateos M, Carbonell V, Salazar DM. Influence of storage conditions on the quality of shelled and roasted almonds. Biosyst Eng. 2003;84:201–9.
Geng Y, Xu L, Ma B, Wu G, Zhu L. The quality and physiological changes in different kind fresh walnuts during refrigeration. Food Sci Technol. 2013;38(3):49–54.
Georgiadou M, Kainourgiou N, Panagou E, Kanavouras, A, Yanniotis S, Application of modified atmosphere packaging to increase shelf-life in fresh pistachio nuts. International Conference. Athens; 2015.
Ghidelli C, Mateos M, Rojas-Argudo C, Pérez-Gago MB. Extending the shelf life of fresh-cut eggplant with a soy protein–cysteine based edible coating and modified atmosphere packaging. Postharvest Biol Technol. 2014;95:81–7.
Ghirardello D, Contessa C, Valentini N, Zeppa G, Rolle L, Gerbi V, Botta R. Effect of storage conditions on chemical and physical characteristics of hazelnut (Corylus avellana L.). Postharvest Biol Technol. 2013;81:37–43.
Giorni P, Battilani P, Pietri A, Magan N. Effect of aw and CO2 level on Aspergillus flavus growth and aflatoxin production in high moisture maize post-harvest. Int J Food Microbiol. 2008;122:109–13.
Gonzalez-Aguilar GA, Buta JG, Wang CY. Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain postharvest quality of papaya ‘Sunrise.’ Postharvest Biol Technol. 2003;28:361–70.
González-Aguilar GA, Valenzuela-Soto E, Lizardi-Mendoza J, Goycoolea F, Martínez-Téllez MA, Villegas-Ochoa MA, Monroy-García IN, Ayala-Zavala JF. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol.’ J Sci Food Agric. 2009;89:15–23.
Gorny JR, Hess-Pierce B, Cifuentes RA, Kader AA. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol Technol. 2002;24:271–8.
Gotoh N, Wada S. The importance of peroxide value in assessing food quality and food safety. JAOCS J Am Oil Chem Soc. 2006;83:473.
Guillén F, Zapata PJ, Martínez-Romero D, Castillo S, Serrano M, Valero D. Improvement of the overall quality of table grapes stored under modified atmosphere packaging in combination with natural antimicrobial compounds. J Food Sci. 2007;72:S185–90.
Han C, Zhao Y, Leonard SW, Traber MG. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria× ananassa) and raspberries (Rubus ideaus). Postharvest Biol Technol. 2004;33:67–78.
Harris JL. Safe, low-distortion tape touch method for fungal slide mounts. J Clin Microbiol. 2000;38:4683–4.
Hashemi SMB, Raeisi S. Evaluation of antifungal and antioxidant properties of edible coating based on apricot (Prunus armeniaca) gum containing Satureja intermedia extract in fresh wild almond (Amygdalus scoparia) kernels. J Food Meas Charact. 2018;12:362–9.
Helander IM, Nurmiaho-Lassila E-L, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int J Food Microbiol. 2001;71:235–44.
Hernandez-Munoz P, Almenar E, Del Valle V, Velez D, Gavara R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria× ananassa) quality during refrigerated storage. Food Chem. 2008;110:428–35.
Holmes RA, Boston RS, Payne GA. Diverse inhibitors of aflatoxin biosynthesis. Appl Microbiol Biotechnol. 2008;78:559–72.
Hontanaya C, Meca G, Luciano FB, Mañes J, Font G. Inhibition of aflatoxin B1, B2, G1 and G2 production by Aspergillus parasiticus in nuts using yellow and oriental mustard flours. Food Control. 2015;47:154–60.
ISIRI. Institute of Standards and Industrial Research of Iran. 6872; 2011
Ito Y, Peterson SW, Wicklow DT, Goto T. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycol Res. 2001;105:233–9.
Jacxsens L, Devlieghere F, Debevere J. Predictive modelling for packaging design: equilibrium modified atmosphere packages of fresh-cut vegetables subjected to a simulated distribution chain. Int J Food Microbiol. 2002;73:331–41.
Jiang T. Effect of alginate coating on physicochemical and sensory qualities of button mushrooms (Agaricus bisporus) under a high oxygen modified atmosphere. Postharvest Biol Technol. 2013;76:91–7.
Jiang T, Feng L, Zheng X. Effect of chitosan coating enriched with thyme oil on postharvest quality and shelf life of shiitake mushroom (Lentinus edodes). J Agric Food Chem. 2012;60:188–96.
Jiang Y, Li J, Jiang W. Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature. LWT-food Sci Technol. 2005;38:757–61.
Kader A, Labivitch J, Mitchell F, Sommer N. Quality and safety of pistachio nuts as influenced by postharvest handling procedure. Pist Assoc Annu Rep. 1979; 45–56.
Kader AA, Watkins CB. Modified atmosphere packaging—toward 2000 and beyond. HortTechnology. 2000;10:483–6.
Kanazawa A, Sawa T, Akaike T, Maeda H. Dietary lipid peroxidation products and DNA damage in colon carcinogenesis. Eur J Lipid Sci Technol. 2002;104:439–47.
Karabulut OA, Baykal N. Integrated control of postharvest diseases of peaches with a yeast antagonist, hot water and modified atmosphere packaging. Crop Prot. 2004;23:431–5.
Kays SJ. Postharvest physiology and handling of perishable plant products. Van Nostrand Reinhold Inc; 1991.
Kazemi MM, Hashemi-Moghaddam H, Mohammadi Nafchi A, Ajodnifar H. Application of modified packaging and nano ZnO for extending the shelf life of fresh pistachio. J Food Process Eng. 2020. https://doi.org/10.1111/jfpe.13548.
Kerch G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: a review. Trends Food Sci Technol. 2015;46:159–66.
Koyuncu MA, Islam A, Küçük M. Fat and fatty acid composition of hazelnut kernels in vacuum packages during storage. Grasas Aceites. 2005;56:263–6.
Li P, Wang W, Liang L, Wang G. Effects of modified atmosphere packaging on changes of physiology and quality of pine nut at ambient temperature. Trans Chinese Soc Agric Eng. 2009;25:324–9.
Liu H, Du Y, Wang X, Sun L. Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol. 2004;95:147–55.
Lopes LF, Bordin K, de Lara GHC, Saladino F, Quiles JM, Meca G, Luciano FB. Fumigation of Brazil nuts with allyl isothiocyanate to inhibit the growth of Aspergillus parasiticus and aflatoxin production. J Sci Food Agric. 2018;98:792–8.
Ma H, Song S, Ma Y, Liu F, Li J, Ma C. Effects of modified atmosphere package on preservation of green walnut fruit. Trans Chinese Soc Agric Eng. 2012;28:262–7.
Ma Y, Liu X, Yuan D, Wang L, Yuan Y. Changes of respiration intensity and quality of different varieties of fresh walnut during cold storage. Trans Chinese Soc Agric Eng. 2010;26:370–4.
Maghsoudlou A, Maghsoudlou Y. Effect of chitosan coating with different molecular weights and concentrations on oxidation activity in the pistachio nut. J Food Res. 2013;23(1):121–31.
Maghsoudlou A, Maghsoudlou Y, Khomeiri M, Ghorbani M. Evaluation of anti-fungal activity of chitosan and its effect on the moisture absorption and organoleptic characteristics of pistachio nuts. Int J Adv Sci Eng Inf Technol. 2012;2:336–40.
Mahfoudhi N, Hamdi S. Use of almond gum and gum arabic as novel edible coating to delay postharvest ripening and to maintain sweet cherry (P runus avium) quality during storage. J Food Process Preserv. 2015;39:1499–508.
Maleki G, Sedaghat N, Woltering EJ, Farhoodi M, Mohebbi M. Chitosan-limonene coating in combination with modified atmosphere packaging preserve postharvest quality of cucumber during storage. J Food Meas Charact. 2018;12:1610–21.
Manjunatha M, Anurag RK. Effect of modified atmosphere packaging and storage conditions on quality characteristics of cucumber. J Food Sci Technol. 2014;51:3470–5.
Markuszewski B, Kopytowski J. Effects of storage conditions on the quality of unripe hazelnuts in the husk. J Hortic Res. 2015;23:59–67.
Márquez IG, Akuaku J, Cruz I, Cheetham J, Golshani A, Smith ML. Disruption of protein synthesis as antifungal mode of action by chitosan. Int J Food Microbiol. 2013;164:108–12.
Martínez-Romero D, Guillén F, Castillo S, Valero D, Serrano M. Modified atmosphere packaging maintains quality of table grapes. J Food Sci. 2003;68:1838–43.
Martiñon ME, Moreira RG, Castell-Perez ME, Gomes C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 C. LWT-Food Sci Technol. 2014;56:341–50.
Maskan M, Karatag S. Sorption characteristics of whole pistachio nuts (Pistacia vera L.). Dry Technol. 1997;15:1119–39.
Maskan M, Karataş Ş. Storage stability of whole-split pistachio nuts (Pistachia vera L.) at various conditions. Food Chem. 1999;66:227–33.
Mason RL, Nottingham SM, Reid CE, Gathambiri C. The quality of macadamia kernels stored in simulated bulk retail dispensers. Food Aust. 2004;56:133–9.
Mencarelli F, Forniti R, DeSantis D, Bellincontro A. Effects of inert atmosphere and temperature for dried hazelnuts storage. Ingredienti Aliment. 2008;39:16–21.
Mexis SF, Badeka AV, Kontominas MG. Quality evaluation of raw ground almond kernels (Prunus dulcis): effect of active and modified atmosphere packaging, container oxygen barrier and storage conditions. Innov Food Sci Emerg Technol. 2009;10:580–9.
Mohammadi A, Hashemi M, Hosseini SM. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov Food Sci Emerg Technol. 2016;33:580–8.
Molamohammadi H, Pakkish Z, Akhavan H-R, Saffari VR. Effect of salicylic acid incorporated chitosan coating on shelf life extension of fresh in-hull pistachio fruit. Food Bioprocess Technol. 2020;13:121–31.
Molteberg EL, Magnus EM, Bjørge JM, Nilsson A. Sensory and chemical studies of lipid oxidation in raw and heat-treated oat flours. Cereal Chem. 1996;73:579–87.
Moscetti R, Frangipane MT, Monarca D, Cecchini M, Massantini R. Maintaining the quality of unripe, fresh hazelnuts through storage under modified atmospheres. Postharvest Biol Technol. 2012;65:33–8.
Nadarajah K, Prinyawiwatkul W, No HK, Sathivel S, Xu Z. Sorption behavior of crawfish chitosan films as affected by chitosan extraction processes and solvent types. J Food Sci. 2006;71:E33–9.
Nasar-Abbas SM, Plummer JA, Siddique KHM, White PF, Harris D, Dods K. Nitrogen retards and oxygen accelerates colour darkening in faba bean (Vicia faba L.) during storage. Postharvest Biol Technol. 2008;47:113–8.
Nazoori F, Kalantari S, Doraki N, Talaie A, Javanshah A. Effect of harvest time, processing type and storage condition on preservation fresh and dried pistachios nuts. J Crop Improv. 2014. https://doi.org/10.22059/jci.2015.53245.
Olivas GI, Barbosa-Cánovas GV. Edible coatings for fresh-cut fruits. Crit Rev Food Sci Nutr. 2005;45:657–70.
Opio P, Photchanachai S. Modified atmosphere influences aflatoxin B1 contamination and quality of peanut (Arachis hypogaea L.) kernels cv. Khon Kaen 84–8. J Stored Prod Res. 2018;78:67–73.
Ozturk I, Sagdic O, Yalcin H, Capar TD, Asyali MH. The effects of packaging type on the quality characteristics of fresh raw pistachios (Pistacia vera L.) during the storage. LWT-Food Sci Technol. 2016;65:457–63.
Padehban L, Ansari S, Koshani R. Effect of packaging method, temperature and storage period on physicochemical and sensory properties of wild almond kernel. J Food Sci Technol. 2018;55:3408–16.
Panahirad S, Zaare-Nahandi F, Mohammadi N, Alizadeh-Salteh S, Safaie N. Effects of salicylic acid on Aspergillus flavus infection and aflatoxin B1 accumulation in pistachio (Pistacia vera L.) fruit. J Sci Food Agric. 2014;94:1758–63.
Paramawati R, Widodo P, Budiharti U, Handaka H. The role of postharvest machineries and packaging in minimizing aflatoxin contamination in peanut. Indones J Agr Sci. 2006;7(1):15–9.
Perez-Gago MB, Rojas C, Del Rio MA. Effect of hydroxypropyl methylcellulose-lipid edible composite coatings on plum (cv. Autumn giant) quality during storage. J Food Sci. 2003;68:879–83.
Phillips CA. Modified atmosphere packaging and its effects on the microbiological quality and safety of produce. Int J food Sci Technol. 1996;31:463–79.
Poverenov E, Zaitsev Y, Arnon H, Granit R, Alkalai-Tuvia S, Perzelan Y, Weinberg T, Fallik E. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol Technol. 2014;96:106–9.
Pushkala R, Parvathy KR, Srividya N. Chitosan powder coating, a novel simple technique for enhancement of shelf life quality of carrot shreds stored in macro perforated LDPE packs. Innov Food Sci Emerg Technol. 2012;16:11–20.
Qi H, Hu W, Jiang A, Tian M, Li Y. Extending shelf-life of fresh-cut ‘Fuji’apples with chitosan-coatings. Innov Food Sci Emerg Technol. 2011;12:62–6.
Rabea EI, Badawy ME-T, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromol. 2003;4:1457–65.
Ranjeet S, Giri SK, Kulkarni SD, Ahirwar R. Study on respiration rate and respiration quotient of green mature mango (Mangifera indica L.) under aerobic conditions. Asian J Bio Sci. 2012;7:210–3.
Reddy MVB, Angers P, Castaigne F, Arul J. Chitosan effects on blackmold rot and pathogenic factors produced by Alternaria alternata in postharvest tomatoes. J Am Soc Hortic Sci. 2000;125:742–7.
Rezagholi F, Hesarinejad M.A. Integration of fuzzy logic and computer vision in intelligent quality control of celiac-friendly products, in: Procedia Computer Science. 2017; 325–332.
Rezaiyan Attar F, Sedaghat N, Pasban A, Yeganehzad S, Hesarinejad MA. Modeling the respiration rate of chitosan coated fresh in-hull pistachios (Pistacia vera L. cv. Badami) for modified atmosphere packaging design. J Food Meas Charact. 2022;16:1049–61.
Rezaiyan Attar F, Sedaghat N, Yeganehzad S, Pasban A, Hesarinejad MA. Shelf life modeling of Badami’s fresh pistachios coated with chitosan under modified atmosphere packaging conditions. Food Sci Technol. 2021;18:181–94.
Romanazzi G. Chitosan treatment to control postharvest gray mold of table grapes. Phytopathology. 2005;95:S90.
Romanazzi G, Gabler FM, Smilanick JL. Preharvest chitosan and postharvest UV irradiation treatments suppress gray mold of table grapes. Plant Dis. 2006;90:445–50.
Romanazzi G, Karabulut OA, Smilanick JL. Combination of chitosan and ethanol to control postharvest gray mold of table grapes. Postharvest Biol Technol. 2007;45:134–40.
Sabaghi M, Maghsoudlou Y, Khomeiri M, Ziaiifar AM. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol Technol. 2015;110:224–8.
Sahai AS, Manocha MS. Chitinases of fungi and plants: their involvement in morphogenesis and host—parasite interaction. FEMS Microbiol Rev. 1993;11:317–38.
Sánchez-González L, Pastor C, Vargas M, Chiralt A, González-Martínez C, Cháfer M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol Technol. 2011;60:57–63.
de Santana LRR, Benedetti BC, Sigrist JMM, Sato HH. Effects of modified atmosphere packaging on ripening of’Douradão’peach related to pectolytic enzymes activities and chilling injury symptoms. Rev Bras Frutic. 2011;33:1084–94.
Saremi H, Okhovvat SM. Effect of Aflatoxin produced by Aspergillus flavus in reduction of our Pistachio marketing all over the world. Iran Food Sci Technol Res J. 2007;3:9–15.
Sayanjali S, Ghanbarzadeh B, Ghiassifar S. Evaluation of antimicrobial and physical properties of edible film based on carboxymethyl cellulose containing potassium sorbate on some mycotoxigenic Aspergillus species in fresh pistachios. LWT-Food Sci Technol. 2011;44:1133–8.
Sayyahi Z, Beigmohammadi F, Shoaiee S. Optimization of starch biopolymer enriched with chitosan containing rosemary essential oil and its application in packaging of peanuts. Nutr Food Sci Res. 2017;4:19–28.
Scussel VM, Giordano BN, Simao V, Manfio D, Galvao S, Rodrigues MNF. Effect of oxygen-reducing atmospheres on the safety of packaged shelled Brazil nuts during storage. Int J Anal Chem. 2011;2011:813591.
Serrano M, Martinez-Romero D, Castillo S, Guillén F, Valero D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov food Sci Emerg Technol. 2005;6:115–23.
Set E, Erkmen O. The aflatoxin contamination of ground red pepper and pistachio nuts sold in Turkey. Food Chem Toxicol. 2010;48:2532–7.
Shayanfar S, Kashaninejad M, Khomeiri M, Emam DZ, Mostofi Y. Effect of MAP and different atmospheric conditions on the sensory attributes and shelf life characteristics of fresh pistachio nuts. J Nuts. 2011;2(3):47–57.
Sheikhi A, Mirdehghan SH, Ferguson L. Extending storage potential of de-hulled fresh pistachios in passive-modified atmosphere. J Sci Food Agric. 2019;99:3426–33.
Sheikhi A, Mirdehghan SH, Karimi HR, Ferguson L. Effects of passive-and active-modified atmosphere packaging on physio-chemical and quality attributes of fresh in-hull pistachios (Pistacia vera L. cv. Badami). Foods. 2019;8:564.
Shi ZF, Chen GY, Zhang XP, Qi XW. Preservation of fresh areca nuts with several herbal medicine extracts advanced materials research. Trans Tech Publ. 2013;647:532–7.
Simões ADN, Tudela JA, Allende A, Puschmann R, Gil MI. Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks. Postharvest Biol Technol. 2009;51:364–70.
Sivakumar D, Korsten L. Influence of modified atmosphere packaging and postharvest treatments on quality retention of litchi cv. Mauritius Postharvest Biol Technol. 2006;41:135–42.
Sivakumar D, Regnier T, Demoz B, Korsten L. Effect of different post-harvest treatments on overall quality retention in litchi fruit during low temperature storage. J Hortic Sci Biotechnol. 2005;80:32–8.
Sivakumar D, Sultanbawa Y, Ranasingh N, Kumara P, Wijesundera R. Effect of the combined application of chitosan and carbonate salts on the incidence of anthracnose and on the quality of papaya during storage. J Hortic Sci Biotechnol. 2005;80:447–52.
Song L, Gao H, Ge L, Mao J, Zhou Y, Chen H, Tao F. Effects of different packaging on lipid peroxidation and antioxidant ability of Torreya grandis nuts during storage at room temperature. Sci Silvae Sin. 2009;45:49–53.
Suseno N, Savitri E, Sapei L, Padmawijaya KS. Improving shelf-life of cavendish banana using chitosan edible coating. Procedia Chem. 2014;9:113–20.
Synowiecki J, Al-Khateeb NA. Production, properties, and some new applications of chitin and its derivatives. Cri Rev Food Sci Nut. 2003;43(2):145–71.
Taniwaki MH, Hocking AD, Pitt JI, Fleet GH. Growth and mycotoxin production by fungi in atmospheres containing 80% carbon dioxide and 20% oxygen. Int J Food Microbiol. 2010;143:218–25.
Taniwaki MH, Hocking AD, Pitt JI, Fleet GH. Growth and mycotoxin production by food spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int J Food Microbiol. 2009;132:100–8.
Tavakolipour H, Javanmard DM, Zirjany L. Inhibitory effect of coated pistachio kernel based in whey protein concentrate (WPC) and thyme essential oil on aflatoxin production. Inn Food Sci Tech. 2011;2(3):53–63.
Tesfay SZ, Magwaza LS. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag Shelf Life. 2017;11:40–8.
Thommohaway C, Kanlayanarat S, Uthairatanakij A, Jitareerat P. Quality of fresh-cut guava (Psidium guajava L.) as affected by chitosan treatment, in: International Conference on Quality Management of Fresh Cut Produce 746. 2007; 449–454
Valverde JM, Guillén F, Martínez-Romero D, Castillo S, Serrano M, Valero D. Improvement of table grapes quality and safety by the combination of modified atmosphere packaging (MAP) and eugenol, menthol, or thymol. J Agric Food Chem. 2005;53:7458–64.
Van der Steen C, Jacxsens L, Devlieghere F, Debevere J. Combining high oxygen atmospheres with low oxygen modified atmosphere packaging to improve the keeping quality of strawberries and raspberries. Postharvest Biol Technol. 2002;26:49–58.
Vanaei M, Sedaghat N, Abbaspour H, Kaviani M, Azarbad HR. Novel edible coating based on aloe vera gel to maintain pistachio quality. Int J Sci Eng Technol. 2014;3:1016–9.
Vargas M, Chiralt A, Albors A, González-Martínez C. Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biol Technol. 2009;51:263–71.
Waghmare RB, Annapure US. Effects of hydrogen peroxide, modified atmosphere and their combination on quality of minimally processed cluster beans. J Food Sci Technol. 2017;54:3658–65.
Waghmare RB, Annapure US. Combined effect of chemical treatment and/or modified atmosphere packaging (MAP) on quality of fresh-cut papaya. Postharvest Biol Technol. 2013;85:147–53.
Waimaleongora-Ek P, Corredor AJH, No HK, Prinyawiwatkul W, King JM, Janes ME, Sathivel S. Selected quality characteristics of fresh-cut sweet potatoes coated with chitosan during 17-day refrigerated storage. J Food Sci. 2008;73:S418–23.
Wang H, Jin X, Wu H. Modified atmosphere packaging bags of peanuts with effect of inhibition of aflatoxin growth. J Appl Polym Sci. 2014;131:8.
Wang J, Li P, Gong B, Li S, Ma H. Phenol metabolism and preservation of fresh in-hull walnut stored in modified atmosphere packaging. J Sci Food Agric. 2017;97:5335–42.
Wang J, Liang S, Ma H, Zhang P, Shi W. Effects of ethephon on fresh in-husk walnut preservation and its possible relationship with phenol metabolism. J Food Sci. 2016;81:C1921–7.
Wang J, Ma YP, Chen JH, Ma HL, Feng WY, Wang DR. Effect of modified atmosphere package conditions on preservation of green walnut fruit and kernel traits. Mod Food Sci Technol. 2014;30:169–76.
Wang S, Ikediala J, Tang J, Hansen J, Mitcham E, Mao R, Swanson B. Radio frequency treatments to control codling moth in in-shell walnuts. Postharvest Biol Technol. 2001;22:29–38.
Wang Y, Long LE. Respiration and quality responses of sweet cherry to different atmospheres during cold storage and shipping. Postharvest Biol Technol. 2014;92:62–9.
Wani SM, Amin S, Javaid I, Masoodi FA, Mir SA, Ganai SA, Yildiz F. Minimal Processing of Tropical and Subtropical Fruits, Vegetables, Nuts, and Seeds, in: Minimally Processed Refrigerated Fruits and Vegetables. Springer, 2017; 469–512. https://doi.org/10.1007/978-1-4939-7018-6_13
Wei W. Effects of modified atmosphere packaging (MAP) on fatty acid oxidation of walnut at room-temperature. J Anhui Agric. Sci. 2008; 23.
Xiao C, Zhu L, Luo W, Song X, Deng Y. Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 2010;121:1003–9.
Xing Y, Li X, Xu Q, Jiang Y, Yun J, Li W. Effects of chitosan-based coating and modified atmosphere packaging (MAP) on browning and shelf life of fresh-cut lotus root (Nelumbo nucifera Gaerth). Innov Food Sci Emerg Technol. 2010;11:684–9.
Xu YX, Kim KM, Hanna MA, Nag D. Chitosan–starch composite film: preparation and characterization. Ind Crops Prod. 2005;21:185–92.
Yang X, Zhang R, Han J, Zhang Y. Effects of different storage methods on postharvest physiology and storage quality of fresh walnut fruit. Sci Agric Sin. 2015;10:16.
Yuan G, Chen X, Li D. Chitosan films and coatings containing essential oils: the antioxidant and antimicrobial activity, and application in food systems. Food Res Int. 2016;89:117–28.
Zagory D, Kader AA. Modified atmosphere packaging of fresh produce. Food Technol. 1988;42:70–7.
Zhang J, Hao X, Li X, Wang W. Effect of chitosan coating combined with sulfur dioxide fumigation on the storage quality of fresh areca nut. J Food Process Preserv. 2017;41:e12974.
Zhang W, Jiang L, Chen C, Xiao H, Qiu L. Effect of different modified atmosphere packaging on storage quality of walnut kernels. Food Sci. 2012;2012:60.
Zheng F, Zheng W, Li L, Pan S, Liu M, Zhang W, Liu H, Zhu C. Chitosan controls postharvest decay and elicits defense response in kiwifruit. Food Bioprocess Technol. 2017;10:1937–45.
Funding
The authors wish to express their profound gratitude sincerely to the Research Deputy of Agricultural Faculty of Ferdowsi University of Mashhad for funding this project.
Author information
Authors and Affiliations
Contributions
Supervision, NS; funding acquisition, NS; conceptualization, FRA and NS; software, FRA, AP, and MAH; methodology, FRA; data curation, FRA; validation, FRA and NS, and SY; formal analysis, FRA, and MAH; writing—original draft, FRA; writing—review and editing, NS and MAH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rezaiyan Attar, F., Sedaghat, N., Pasban, A. et al. Modified atmosphere packaging with chitosan coating to prevent deterioration of fresh in-hull Badami’s pistachio fruit. Chem. Biol. Technol. Agric. 10, 16 (2023). https://doi.org/10.1186/s40538-023-00393-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00393-9