Abstract
Circulating tumor cells (CTCs) are cells that shed from a primary tumor and travel through the bloodstream. Studying the functional and molecular characteristics of CTCs may provide in-depth knowledge regarding highly lethal tumor diseases. Researchers are working to design devices and develop analytical methods that can capture and detect CTCs in whole blood from cancer patients with improved sensitivity and specificity. Techniques using whole blood samples utilize physical prosperity, immunoaffinity or a combination of the above methods and positive and negative enrichment during separation. Further analysis of CTCs is helpful in cancer monitoring, efficacy evaluation and designing of targeted cancer treatment methods. Although many advances have been achieved in the detection and molecular characterization of CTCs, several challenges still exist that limit the current use of this burgeoning diagnostic approach. In this review, a brief summary of the biological characterization of CTCs is presented. We focus on the current existing CTC detection methods and the potential clinical implications and challenges of CTCs. We also put forward our own views regarding the future development direction of CTCs.
Similar content being viewed by others
Introduction
Cancer metastasis is the primary cause of death worldwide and remains one of the prevailing challenges in curing cancer [1]. Most patients with metastatic disease receive systemic drugs to prolong survival and improve symptoms, but there is usually no cure and patients cannot achieve long-term survival. Metastasis is a multistep process involving intravasation, extravasation, migration and regeneration, in which cancer cells from a primary tumor detach and invade distant tissues using the bloodstream as a transport system [2, 3]. Cells that are separated from the primary tumor and travel through the bloodstream are called circulating tumor cells (CTCs) [4]. Understanding their part in the metastasis may contribute to better therapeutic management. In addition, CTCs can be extracted to detect the biological characteristics and molecular type of primary tumor cells. CTCs were reported for the first time as the presence of cells in blood that had the same size, shape, and appearance as those in primary tumors 150 years ago in 1869 by Ashworth Thomas Ramsden [5]. Since then, many studies have focused on exploring and developing efficient detection techniques as CTCs are noninvasive and accessible and could overcome the problem of tumor heterogeneity [5,6,7]. Significant leaps in the detection and characterization of CTCs have been achieved over the past two decades with new methods and devices emerging for CTC analysis. However, several challenges are associated with the isolation, detachment and detection of CTCs. Firstly, CTCs are infrequent and rare. Approximately 1–100 cells along with 106–108 red blood cells can be found per milliliter of blood [8, 9]. Secondly, as cancer cells are heterogeneous, a variety of groups of CTCs have significant variations in the expression of surface biomarkers [10,11,12]. Therefore, it is not easy to recognize different types of CTCs by the identical standard [13]. Finally, the nondestructive release of CTCs after the cells are captured on the surface effectively poses a challenge [14]. Herein, we systematically review CTCs, briefly provide an overview of their biology, and mainly investigate the current and emerging CTC detection techniques. Moreover, the clinical aspects of CTCs are described, and examples of how CTCs can participate in monitoring cancer development and drug therapy responses are discussed. Although the detection of CTCs is a promising technique for precision medicine, notably, there are still many unsolved problems. In this review, we present the existing challenges and offer our own insights into the future development of CTCs.
Biology of CTCs
CTCs are considered to detach themselves from a primary tumor and pass through the bloodstream which can reflect metastasis, and several studies have shown their diagnostic and prognostic significance [5, 15, 16]. Through some newly developed high-throughput technologies, we can isolate these cells from the blood and conduct research at the single-cell level [14]. Over the course of disease or treatment, CTCs can provide a precise, dynamic, and treatment-related method to treat cancer.
Steps of metastasis
Tumor cell dissemination may occur in the following outlined steps: 1) localized invasion through the basement membrane during malignant progression [17]; 2) intravasation into hematogenous or lymphatic circulation systems, which allows for transport via circulation and interactions with blood components [18]; 3) survival in circulation by competition with circulating immune cells, loss of cell–cell junctions and shear stress [19, 20]; 4) arrest in the capillary bed of various organs [3]; 5) extravasation and migration into a foreign microenvironment, followed by colonization to form micrometastases [21]; and 6) stimulation of angiogenesis leading to growth into metastatic tumors (Fig. 1). However, this process is highly inefficient, and less than 0.01% of CTCs metastasize [22].
Tumor cell dissemination. 1) localized invasion; 2) intravasation; 3) survival in circulation; 4) arrest in the capillary bed; 5) extravasation and migration; 6) stimulation of angiogenesis. In addition to individual CTCs, CTC clusters are also found in patient blood, which have a significantly higher metastatic potential and increased ability to survive. CTCs get help from the platelets as well as immune cells during the escape phase
Most CTCs introduced into circulation are quickly killed by processes, such as immune attacks, shear stress, anoikis, oxidative stress and the lack of cytokines and growth factors [22]. Therefore, CTCs undergo a series of adaptations in order to survive in such a hostile environment. The epithelial to mesenchymal transition (EMT) has been identified as a vital process allowing CTCs to behave similar to mesenchymal cells [23]. During the EMT, epithelial cells lose epithelial characteristics such as the expression of EpCAM, keratins, and E-cadherin and upregulate matrix metalloproteinase (MMP) activity, which enables these cells to navigate through the local extracellular matrix (ECM) and enter the microvasculature [10, 24,25,26]. Thus, CTCs can be easily separated from a primary tissue, invade the capillaries and possess a significantly improved ability to survive and metastasize. In addition to individual CTCs, CTC clusters are also found in patients’ blood. CTC clusters are composed of 2 to 50 cells, including fibroblasts, endothelial cells, leukocytes and platelets, which have a prominently higher metastatic capacity and increased ability to survive [27].
Characterization of CTCs
The isolation of viable CTCs enables the analysis of their molecular and functional characterization as CTCs are biochemically different from blood cells. One of the most common surface molecules on CTCs is the epithelial cell adhesion molecule (EpCAM), which originates from the epithelium [10, 25, 28]. EpCAM is a transmembrane glycoprotein that is present in 80% of solid cancers (such as breast, colorectal, and prostate cancer), but is absent in peripheral blood cells [10, 29]. Alternatives such as keratin 19, tumor-specific antigen 9, and progastrin-releasing peptides have also been reported [15]. Similarly, many tumor immune markers, such as prostate specific antigen (PSA), human epidermal growth factor receptor 2 (HER2) and endothelial growth factor receptor (EGFR), can also be used as antibodies for the specific recognition of CTCs [30, 31].
Nevertheless, in recent years, many studies have found that EpCAM is heterogeneously expressed or even not expressed on some cancers and cancer subtypes [32]. The process of the EMT has been found to be the most critical modulator of EpCAM expression [10]. The EMT is a strictly controlled process that allows cells to switch phenotypes. The EMT is believed to be essential for metastasis by promoting invasion, motility and dissemination in epithelial cancer cells [33]. Gorges and colleagues observed that EpCAM-negative breast cancer cells express high amounts of EMT-related genes [34]. The interrelated molecular mechanisms underlying EpCAM withdrawal from the cell surface may be associated with the endocytosis and subsequent degradation of EpCAM in intracellular compartments [25, 28, 32].
In addition to biochemical differences, there are distinct physical differences between CTCs and blood cells [35]. It is generally agreed that cell lines originating from solid tumors have greater cell sizes than blood cells. The cross-sectional areas of five tumor cell lines (MCF-7, Hep3B, HepG2, LNCaP, and HeLa) under a microscope were measured to be 396–796 μm2, which is significantly larger than that of leukocytes (average 140 μm2) measured under the same conditions [36]. Cell size extracted from dielectrophoresis (DEP) data clearly demonstrated the size difference among leukocytes (6.2–9.4 μm), leukemia cells (8.9–15.3 μm) and solid tumor cell lines (11.7–23.8 μm) [37]. Various biomechanical tools have been exploited to measure the mechanical properties of living cells, indicating that tumor cells with greater metastatic potential are more susceptible to deformation [24]. S. E. Cross and colleagues applied AFM to measure the stiffness of live metastatic cancer cells obtained from pleural fluids from patients with lung, breast and pancreatic cancer. The cell stiffness of metastatic cancer cells is more than 70% softer than that of benign cells within the same pleural fluid samples (Young’s modulus 0.53 ± 0.10 kPa versus 1.97 ± 0.07 kPa) [38]. Moreover, cancer cells contain a variety of polarizable particles, including peptides, proteins and nucleic acids. Gascoyne’s group applied dielectrophoretic field-flow fractionation (DEP-FFF) to study the dielectric properties of cancer cells and reported that the capacitances of cancer cells are significantly larger than those of blood cells. All data show that the total cell capacitance scales with the cube of the cell diameter, which is consistent with the general conclusion that cancer cells are larger than blood cells [37].
Cells contribute to the survival of CTCs
Circulating tumor cells receive help from other nontumor cells during the escape phase (Fig. 1). Morphologic observations of tumor cells arrested in capillaries have documented the close association of tumor cells with activated platelets [39]. Platelets can rapidly enfold CTCs, protecting them from fierce shear forces [20]. Platelet aggregation induced by tumor cells can promote extravasation and adhesion [40]. Platelets also provide a defense against the immune system. Platelet-secreted transforming growth factor-β(TGF-β) is able to inactivate natural killer (NK) cells [41]. Transferring the MHC I complex from granular platelets to CTCs shields CTCs from the cytotoxic attack of NK cells [42].
In addition to platelets, increasing evidence suggests that many other blood cells are associated with the metastasis of CTCs in the bloodstream, such as neutrophils, monocytes and Treg cells. CTCs interact with endothelial-bound neutrophils in the vascular network, promoting adhesion and migration activities through different molecular targets (IL-8, CAM-1) expands the metastatic potential [19, 43, 44].
It has also been demonstrated that monocytes may play an important role in metastasis. Monocytes were observed to be associated with five or more CTCs in metastatic breast cancer (MBC) [45]. Classical monocytes can extravasate and differentiate into macrophages, promoting tumor cell extravasation, survival, and subsequent growth [46]. A subpopulation of CCR2 (receptor for chemokine CCL2) expressing monocytes was recruited by metastatic tumor cells which enhanced the subsequent extravasation of the tumor cells through the targeted delivery of molecules such as vascular endothelial growth factor(VEGF) [47].
CTCs have also adapted to avoid attack by immune cells in the bloodstream. Tumor cells are able to achieve immune escape by upregulating the expression of FASL on their surface, reducing the threshold for apoptosis in cytotoxic T lymphocytes (CTLs) [48]. Moreover, CTCs express programmed cell death-ligand 1(PD-L1), representing a potential mechanisms responsible for immune escape [49, 50]. Researchers have proposed that CTCs positive for PD-L1 can mediate Treg cells to play a role of immunosuppression. Treg cells can protect CTCs against being attacked by the immune system, weaken CTL killing ability and trigger more myeloid-derived suppressor cells (MDSCs) [51]. It has also been found that CTCs of colorectal cancer exhibit a distinct nonimmunogenic phenotype by overexpressing CD47 [52].
Techniques used in CTC
As a consequence of the low concentration of CTCs in peripheral blood (1-100 cells per ml), a high specificity and an excellent affinity are both obligatory requirements for effective CTC capture as stated above. Most of the extant technologies consist of a two-step process of cell enrichment and subsequent detection. Most CTCs enrichment methods utilize the unique surficial antigen expression of CTCs to separate them from the great number of leukocytes, erythrocytes and other blood components [4, 53]. There are also technologies capturing CTCs that utilize the physical properties of CTCs including their size, density, and capacitive character [6, 54, 55]. The subsequent challenge is to effectively release CTCs from surfaces using enrichment methods without damaging the target cells14. Enzymatic digestion, oligonucleotide-mediated aptamer release, and stimuli-responsive polymers hold marvelous potential for CTC detachment [56,57,58].
CTC isolation
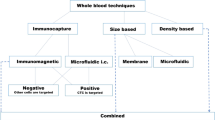
In view of the low abundance of CTCs in whole peripheral blood, separating CTCs from the background contamination of blood cells is a crucial step for subsequent analysis. However, the low frequency of CTCs along with the heterogeneity observed in CTCs render high-precision detection laborious. Currently, there is no ideal device capable of isolating a pure population of CTCs. Most separation methods are based on the physical properties or biological properties of CTCs. Due to limitations, such as low cell recovery, poor purity, and diminished viability, the widespread use of CTCs in laboratory and clinical environments is hindered (Fig. 2).
Outline of existing isolation techniques. The majority of CTC enrichment methods utilize the unique surficial antigen expression of CTCs or the physical or functional property of CTCs to separate CTCs from the great number of erythrocytes, leukocytes, and other blood. EpCAM: epithelial cell adhesion molecule; RBC: red blood cell; WBC: white blood cell; CTC: circulating tumor cell; GFP: green fluorescent protein; hTERT: human telomerase reverse transcriptase; SPPCNs: superparamagnetic positively charged nanoparticles
Devices utilizing physical properties
Enrichment methodologies via physical properties are based on unique CTC properties such as their size [50], membrane capacitance [6], and density [55]. The most essential advantage associated with the technologies above is that they are independent of the recognition of surface markers. Therefore, these techniques are appropriate for isolating CTCs with low/negative EpCAM expression levels as CellSearch® system fails to detect CTCs in approximately 36% of metastatic breast cancer and lung cancer patients [59, 60]. Isolation by the size of epithelial tumor cells (ISET®), Metacell filtration device, ScreenCellCyto, Parsotrix™, and dead flow fractionation techniques are all size-based CTC selections [50, 61,62,63,64,65], that utilize filtration to separate individual tumor cells on the basis of size. Coumans and colleagues compared three filtrations to investigate the properties of the ideal filter for CTC recovery, such as pore size, number of pores, spacing between pores, filter thickness and filter surface material. The authors summarized the experiment and arrived at the conclusion that the optimum filter for CTC enrichment from 10 ml of whole blood has a pore size of about 5 mm, a thickness of at least 10 mm, no less than 100,000 regularly spaced pores, and a porosity of 10% or less [66]. Unfortunately, these technologies have certain limitations, since the current technology lacks specificity, and due to the heterogeneity of cells, the results obtained are not as pure as those of functional tests. Meanwhile, filter pores can cause deformation and damage of CTCs and may lose CTCs with smaller sizes than average. On the other hand, larger cells that are not tumor cells, such as megakaryocytes, can be kept together with isolated cell populations. In conclusion, size-based CTC isolation methods provide high throughput; however, these methods have limited applicability in clinical settings due to the heterogeneity of CTCs in terms of their size.
Cell sorting based on deformability is particularly relevant to the separation of CTCs from whole blood because CTCs may not be simply distinguished from white blood cells based on size alone. This situation is particularly related to colorectal cancer and prostate cancer, as it is recognized that patients’ CTCs are small and have important overlap with contaminated leukocytes [67]. Tumor cells form an enlarged nucleus and, therefore, may exhibit a greater nucleocytoplasmic ratio than leukocytes. In fact, the nucleoplasm is two times more viscous and nearly three to four times more rigid than the cytoplasm. Thus, it seems to be a feasible option to take advantage of cell deformability to sort CTCs. Park and colleagues proved that separation based on deformability improves enrichment ≈100× over size-only separation, providing a significantly selective biophysical enrichment process. Their measurement involving the diameter of enriched CTCs and patients leukocytes before and after enrichment demonstrated that these cells were primarily discriminated on the basis of their cell deformability [67].
Centrifugation, which uses the specific density of leukocytes, red blood cells, and cancer cells, is among the first reported techniques used for CTC separation [68]. In density centrifugation methods, erythrocytes, platelets, and polymorphic nuclear cells are separated in the pellet, and mononuclear cells (MNCs), including tumor cells, gather in the so-called interphase [9]. A comparison of two density gradient centrifugation systems demonstrated that OncoQuick improved tumor cell enrichment in comparison with Ficoll, which was achieved by an increased consumption of MNCs and a comparable tumor cell recovery [55]. The AccuCyte® system is differentiated from existing density-based methods which separates the buffy coat from red blood cells and plasma by using a unique separation tube and collector device. The device allows virtually complete harvesting of the buffy coat into a small volume for application on a microscopic slide without cell lysis or wash steps, which is considered a potential source of CTC loss [69]. RosetteSep is an immune density cell separation kit designed to separate and enrich circulating epithelial tumor cells from normal hematopoietic cells. This kit contains an antibody cocktail for the removal of unwanted cells by changing their density. The excess cells settle through density gradient centrifugation, and purified tumor cells appear at the interface between the density gradient medium and the plasma [70].
Cancer cells have larger folding factors and radii than both normal cells of comparable origin and blood cells. The NCI-60 panel of cancer cell types has a DEP characterization and all cell lines derived from solid tumors have crossover frequencies that should allow their efficient isolation from normal blood cell types [6]. DEP is an electrokinetic method which allows inherent dielectric properties of suspended cells for discrimination and separation [71]. DEP has emerged as a promising method for isolating CTCs from whole blood. DEP isolation of CTCs is independent of cell surface markers [6]. The continuous flow ApoStream® device was developed to overcome the cell throughput limitation of the DEP batch mode configuration, providing an effective enrichment and separation of CTCs from full blood. The linearity data and recovery accuracy of the ApoStream® device confirmed consistent cancer cell recovery performance in both high- and low-EpCAM expressing cancer cell types over a wide range of spiking levels [72].
Bioelectricity is an essential biophysical indicator of cell behaviors and is directly modulated via the metabolic mode [73]. Successful CTC isolation based on the surface charge is uniquely related to the unique characteristics of cancer cells, i.e., a high glycolysis rate and strong lactate acid secretion. Studies have shown that the acidic cancer microenvironment associated with the “Warburg effect” is related to the negative charge of cancer cells, which is related to the following hallmark feature of cancer cell energy metabolism: a high aerobic glycolysis rate [74]. Superparamagnetic positively charged nanoparticles (SPPCNs) electrostatically and strongly bind malignant cells characterized by high rates of glycolysis, enabling the effective capture of CTCs and subsequent magnetic isolation from clinical blood samples of cancer patients [75].
Devices utilizing functional assays
Recent studies have reported that the EMT plays a pivotal role in the invasion of tumor cells [23, 76]. CTCs are hypothesized to contain a significant number of EMT tumor cells, which are reported to have a low expression of epithelial surface antigens, especially EpCAM [77]. Thus, such tumor cells that lose EpCAM expression are less likely to be detected by assays utilizing cell immunological characteristics. Functional assays use the viable CTC cellular functions to overcome certain limitations of tumor cell heterogeneity. However, the current circumscription of product purity is a dominant problem in the method of enriching CTCs based on cell functional characteristics. An adaptation of enzyme-linked immunospot technology, Epithelial Immunospot (ELISPOT), was introduced for the detection of viable CTCs in cancer patients. Secreted, shed or released proteins are immunocaptured on the membrane during short-term cultures, and the EPISPOT assay has a sensitivity to detect them [78]. In order to collect CTCs with aggressive phenotypes and explore their molecular features, researchers applied a functional cell separation method called the collagen adhesion matrix (CAM) assay, which identified CTC invasiveness via CAM protein uptake while recognizing epithelial antigens and produces results with high sensitivity and specificity [79].
Chen and colleagues reported a strategy for CTC enrichment by exploiting the differential adhesion preference of cancer cells to nanorough surfaces. Bare glass surfaces treated with reactive ion etching (RIE) for different durations could acquire different levels of roughness. Subsequently, RIE-generated nanorough surfaces could capture different types of cancer cells efficiently without any use of capture antibody [80]. This method is a promising strategy for achieving efficient capture at a quite low cost and is expected to provide a better isolation and enrichment strategy for viable CTCs from blood specimens. However, these nanorough glass surfaces show a low CTC capture purity as a result of significant nonspecific binding of other blood cells.
The telomere length has been frequently used as a means to predict the future life of cells [81]. TelomeScan detects viable CTCs via a telomerase-specific replication selective adenovirus in human peripheral blood. Viral infection increases the signal-to-background ratio as a tumor-specific probe and emits fluorescence. The transfected cells are easily recognizable, as the special adenovirus can be amplified only in tumor cells [82]. The TelomeScan may be more applicable for the detection of EMT tumor cells given that it is not influenced by the level of EpCAM expression. The assay seems promising, but future studies covering a large number of patients are still needed confirmation [83].
Tannic acid-functionalized magnetic nanoparticles (MNPs-TA) were recently developed for binding between the polyphenol structure of TA and the special glycocalyx on cancer cells. Furthermore, TA has a great antileukocyte adhesion effect, reducing the interaction with nontarget cells [84].
Devices utilizing Immunomagnetic assays
The most common technique used for CTC isolation is the immune isolation, which is based on specific CTC cell surface markers. Immunobead methods use either positive selection, targeting tumor-associated antigens expressed by CTCs, or negative selection, removing blood cells with common leukocyte biomarkers. EpCAM is an antigen often used in positive selection, while CD45 is used for negative selection [7, 85]. In 2004, the CellSearch® system was introduced as the first and only Food and Drug Administration (FDA)-approved method for the enumeration of CTCs in 7.5 mL of blood. The highest proportion of positive specimens was detected in patients with metastatic prostate cancer, followed by metastatic ovarian cancer and breast cancer [5]. The limitation of the fact that CellSearch® detects EpCAM+ cells leading to the loss of EpCAM-cells has been improved in other methods by combining different specific tumor markers, including EGFR, cytokeratin, HER2, folic acid receptors (FRs), and recombinant VAR2CSA (rVAR2) [16, 86,87,88]. In recent years, several alternative immunobead technologies that can improve the purity and recovery and retrieve CTCs off-chip with high fidelity have been developed, involving MagSweeper [53], AdnaTest® [43, 89], IsoFlux™ [90], and CTC-μChip [91].
Recently, researchers have combined CTC specific surface markers with other methods to improve the sensitivity of CTC detection and maintain the integrity and biological characteristics of CTCs for subsequent studies. Park and colleagues developed a novel 3D printed immunomagnetic concentrator (3DPIC) with an ATP luminescence assay for CTC enrichment and rapid detection. The ATP luminescence assay is used to measure cell intracellular ATP but has not been applied to CTC detection as a consequence of interference from non-CTC-derived ATP. An antibody (Ab)-conjugated magnetic nanoparticles (MNPs) conjugated with EpCAM provided spectacular enrichment in 3DPIC, and then, these cells were enumerated using an ATP luminescence assay [92]. Researchers have presented chemically stable and instantly degradable (CSID) hydrogel immunospheres for CTC isolation. These researchers modified the CSID hydrogel spheres with the anti-EpCAM antibody to successfully isolate and effortlessly retrieve the target cells with an average of 10.8 ± 5.9 CTCs/ml [93].
Negative selection is also a possible solution to overcoming the low recovery rate associated with EpCAM-cells. CD45 expressed in hematopoietic cells is the most prevailing antigen used in negative selection. CD45 depletion is often combined with other label-free methodologies, such as density gradient centrifugation or red blood cell lysis to improve the yield [85, 94]. Using these strategies, unconventional CD45-expressing CTCs may be accidentally removed, resulting in the underestimation of the number of CTCs [2]. DynaBeads® and EasySep are immunomagnetic methods in which antibodies recognizing cell surface antigens are coupled to magnetic beads and used to remove unwanted cells. However, it is not easy to enumerate massive Dynabead-bound cells due to the autofluorescence of the beads, the large number of beads, and the low efficiency of the labeling of cell antibodies when the beads are bound. In contrast, the smaller EasySep nanoparticles do not interfere with downstream immunocytochemical processing and are able to achieve higher purity [95].
Devices utilize microdevices
Nowadays microfluidic platforms have become among the most prevalent technologies because of their tremendous applications, including biological and chemical analyses, fertility analyses, cell sorting, infectious disease diagnostics, DNA sequencing, ect [96]. Microfluidic platforms provide many attractive advantages, such as continuous sample processing to reduce target cell loss. Microfluidic platforms can capture CTCs through different methods, which can be roughly divided into: 1) using epithelial cell markers as antigens; 2) using the physical properties of tumor cells; 3) using the electrical properties of CTCs; and 4) other methods [97].
A mass of microdevices utilize the unique antigen expression of CTCs as enrichment and capture methods. EpCAM, as already mentioned, is logically one of the most common surface markers applied to distinguish CTCs from hematopoietic cells [98,99,100,101]. An effective microfluidic named CTC-chip using antibody-coated microposts to capture these EpCAM-positive cells was demonstrated. Stott and colleagues developed a high-throughput microfluidic mixing device, the herringbone-chip (HB-Chip) which could provide an enhanced efficiency of CTC isolation. The HB-Chip design applies passive mixing of blood cells through the generation of microvortices to significantly increase the interactions between the target CTCs and the antibody-coated chip surface [98]. The electrochemical Lab-on-a-Disc (eLoaD) platform captures cancer cells from separated plasma through anti-EpCAM antibodies immobilized on gold electrodes and quantifies them by the use of label-free electrochemical impedance [99]. Lin and colleagues developed three generations of NanoVelcro CTC chips, a nanostructured substrate, coated with anti-EpCAM antibodies. These authors developed a third generation NanoVelcro device using biotin-streptavidin interaction linked anti-EpCAM antibodies to efficiently capture CTCs. At 37 °C, functionalized domains are present on the surface of the chips; thus, CTCs that interact with the substrate can be caught. The thermally responsive polymer brushes of a poly N-isopropylacrylamide (PIPAAm) substrate undergo conformational changes when the temperature decreases at 4 °C, leading to the internalization of the anti-EpCAM antibodies. Thus, the captured CTCs are capable of being released from the device [100]. A recent study presented a fully automated and rapid microfluidic system for efficient CTC identification. A lateral flow-based four-channel microfluidic chip was applied to separate and distribute CTCs as a single-cell array. An approximately 90% capture rate was achieved in different cell lines when spiking 100 cells in 2 mL of healthy donor blood samples from healthy donors, revealing the wide application of this platform to different tumors [102].
It has been proven that metastatic cancer cells from patients with lung, breast and pancreatic cancer are 70% softer than benign cells from the body cavity through atomic force microscopy (AFM) [52]. The iterative mechanical characteristics (iMECH) analyzer provides a low-cost yet high-throughput solution for single-cell level metastatic detection. It directs the cyclic deformation regimen by pulling CTCs and other cells through a trial channel composed of narrow deformation channels interspersed with wider relaxation regions to simulate the dynamic microenvironment jointly. Researchers revealed that cells from nonmetastatic breast cell lines were more resistant to deformation when passing through cyclic deformations, and their average velocity through the channels decreased after each relaxation [103].
Alternatively, inertial microfluidics devices isolating cells based on size by utilizing the fluidic forces in straight or curved channels have already been developed [104, 105]. These devices demonstrated remarkably higher flow rates than immunoaffinity based devices, allowing a high throughput process. Researchers devised an efficient inertial device, the CTCKey™ without additional preprocessing steps. The study reported that CTCKey™-enriched blood could be further processed utilizing the CellSearch® system, enabling processing higher volumes of blood (up to 5fold) [105].
Over the past decade microdevices have emerged as promising techniques to address challenges, given rarity, phenotypic and size heterogeneities of CTC, and the need to preserve CTC viability for downstream analysis. Nevertheless, there are still certain limitations of these novel technologies. Physical-based microdevices face with the risk of clogging, low purity, and challenging downstream analysis. For example, the throughput of microfluidic ratchets is relatively low with 1 mL/h. The device fails to process 7.5 mL of blood (standard volume for protocols), which may overstate the probability of recovering CTCs in the clinic trail with microfluidic ratchets [67]. The use of nanostructured substrates, such as silicon nanopillars (NanoVelcro Chip), was also reported to enhance CTC isolation sensitivity as the consequence of high surface area-to-volume ratio of nanostructured substrates and similar size to cellular surface components [90]. Moreover, subsequent enzymatic degradation may compromise the viability of CTCs due to over exposure to the degrading membrane itself and enzymatic hydrolysis solutions. The irreversible capture of CTCs on these nanostructures greatly limits downstream analysis and subsequent cell culture [56]. The immunomagnetic separation can either target CTCs or WBCs. As previously mentioned, such tumor–antigen dependent immunomagnetic separation methods are unable to overcome marker expression variability among CTCs. Nonetheless, given the high concentration of WBCs in blood, it is more challenging to deplete WBCs completely without CTC damage. In addition, these devices have to processed large volumes of blood to ensure the sufficient number of CTCs, which might lead to clogging on account of the large number of WBCs.
GILUPI GmbH CellCollector, an in vivo and novel technology, uses an anti-EpCAM wire directly into the peripheral arm vein and captures targeted cells with high efficiency. In the study, all volunteers tolerated the 30 min in vivo exposure to the nanodetectors with no sign of adverse events. Within the test 24 cancer patients were examined; of those, 22 of 24 were detected with a median of 5.5 (0–50) CTCs in breast cancer (n = 12) and 16 (2–515) CTCs in non-small cell lung cancer (NSCLC) (n = 12). This technology has the ability to process approximately 1.5 L of blood in 30 minutes, which improves the device’s sensitivity, thereby rendering it a promising candidate for future CTC studies [106].
Since the number of CTCs is very tiny and the time of tumor cells shedding into the blood might be related to biological rhythms. The fluorescence in vivo flow cytometry (IVFC) is developed as an emerging and powerful optical technique. The biggest advantage of IVFC is that the blood collection is not required. This technique could detect fluorescent circulating cells in living animals through a noninvasive manner over a long period of time to reduce the error caused by acquisition time [107]. This method helps to identify the effects of treatments, as sorafenib was revealed to reduce CTC count through fluorescence IVFC [108]. Nevertheless, IVFC has limitations: 1) its detection speed is 1 μL/min, while ~ 5 L/min blood passes through human blood vessels; 2) this emerging technology is still at the stage of animal models due to the use of fluorescent dyes; 3) it is unfavorable for the detection of CTC molecular typing and the study of biological characteristics. However, this technique is particularly useful for CTC detection and counting, which should be valuable for clinical monitoring and prognosis evaluation.
The advantages and limitations of various CTC isolation and detection technologies are summarized in Table 1.
CTCs detachment
How to release CTCs nondestructively after catching them from the surface effectively remains a challenging problem that needs to be solved. Detachment from filters, immunoaffinity chips and other substrates using excessive stress may reduce the cell viability and potentially induce phenotypic change, resulting in the loss of valuable information regarding the isolated cells [128]. The current technologies that hold great potential for CTC detachment include enzymatic digestion, oligonucleotide-mediated aptamer release, and stimuli-responsive polymers [56,57,58].
Although enzymatic digestion is applied to digest the extracellular matrix and detach cells, which may reduce other cell membrane proteins and damage cell-to-cell junctions, it is still the standard method of CTC release. In recent years, many new enzymatic degradations, including alginate lyase and endonuclease, have been developed to ameliorate the cell viability and reduce cell damage [56, 129]. Aptamers are burgeoning and powerful tools used to study CTCs that provide high stability resistance to a spectrum of harsh conditions, thereby offering a noninvasive and efficient detachment technique. In addition, aptamers can be developed against binding targets in the range between small compounds and large cell membranes or transmembrane proteins on CTCs [57, 130]. Similarly, polymers can reversibly change their conformation via deformation or dissolution in response to changes in external conditions [131]. Temperature-responsive polymers have been used to control cell adhesion with the aim of recovering cells for additional analyses. The third-generation NanoVelcro chips have demonstrated the capture of CTCs at 37 °C and release at 4 °C. The temperature-dependent conformational changes of polymer brushes can alter the accessibility of the capture agent effectively with desired CTC viability and molecular integrity [100]. A dual-mode gelatin-based nanostructured coating that can achieve temperature-responsive release of CTCs from peripheral blood was presented. The cell viability was 88.3%, and the recovery rate was 93.2% [132]. Another type of polymer commonly used are pH-responsive polymers which are synthesized by linking structures with weakly acidic and basic functional groups to a hydrophobic base. They are specifically triggered by the pH of the environment (by either accepting or releasing protons), which undergoes changes in physicochemical properties [58]. The ionization of polymers can directly affect their affinity to ECM proteins because these proteins are negatively charged under physiological conditions, resulting in high cell viability and recovery [80].
Clinical relevance of CTC
Liquid biopsy, as a noninvasive detection method, can be extracted from peripheral blood to detect the biological characteristics and molecular typing of primary tumor cells (Table 2).
Studies based on CTC count
Higher CTC counts in patients’ peripheral blood have been reported to be associated with a poor prognosis in various types of cancers, including colorectal cancer, breast cancer, lung cancer,pancreatic cancer and so on [16, 79, 160,161,162]. It has been proven that the presence of ≥3 CTCs per 7.5 mL of peripheral blood is a strong predictor of progression-free survival (PFS) reduction, whereas the detection of < 3 CTCs per 7.5 mL indicates better overall survival (OS) [133, 163]. Initial CTC counts as well as early changes after treatment initiation are closely related to the primary tumor size, the number of metastases, and the PFS reduction in patients with breast cancer [27, 164, 165]. CTC counts increase with tumor progression and development of distant metastases [166]. It has been reported that the area under receiver operating characteristic (ROC) curve for CTC count in forecast of distant metastasis was 0.783 [167].
CTC detection is a potential novel approach to assess the efficacy of neoadjuvant chemotherapy (NAC) [168]. Indeed, the results of studies published in the past 5 years, involving thousands of patients with breast cancer, have demonstrated that the CTC counts before and after neoadjuvant therapy are predictive of the risk of disease relapse [134]. Patients with ≥4 CTCs were more likely to be resistant to chemotherapy than those with < 4 CTCs, indicating that the CTC count is a promising indicator in the evaluation of biological activities and the chemotherapy response in gastric carcinoma (GC) patients [127]. CTCs may be a practical surrogate marker with the chemotherapy response since chemotherapy leads to a rapid decline in CTC counts with a 50% reduction in baseline apoptotic CTC count [135, 160].
Data obtained in animal models indicate that blood dissemination of cancer cells occurs early during tumor development, which may provide the possibility to explore CTCs as marker for early detection [169]. It has been demonstrated that CTC-positive chronic obstructive pulmonary disorder (COPD) patients were examined with lung nodules 1 to 4 years after CTC detection, leading to prompt surgical resection and histopathological type of early-stage lung cancer. Follow-up studies conducted one-year post-surgery showed no tumor recurrence [170]. It seemed that CTC as a sentinel of tumor development could save patient lives – especially in asymptomatic cancers for which no routine screening methods are available. The initial encouraging results of the pilot study in patients with COPD generated public attention, but the results of the later validation cohort study confirmed that CTC detection is not suitable for lung cancer early detection [142]. The low sensitivity of CTCs for early cancer detection might be explained as the gradient difference of tumor cells counts between the tumor-draining vessels and the peripheral veins [171, 172]. Metastases present in lymph nodes or distant organs promote the pool of CTCs in peripheral blood in later tumor stages, which considerably increases CTC counts. In conclusion, CTC plays a significant role in early detection, dynamic monitoring, efficacy evaluation and prognosis judgment.
Studies based on molecular characteristics of CTC
In addition to pure quantitative analyses, the use of CTCs as a tumor surrogate was concerned as one of the main concepts studied in clinical trials. CTCs from patient peripheral blood may be a novel and attractive noninvasive alternative for assessing tumor heterogeneity, molecular tumor characteristics and changes during treatment.
Many studies have identified genes that can be used as prognostic markers by CTC detection, including HER2, ESR1, PI3KCA, PSMA, MYC, TP53 and so on [173,174,175,176]. Several studies have demonstrated the feasibility of evaluating HER2 status of CTCs in BC using CellSearch® [136, 177, 178]. Jaeger and colleagues have found unusual inconsistency of HER2 expression between CTCs and the primary tumor in early breast cancer. They have detected HER2-positive CTCs in peripheral blood from patients with HER2-negative breast cancer [178]. Current studies have reported that HER2-negative breast cancer patients with HER2-expressing CTCs can still benefit from trastuzumab therapy [137]. ESR1 gene mutations have reported as a biomarker for resistance to endocrine therapy in BC. ESR1 mutations which rarely detected at the beginning of first-line endocrine therapy were significantly enriched during disease progression, suggesting that ESR1 mutations conferred endocrine resistance in metastatic breast cancer [179]. Mastoraki et al. investigated epigenetic silencing of ESR1 and its effect on endocrine therapy response. ESR1 methylation was observed in 27.8% (10/36) of CTC-positive samples and was associated with non-response to treatment in peripheral blood samples from everolimus/exemestane-treated patients [180]. Changes in CTC count based on PSMA status were determining by EPIC Sciences technology in a phase 2 trial evaluated the efficacy and safety of BIND-014 in prostate cancer patients. Interestingly, PSMA-positive CTCs were reduced preferentially compared with the baseline, indicating the effect of PSMA-positive CTCs as biomarkers and monitors for PSMA-based treatment [181]. Gene expression profiling of CTCs in metastatic breast cancer suggested that CTCs associated with brain metastasis had increased activity of the Notch signaling pathways [182]. Another study revealed that overexpression of MYC and copy-number gain of SEMA4D (a mediator of blood–brain barrier transmigration) were novel markers for brain metastasis through a genome-wide assessment of CTC lines established from breast cancer patients [183].
The appearance of inhibitors such as PD-1 or PD-L1 has demonstrated interesting results in certain metastatic cancers. In NSCLC, CTC status was assessed with CellSearch® and PD-L1 staining methods at baseline, and at 3 and 6 months in patients treated with nivolumab. Patients with PD-L1 negative CTCs at 6 months gained a clinical benefit, while patients with PD-L1 positive CTCs experienced tumor progression [184]. A recent study using CellSearch® to continuously collect blood, utilized PD-L1 antibodies to measure CTCs and platelets in both patients with metastatic breast cancer and healthy subjects. More than 40% patients (52/124, 42%) detected ≥5 CTCS / 7.5 mL whole blood, and 21 (40%) were PD-L1 positive for CTCs [138]. These studies showed that PD-L1 expression existed independently on CTCs and could play a role as a pharmacodynamic biomarker predicting which patients should receive immune checkpoint suppression and therapy.
Conclusions and future perspectives
The novel discovery of CTCs as a liquid biopsy had a revolutionary effect on early diagnosis, metastasis detection and individualized treatment of tumors [134, 162, 163]. Despite the advantages above, the clinical use of CTCs is hindered by considerable challenges because of the heterogeneity, fragility, singularity and incomplete gene expression expertise of CTCs. Methods for isolating, detaching, and detecting these cells in blood from cancer patients have been rapidly developed to address the need for increased specificity, sensitivity, and throughput. The most commonly used detection methods are based on specific surface antigens, physical properties and functional properties of CTCs [43, 80, 185]. CTCs extracted from patient samples can be used for further studies to develop the best treatment regimen, conduct effective disease surveillance and discover new drug targets for molecular and genetic analyses [14].
However, many problems have not been solved. First, several biological questions remain, such as, what determines the tendency of CTC metastases and which pathways could be targeted for metastatic restraint of CTCs. Also, since trafficking of CTCs may be regulated by circadian rhythm, the distribution of CTCs in circulation may not be uniform. Patients with zero CTC detected at a given time point may not be CTC-free [186]. Repeated blood draws to clarify the temporal distribution of CTCs in patients are not realistic. IVFC could help monitoring CTCs dynamically to reduce the false negative rate, but it is still need more preclinical research to prove whether it can be applied in patients to solve the false negative results caused by detection time.
Secondly, although there are many CTC isolation and detection techniques, different CTC-positive ratios are reported from the usage of different methods. Therefore, it is necessary to establish and improve the standardized protocols off CTC-related detection methods as soon as possible. The Horizon 2020 SPIDA4P aims to develop and implement a comprehensive portfolio of 22 pan-European CEN and ISO standards documents, driving the standardization of preanalytical workflows applied to personalized medicine (www.spidia.eu). Due to the high senescence of CTCs, blood is usually placed in blood collection tubes with preservatives for long term preservation, which could result in the loss of viable CTC cells [187]. It is also significant to consider the volume of blood samples to unify CTC isolation and identification protocols since CTCs are rare in the bloodstream [187]. In addition, how many CTCs are required for a genic panorama of the donor is another problem under solved.
Thirdly, different CTC techniques used for detecting the same sample may obtain completely different results; thus, how to choose the most suitable CTC detection method is also a major problem that currently needs to be solved [83, 188]. A study aimed to evaluate how two different isolation techniques, evolving the physical (Parsortix®) and biological (MACS®) separation techniques, affect cell morphology. The researchers found that in the MBC patient cohort, the morphological features of CTCs were dependent on the separation process. CTCs with a preserved cell morphology were detected after physical separation while the identification of the cell morphology was difficult due to the degeneration of CTCs after biological separation [111]. A comparative study indicated that although the EpCAM-dependent CTC enrichment was superior in terms of specificity compared to label-free CTC enrichment, it is more suitable to choose size-dependent enrichment approaches in consideration of the evaluation of CTC molecular characterization [189]. Regardless, not all CTC methods are appropriate for downstream analysis, such as DNA analysis. It is also challenge to find the most suitable detection method to be applied in different tumor screening setting. Devices based on positive selection achieve the high purity in clinical applications but lose CTC subpopulations including EMTed CTCs, clusters, and CTCs cloaked by blood cells. On the contrary, negative selection-based techniques are theoretically capable of enriching all potential CTC subpopulations but with low purity. Along with the devices based on biological properties, techniques isolating and detecting CTCs based on their physical properties appear suitable for use in a clinical cytopathology laboratory for identification of CTC morphology and evaluation of CTC molecular characterization [112].
In addition, the current studies investigating the clinical application of CTCs mostly focus on advanced or metastatic cancers and rarely involve their application in early-stage cancer. Can CTCs be detected reliably in early disease and ca be used to routinely guide cancer patient care are still unanswerable problems. The number of CTCs detected in the blood of patients with early-stage cancer is lower than that in patients with metastatic disease, requiring higher sensitivity. Therefore, is it necessary to apply such a high-cost technique for the detection of rare CTCs in the patients with early-stage cancer?
Finally, whether the CTCs obtained by these CTC detection techniques are truly representative of the heterogeneity of the primary tumor or whether these techniques could detect those so-called CTCs remains an unanswered question. A recent study demonstrated that transcriptional profiles may be altered when cells leave hypoxic primary lesions and enter the well-oxygenated bloodstream [190].
CTC analysis is a simple and feasible liquid biopsy technique that has attracted great attention and achieved great success, although there are still some problems to be solved. The further development of CTC diagnostic technology should be of great value in the individualized treatment of cancers.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- CAM:
-
Collagen adhesion matrix
- CCL2:
-
Chemokine C-C motif ligand 2
- COPD:
-
Chronic obstructive pulmonary disorder
- CTC:
-
Circulating tumor cell
- CTL:
-
Cytotoxic T lymphocyte
- DEP:
-
Dielectrophoresis
- DEP-FFF:
-
Dielectrophoretic field-flow fractionation
- ECM:
-
Extracellular matrix
- EGFR:
-
Endothelial growth factor receptor
- ELISPOT:
-
Epithelial Immuno-spot
- EMT:
-
Epithelial-mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- HER2:
-
Human epidermal growth factor receptor 2
- IVFC:
-
Fluorescence in vivo flow cytometry
- MDSC:
-
Myeloid-derived suppressor cell
- MHC-I:
-
Major histocompatibility complex-I
- MMP:
-
Matrix metalloproteinase
- NAC:
-
Neoadjuvant chemotherapy
- NK cell:
-
Natural killer cell
- PD-L1:
-
Programmed cell death-ligand 1
- PSA:
-
Prostate specific antigen
- ROC:
-
Receiver operating characteristic
- SPPCN:
-
Superparamagnetic positively charged nanoparticle
- TGF-β:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
References
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. https://doi.org/10.1016/j.jhep.2018.09.014.
Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2015;7(1):1–11. https://doi.org/10.15252/emmm.201303698.
Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11(1):40–61. https://doi.org/10.1002/1878-0261.12022.
Lara O, Tong X, Zborowski M, Chalmers JJ. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through, immunomagnetic cell separation. Exp Hematol. 2004;32(10):891–904. https://doi.org/10.1016/j.exphem.2004.07.007.
Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–904. https://doi.org/10.1158/1078-0432.CCR-04-0378.
Gascoyne PRC, Shim S. Isolation of circulating tumor cells by Dielectrophoresis. Cancers. 2014;6(1):545–79. https://doi.org/10.3390/cancers6010545.
Andree KC, van Dalum G, Terstappen LWMM. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol. 2016;10(3):395–407. https://doi.org/10.1016/j.molonc.2015.12.002.
Miller MC, Doyle GV, Terstappen LWMM. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol. 2010;2010. https://doi.org/10.1155/2010/617421.
Kulasinghe A, Kenny L, Perry C, et al. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget. 2016;7(44):71223–34. https://doi.org/10.18632/oncotarget.12086.
Gires O, Stoecklein NH. Dynamic EpCAM expression on circulating and disseminating tumor cells: causes and consequences. Cell Mol Life Sci. 2014;71(22):4393–402. https://doi.org/10.1007/s00018-014-1693-1.
Mai J, Abubrig M, Lehmann T, et al. T2 mapping in prostate Cancer. Investig Radiol. 2019;54(3):146–52. https://doi.org/10.1097/RLI.0000000000000520.
Brasó-Maristany F, Griguolo G, Pascual T, et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun. 2020;11. https://doi.org/10.1038/s41467-019-14111-3.
Praharaj PP, Bhutia SK, Nagrath S, Bitting RL, Deep G. Circulating tumor cell-derived organoids: current challenges and promises in medical research and precision medicine. Biochim Biophys Acta. 2018;1869(2):117–27. https://doi.org/10.1016/j.bbcan.2017.12.005.
Sharma S, Zhuang R, Long M, et al. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol Adv. 2018;36(4):1063–78. https://doi.org/10.1016/j.biotechadv.2018.03.007.
Liu L, Liao GQ, He P, et al. Detection of circulating cancer cells in lung cancer patients with a panel of marker genes. Biochem Biophys Res Commun. 2008;372(4):756–60. https://doi.org/10.1016/j.bbrc.2008.05.101.
Weissenstein U, Schumann A, Reif M, Link S, Toffol-Schmidt UD, Heusser P. Detection of circulating tumor cells in blood of metastatic breast cancer patients using a combination of cytokeratin and EpCAM antibodies. BMC Cancer. 2012;12(1):206. https://doi.org/10.1186/1471-2407-12-206.
Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18(9):533–48. https://doi.org/10.1038/s41568-018-0038-z.
Zavyalova MV, Denisov EV, Tashireva LA, et al. Intravasation as a key step in Cancer metastasis. Biochem Mosc. 2019;84(7):762–72. https://doi.org/10.1134/S0006297919070071.
Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–7. https://doi.org/10.1038/s41586-019-0915-y.
McCarty OJT, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96(5):1789–97. https://doi.org/10.1182/blood.V96.5.1789.
Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–70. https://doi.org/10.1038/nrc3628.
Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31(18):1827–40. https://doi.org/10.1101/gad.305805.117.
Yeung KT, Yang J. Epithelial–mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11(1):28–39. https://doi.org/10.1002/1878-0261.12017.
Rejniak KA. Investigating dynamical deformations of tumor cells in circulation: predictions from a theoretical model. Front. Oncol. 2012;2. https://doi.org/10.3389/fonc.2012.00111.
Gosens MJEM, van Kempen LCL, van de Velde CJH, van Krieken JHJM, Nagtegaal ID. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20(2):221–32. https://doi.org/10.1038/modpathol.3800733.
Dhar M, Lam JN, Walser T, Dubinett SM, Rettig MB, Di Carlo D. Functional profiling of circulating tumor cells with an integrated vortex capture and single-cell protease activity assay. Proc Natl Acad Sci U S A. 2018;115(40):9986–91. https://doi.org/10.1073/pnas.1803884115.
Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are Oligoclonal precursors of breast Cancer metastasis. Cell. 2014;158(5):1110–22. https://doi.org/10.1016/j.cell.2014.07.013.
Mannucci PM, Cattaneo M, Teresa Canciani M, Maniezzo M, Vaglini M, Cascinelli N. Early presence of activated (‘exhausted’) platelets in malignant tumors (breast adenocarcinoma and malignant melanoma). Eur J Cancer Clin Oncol. 1989;25(10):1413–7. https://doi.org/10.1016/0277-5379(89)90098-9.
Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–77. https://doi.org/10.1016/j.semcancer.2010.04.005.
Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-β Down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69(19):7775–83. https://doi.org/10.1158/0008-5472.CAN-09-2123.
Placke T, Örgel M, Schaller M, et al. Platelet-derived MHC class I confers a Pseudonormal phenotype to Cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72(2):440–8. https://doi.org/10.1158/0008-5472.CAN-11-1872.
Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res. 2010;316(1):138–48. https://doi.org/10.1016/j.yexcr.2009.09.003.
Saini M, Szczerba BM, Aceto N. Circulating tumor cell-neutrophil tango along the metastatic process. Cancer Res. 2019;79(24):6067–73. https://doi.org/10.1158/0008-5472.CAN-19-1972.
De Giorgi U, Mego M, Scarpi E, et al. Association between circulating tumor cells and peripheral blood monocytes in metastatic breast cancer. Ther Adv Med Oncol. 2019;11. https://doi.org/10.1177/1758835919866065.
Qian B, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014.
Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast tumor metastasis. Nature. 2011;475(7355):222–5. https://doi.org/10.1038/nature10138.
Gordon N, Kleinerman ES. The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases. Cancer Treat Res. 2009;152:497–508. https://doi.org/10.1007/978-1-4419-0284-9_29.
Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9(9):1773–82. https://doi.org/10.1016/j.molonc.2015.05.009.
Oliveira-Costa JP, de Carvalho AF, da Silveira GG, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget. 2015;6(25):20902–20.
Wang X, Sun Q, Liu Q, Wang C, Yao R, Wang Y. CTC immune escape mediated by PD-L1. Med Hypotheses. 2016;93:138–9. https://doi.org/10.1016/j.mehy.2016.05.022.
Steinert G, Schölch S, Niemietz T, et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal Cancer. Cancer Res. 2014;74(6):1694–704. https://doi.org/10.1158/0008-5472.CAN-13-1885.
Winkler J, Martin-Killias P, Plückthun A, Zangemeister-Wittke U. EpCAM-targeted delivery of nanocomplexed siRNA to tumor cells with designed Ankyrin repeat proteins. Mol Cancer Ther. 2009;8(9):2674–83. https://doi.org/10.1158/1535-7163.MCT-09-0402.
Müller V, Riethdorf S, Rack B, et al. Prognostic impact of circulating tumor cells assessed with the CellSearch System™ and AdnaTest Breast™ in metastatic breast cancer patients: the DETECT study. Breast Cancer Res. 2012;14(4):R118. https://doi.org/10.1186/bcr3243.
Deutsch TM, Riethdorf S, Fremd C, et al. HER2-targeted therapy influences CTC status in metastatic breast cancer. Breast Cancer Res Treat. 2020;182(1):127–36. https://doi.org/10.1007/s10549-020-05687-2.
Day KC, Hiles GL, Kozminsky M, et al. HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 2017;77(1):74–85. https://doi.org/10.1158/0008-5472.CAN-16-1656.
Driemel C, Kremling H, Schumacher S, et al. Context-dependent adaption of EpCAM expression in early systemic esophageal cancer. Oncogene. 2014;33(41):4904–15. https://doi.org/10.1038/onc.2013.441.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. https://doi.org/10.1038/s41580-018-0080-4.
Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. https://doi.org/10.1186/1471-2407-12-178.
Harouaka RA, Nisic M, Zheng SY. Circulating tumor cell enrichment based on physical properties. J Lab Autom. 2013;18(6). https://doi.org/10.1177/2211068213494391.
Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells. Am J Pathol. 2000;156(1):57–63.
Gascoyne PRC, Shim S, Noshari J, Becker FF, Stemke-Hale K. Correlations between the dielectric properties and exterior morphology of cells revealed by Dielectrophoretic field-flow fractionation. Electrophoresis. 2013;34(7):1042–50. https://doi.org/10.1002/elps.201200496.
Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2(12):780–3. https://doi.org/10.1038/nnano.2007.388.
Talasaz AH, Powell AA, Huber DE, et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc Natl Acad Sci. 2009;106(10):3970–5. https://doi.org/10.1073/pnas.0813188106.
Fan X, Jia C, Yang J, et al. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens Bioelectron. 2015;71:380–6. https://doi.org/10.1016/j.bios.2015.04.080.
Rosenberg R, Gertler R, Friederichs J, et al. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49(4):150–8. https://doi.org/10.1002/cyto.10161.
Li W, Reátegui E, Park MH, et al. Biodegradable nano-films for capture and non-invasive release of circulating tumor cells. Biomaterials. 2015;65:93–102. https://doi.org/10.1016/j.biomaterials.2015.06.036.
Dickey DD, Giangrande PH. Oligonucleotide Aptamers: a next-generation Technology for the Capture and Detection of circulating tumor cells. Methods San Diego Calif. 2016;97:94–103. https://doi.org/10.1016/j.ymeth.2015.11.020.
Tao W, Wang J, Parak WJ, Farokhzad OC, Shi J. Nanobuffering of pH-responsive polymers: a known but sometimes overlooked phenomenon and its biological applications. ACS Nano. 2019;13(5):4876–82. https://doi.org/10.1021/acsnano.9b01696.
Bozzetti C, Quaini F, Squadrilli A, et al. Isolation and characterization of circulating tumor cells in squamous cell carcinoma of the lung using a non-EpCAM-based capture method. PLoS One. 2015;10(11). https://doi.org/10.1371/journal.pone.0142891.
Mego M, Giorgi UD, Dawood S, et al. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer 2011;129(2):417-423. doi:https://doi.org/https://doi.org/10.1002/ijc.25690.
Dolfus C, Piton N, Toure E, Sabourin JC. Circulating tumor cell isolation: the assets of filtration methods with polycarbonate track-etched filters. Chin J Cancer Res. 2015;27(5):479–87. https://doi.org/10.3978/j.issn.1000-9604.2015.09.01.
Hou HW, Warkiani ME, Khoo BL, et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep. 2013;3(1):1259. https://doi.org/10.1038/srep01259.
De Giorgi V, Pinzani P, Salvianti F, et al. Circulating benign nevus cells detected by ISET technique: warning for melanoma molecular diagnosis. Arch Dermatol. 2010;146(10):1120–4. https://doi.org/10.1001/archdermatol.2010.264.
Tamminga M, Andree KC, Hiltermann TJN, et al. Detection of Circulating Tumor Cells in the Diagnostic Leukapheresis Product of Non-Small-Cell Lung Cancer Patients Comparing CellSearch® and ISET. Cancers. 2020;12(4). https://doi.org/10.3390/cancers12040896.
Xu L, Mao X, Imrali A, et al. Optimization and evaluation of a novel size based circulating tumor cell isolation system. PLoS One. 2015;10(9). https://doi.org/10.1371/journal.pone.0138032.
Coumans FAW, van Dalum G, Beck M, Terstappen LWMM. Filter characteristics influencing circulating tumor cell enrichment from whole blood. Secomb TW, ed. PLoS One. 2013;8(4):e61770. https://doi.org/10.1371/journal.pone.0061770.
Park ES, Jin C, Guo Q, et al. Continuous flow deformability-based separation of circulating tumor cells using microfluidic ratchets. Small. 2016;14:11.
Bankó P, Lee SY, Nagygyörgy V, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol OncolJ Hematol Oncol. 2019;12(1):48. https://doi.org/10.1186/s13045-019-0735-4.
Campton DE, Ramirez AB, Nordberg JJ, et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer. 2015;15. https://doi.org/10.1186/s12885-015-1383-x.
Bischoff FZ, Marquéz-Do DA, Martinez DI, et al. Intact fetal cell isolation from maternal blood: improved isolation using a simple whole blood progenitor cell enrichment approach (RosetteSepTM). Clin Genet 2003;63(6):483-489. doi:https://doi.org/https://doi.org/10.1034/j.1399-0004.2003.00087.x.
Shim S, Stemke-Hale K, Noshari J, Becker FF, Gascoyne PRC. Dielectrophoresis has broad applicability to marker-free isolation of tumor cells from blood by microfluidic systems. Biomicrofluidics. 2013;7(1):011808. https://doi.org/10.1063/1.4774307.
Gupta V, Jafferji I, Garza M, et al. ApoStreamTM, a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics. 2012;6(2). https://doi.org/10.1063/1.4731647.
Wang Y, Han X, Cui Z, Shi D. Bioelectricity, its fundamentals, characterization methodology, and applications in Nano-bioprobing and Cancer diagnosis. Adv Biosyst. 2019;3(10):1900101. https://doi.org/10.1002/adbi.201900101.
Zhu Z, Zhang YHP. In vitro metabolic engineering of bioelectricity generation by the complete oxidation of glucose. Metab Eng. 2017;39:110–6. https://doi.org/10.1016/j.ymben.2016.11.002.
Wu S, Gu L, Qin J, et al. Rapid label-free isolation of circulating tumor cells from patients’ peripheral blood using electrically charged Fe 3 O 4 nanoparticles. ACS Appl Mater Interfaces. 2020;12(4):4193–203. https://doi.org/10.1021/acsami.9b16385.
Genna A, Vanwynsberghe AM, Villard AV, et al. EMT-associated heterogeneity in circulating tumor cells: sticky friends on the road to metastasis. Cancers. 2020;12(6). https://doi.org/10.3390/cancers12061632.
Po JW, Roohullah A, Lynch D, et al. Improved ovarian cancer EMT-CTC isolation by immunomagnetic targeting of epithelial EpCAM and mesenchymal N-cadherin. J Circ Biomark. 2018;7. https://doi.org/10.1177/1849454418782617.
Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69–76. https://doi.org/10.1007/978-3-642-28160-0_6. PMID: 22527495.
Lu J, Fan T, Zhao Q, et al. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int J Cancer. 2010;126(3):669–83. https://doi.org/10.1002/ijc.24814.
Chen W, Weng S, Zhang F, et al. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. ACS Nano. 2013;7(1):566–75. https://doi.org/10.1021/nn304719q.
Aguado J, d’Adda di Fagagna F, Wolvetang E. Telomere transcription in ageing. Ageing Res Rev. 2020;62:101115. https://doi.org/10.1016/j.arr.2020.101115.
Kojima T, Hashimoto Y, Watanabe Y, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest. 2009;119(10):3172–81. https://doi.org/10.1172/JCI38609.
Kim SJ, Masago A, Tamaki Y, et al. A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat. 2011;128(3):765–73. https://doi.org/10.1007/s10549-011-1603-2.
Ding P, Wang Z, Wu Z, et al. Tannic acid (TA)-functionalized magnetic nanoparticles for EpCAM-independent circulating tumor cell (CTC) isolation from patients with different cancers. ACS Appl Mater Interfaces. 2021;13(3):3694–700. https://doi.org/10.1021/acsami.0c20916.
Meye A, Bilkenroth U, Schmidt U, et al. Isolation and enrichment of urologic tumor cells in blood samples by a semi-automated CD45 depletion autoMACS protocol. Int J Oncol. 2002;21(3):521–30. https://doi.org/10.3892/ijo.21.3.521.
Hu L, Chen X, Chen M, Fang J, Nie J, Dai H. Enrichment and detection of circulating tumor cells by immunomagnetic beads and flow cytometry. Biotechnol Lett. 2021;43(1):25–34. https://doi.org/10.1007/s10529-020-03007-8.
Agerbæk MØ, Bang-Christensen SR, Yang MH, et al. The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat Commun. 2018;9. https://doi.org/10.1038/s41467-018-05793-2.
Nie L, Li F, Huang X, et al. Folic acid targeting for efficient isolation and detection of ovarian Cancer CTCs from human whole blood based on two-step binding strategy. ACS Appl Mater Interfaces. 2018;10(16):14055–62. https://doi.org/10.1021/acsami.8b02583.
Van der Auwera I, Peeters D, Benoy IH, et al. Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT–PCR in patients with metastatic breast cancer. Br J Cancer. 2010;102(2):276–84. https://doi.org/10.1038/sj.bjc.6605472.
Harb W, Fan A, Tran T, et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol. 2013;6(5):528–38.
Cho H, Kim J, Jeon CW, Han KH. A disposable microfluidic device with a reusable magnetophoretic functional substrate for isolation of circulating tumor cells. Lab Chip. 2017;17(23):4113–23. https://doi.org/10.1039/C7LC00925A.
Park C, Abafogi AT, Ponnuvelu DV, Song I, Ko K, Park S. Enhanced luminescent detection of circulating tumor cells by a 3D printed Immunomagnetic concentrator. Biosensors. 2021;11(8):278. https://doi.org/10.3390/bios11080278.
Kim YJ, Cho YH, Min J, Han SW. Circulating tumor marker isolation with the chemically stable and instantly degradable (CSID) hydrogel ImmunoSpheres. Anal Chem. 2021;93(2):1100–9. https://doi.org/10.1021/acs.analchem.0c04152.
Liu Z, Fusi A, Klopocki E, et al. Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients. J Transl Med. 2011;9:70. https://doi.org/10.1186/1479-5876-9-70.
Drucker A, Teh EM, Kostyleva R, Rayson D, Douglas S, Pinto DM. Comparative performance of different methods for circulating tumor cell enrichment in metastatic breast cancer patients. PLoS One. 2020;15(8). https://doi.org/10.1371/journal.pone.0237308.
Yang H, Gijs MAM. Micro-optics for microfluidic analytical applications. Chem Soc Rev. 2018;47(4):1391–458. https://doi.org/10.1039/C5CS00649J.
Burinaru TA, Avram M, Avram A, et al. Detection of circulating tumor cells using microfluidics. ACS Comb Sci. 2018;20(3):107–26. https://doi.org/10.1021/acscombsci.7b00146.
Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci. 2010;107(43):18392–7. https://doi.org/10.1073/pnas.1012539107.
Nwankire CE, Venkatanarayanan A, Glennon T, Keyes TE, Forster RJ, Ducrée J. Label-free impedance detection of cancer cells from whole blood on an integrated centrifugal microfluidic platform. Biosens Bioelectron. 2015;68:382–9. https://doi.org/10.1016/j.bios.2014.12.049.
Lin M, Chen JF, Lu YT, et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res. 2014;47(10):2941–50. https://doi.org/10.1021/ar5001617.
Jou HJ, Chou LY, Chang WC, et al. An automatic platform based on nanostructured microfluidic Chip for isolating and identification of circulating tumor cells. Micromachines. 2021;12(5):473. https://doi.org/10.3390/mi12050473.
Wang J, Li Y, Wang R, et al. A fully automated and integrated microfluidic system for efficient CTC detection and its application in hepatocellular carcinoma screening and prognosis. ACS Appl Mater Interfaces. 2021;13(25):30174–86. https://doi.org/10.1021/acsami.1c06337.
Babahosseini H, Strobl JS, Agah M. Microfluidic iterative mechanical characteristics (iMECH) analyzer for single-cell metastatic identification. Anal Methods. 2017;9(5):847–55. https://doi.org/10.1039/C6AY03342C.
Lin E, Rivera-Báez L, Fouladdel S, et al. High-throughput microfluidic labyrinth for the label-free isolation of circulating tumor cells. Cell Syst. 2017;5(3):295–304.e4. https://doi.org/10.1016/j.cels.2017.08.012.
Smith KJ, Jana JA, Kaehr A, et al. Inertial focusing of circulating tumor cells in whole blood at high flow rates using the microfluidic CTCKeyTM device for CTC enrichment. Lab Chip. 2021;21(18):3559–72. https://doi.org/10.1039/D1LC00546D.
Saucedo-Zeni N, Mewes S, Niestroj R, et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol. 2012;41(4):1241–50. https://doi.org/10.3892/ijo.2012.1557.
Zhu X, Suo Y, Fu Y, et al. In vivo flow cytometry reveals a circadian rhythm of circulating tumor cells. Light Sci Appl. 2021;10:110. https://doi.org/10.1038/s41377-021-00542-5.
Yan J, Fan Z, Wu X, et al. Circulating tumor cells are correlated with disease progression and treatment response in an orthotopic hepatocellular carcinoma model. Cytom Part J Int Soc Anal Cytol. 2015;87(11):1020–8. https://doi.org/10.1002/cyto.a.22782.
Gorges TM, Stein A, Quidde J, et al. Improved Detection of Circulating Tumor Cells in Metastatic Colorectal Cancer by the Combination of the CellSearch® System and the AdnaTest®. PLoS One, e0155126. 2016;11(5). https://doi.org/10.1371/journal.pone.0155126.
Cho H, Chung JS, Han KH. A direct comparison between the lateral Magnetophoretic microseparator and AdnaTest for isolating prostate circulating tumor cells. Micromachines. 2020;11(9). https://doi.org/10.3390/mi11090870.
Jesenko T, Modic Z, Kuhar CG, et al. Morphological features of breast Cancer circulating tumor cells in blood after physical and biological type of isolation. Radiol Oncol. 2021;55(3):292–304. https://doi.org/10.2478/raon-2021-0033.
Lozar T, Jesenko T, Kloboves Prevodnik V, et al. Preclinical and clinical evaluation of magnetic-activated cell separation technology for CTC isolation in breast Cancer. Front Oncol. 2020;10:554554. https://doi.org/10.3389/fonc.2020.554554.
Sharifi M, Zarrin B, Bahri Najafi M, et al. Integrin α6 β4 on circulating tumor cells of metastatic breast Cancer patients. Adv Biomed Res. 2021;10:16. https://doi.org/10.4103/abr.abr_76_21.
Duan GC, Zhang XP, Wang HE, et al. Circulating tumor cells as a screening and diagnostic marker for early-stage non-small cell lung Cancer. OncoTargets Ther. 2020;13:1931–9. https://doi.org/10.2147/OTT.S241956.
Theil G, Boehm C, Fischer K, et al. In vivo isolation of circulating tumor cells in patients with different stages of prostate cancer. Oncol Lett. 2021;21(5):357. https://doi.org/10.3892/ol.2021.12618.
Todenhöfer T, Park ES, Duffy S, et al. Microfluidic enrichment of circulating tumor cells in patients with clinically localized prostate cancer. Urol Oncol. 2016;34(11):483.e9–483.e16. https://doi.org/10.1016/j.urolonc.2016.06.004.
Bobek V, Gurlich R, Eliasova P, Kolostova K. Circulating tumor cells in pancreatic cancer patients: enrichment and cultivation. World J Gastroenterol. 2014;20(45):17163–70. https://doi.org/10.3748/wjg.v20.i45.17163.
Barr J, Chudasama D, Rice A, Karteris E, Anikin V. Lack of association between Screencell-detected circulating tumour cells and long-term survival of patients undergoing surgery for non-small cell lung cancer: a pilot clinical study. Mol Clin Oncol. 2020;12(3):191–5. https://doi.org/10.3892/mco.2020.1981.
Philippron A, Depypere L, Oeyen S, et al. Evaluation of a marker independent isolation method for circulating tumor cells in esophageal adenocarcinoma. PLoS One. 2021;16(5):e0251052. https://doi.org/10.1371/journal.pone.0251052.
Çağlayan Arslan Z, Demircan Yalçın Y, Külah H. Label-free enrichment of MCF7 breast cancer cells from leukocytes using continuous flow dielectrophoresis. Electrophoresis. 2022;43(13-14):1531–44. https://doi.org/10.1002/elps.202100318.
Lagoudianakis EE, Kataki A, Manouras A, et al. Detection of epithelial cells by RT-PCR targeting CEA, CK20, and TEM-8 in colorectal carcinoma patients using OncoQuick density gradient centrifugation system. J Surg Res. 2009;155(2):183–90. https://doi.org/10.1016/j.jss.2007.10.013.
Riebensahm C, Joosse SA, Mohme M, et al. Clonality of circulating tumor cells in breast cancer brain metastasis patients. Breast Cancer Res BCR. 2019;21(1):101. https://doi.org/10.1186/s13058-019-1184-2.
van der Toom EE, Groot VP, Glavaris SA, et al. Analogous detection of circulating tumor cells using the AccuCyte® -CyteFinder® system and ISET system in patients with locally advanced and metastatic prostate cancer. Prostate. 2018;78(4):300–7. https://doi.org/10.1002/pros.23474.
O’Shannessy DJ, Davis DW, Anderes K, Somers EB. Isolation of circulating tumor cells from multiple epithelial cancers with ApoStream(®) for detecting (or monitoring) the expression of Folate receptor alpha. Biomark Insights. 2016;11:7–18. https://doi.org/10.4137/BMI.S35075.
Le Du F, Fujii T, Kida K, et al. EpCAM-independent isolation of circulating tumor cells with epithelial-to-mesenchymal transition and cancer stem cell phenotypes using ApoStream® in patients with breast cancer treated with primary systemic therapy. PLoS One. 2020;15(3):e0229903. https://doi.org/10.1371/journal.pone.0229903.
Togo S, Katagiri N, Namba Y, et al. Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase-selective replicating adenovirus in non-small cell lung cancer patients. Oncotarget. 2017;8(21):34884–95. https://doi.org/10.18632/oncotarget.16818.
He W, Hou M, Zhang H, et al. Clinical significance of circulating tumor cells in predicting disease progression and chemotherapy resistance in patients with gestational choriocarcinoma. Int J Cancer. 2019;144(6):1421–31. https://doi.org/10.1002/ijc.31742.
Zheng Q, Iqbal SM, Wan Y. Cell detachment: Post-isolation challenges. Biotechnol Adv. 2013;31(8):1664–75. https://doi.org/10.1016/j.biotechadv.2013.08.013.
Li S, Chen N, Zhang Z, Wang Y. Endonuclease-responsive aptamer-functionalized hydrogel coating for sequential catch and release of cancer cells. Biomaterials. 2013;34(2):460–9. https://doi.org/10.1016/j.biomaterials.2012.09.040.
Sun N, Liu M, Wang J, et al. Chitosan Nanofibers for specific capture and nondestructive release of CTCs assisted by pCBMA brushes. Small. 2016;12(36):5090-5097. doi:https://doi.org/https://doi.org/10.1002/smll.201600475.
De las Heras Alarcón C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34(3):276–85. https://doi.org/10.1039/B406727D.
Reátegui E, Aceto N, Lim EJ, et al. Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells. Adv Mater Deerfield Beach Fla. 2015;27(9):1593–9. https://doi.org/10.1002/adma.201404677.
Rossi G, Mu Z, Rademaker AW, et al. Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast Cancer. Clin Cancer Res. 2018;24(3):560–8. https://doi.org/10.1158/1078-0432.CCR-17-2092.
Radovich M, Jiang G, Hancock BA, et al. Association of Circulating Tumor DNA and circulating tumor cells after Neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast Cancer: preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol. 2020;6(9):1410–5. https://doi.org/10.1001/jamaoncol.2020.2295.
Deutsch TM, Stefanovic S, Feisst M, et al. Cut-off analysis of CTC change under systemic therapy for defining early therapy response in metastatic breast Cancer. Cancers. 2020;12(4):E1055. https://doi.org/10.3390/cancers12041055.
Nanou A, Zeune LL, Bidard FC, Pierga JY, Terstappen LWMM. HER2 expression on tumor-derived extracellular vesicles and circulating tumor cells in metastatic breast cancer. Breast Cancer Res BCR. 2020;22:86. https://doi.org/10.1186/s13058-020-01323-5.
Wang C, Mu Z, Ye Z, et al. Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients with HER2-negative tumors. Breast Cancer Res Treat. 2020;181(3):679–89. https://doi.org/10.1007/s10549-020-05662-x.
Darga EP, Dolce EM, Fang F, et al. PD-L1 expression on circulating tumor cells and platelets in patients with metastatic breast cancer. PLoS One. 2021;16(11):e0260124. https://doi.org/10.1371/journal.pone.0260124.
Paoletti C, Miao J, Dolce EM, et al. Circulating tumor cell clusters in patients with metastatic breast Cancer: a SWOG S0500 translational medicine study. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(20):6089–97. https://doi.org/10.1158/1078-0432.CCR-19-0208.
Strati A, Nikolaou M, Georgoulias V, Lianidou ES. Prognostic significance of TWIST1, CD24, CD44, and ALDH1 transcript quantification in EpCAM-positive circulating tumor cells from early stage breast Cancer patients. Cells. 2019;8(7):E652. https://doi.org/10.3390/cells8070652.
Magbanua MJM, Savenkov O, Asmus EJ, et al. Clinical significance of circulating tumor cells in hormone receptor-positive metastatic breast Cancer patients who received Letrozole with or without Bevacizumab. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(18):4911–20. https://doi.org/10.1158/1078-0432.CCR-20-1329.
Marquette CH, Boutros J, Benzaquen J, et al. Circulating tumour cells as a potential biomarker for lung cancer screening: a prospective cohort study. Lancet Respir Med. 2020;8(7):709–16. https://doi.org/10.1016/S2213-2600(20)30081-3.
Wang PP, Liu SH, Chen CT, et al. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J Cancer. 2020;11(8):2113–22. https://doi.org/10.7150/jca.35308.
Li Z, Xu K, Tartarone A, Santarpia M, Zhu Y, Jiang G. Circulating tumor cells can predict the prognosis of patients with non-small cell lung cancer after resection: a retrospective study. Transl Lung Cancer Res. 2021;10(2):995–1006. https://doi.org/10.21037/tlcr-21-149.
Matsushita D, Uenosono Y, Arigami T, et al. Clinical significance of circulating tumor cells in the response to trastuzumab for HER2-negative metastatic gastric cancer. Cancer Chemother Pharmacol. 2021;87(6):789–97. https://doi.org/10.1007/s00280-021-04251-z.
Kuroda K, Yashiro M, Miki Y, et al. Circulating tumor cells with FGFR2 expression might be useful to identify patients with existing FGFR2-overexpressing tumor. Cancer Sci. 2020;111(12):4500–9. https://doi.org/10.1111/cas.14654.
Miki Y, Yashiro M, Kuroda K, et al. Circulating CEA-positive and EpCAM-negative tumor cells might be a predictive biomarker for recurrence in patients with gastric cancer. Cancer Med. 2021;10(2):521–8. https://doi.org/10.1002/cam4.3616.
Sastre J, de la Orden V, Martínez A, et al. Association between baseline circulating tumor cells, molecular tumor profiling, and clinical characteristics in a large cohort of chemo-naïve metastatic colorectal Cancer patients prospectively collected. Clin Colorectal Cancer. 2020;19(3):e110–6. https://doi.org/10.1016/j.clcc.2020.02.014.
Messaritakis I, Sfakianaki M, Vogiatzoglou K, et al. Evaluation of the role of circulating tumor cells and microsatellite instability status in predicting outcome of advanced CRC patients. J Pers Med. 2020;10(4):E235. https://doi.org/10.3390/jpm10040235.
Hugenschmidt H, Labori KJ, Brunborg C, et al. Circulating tumor cells are an independent predictor of shorter survival in patients undergoing resection for pancreatic and Periampullary adenocarcinoma. Ann Surg. 2020;271(3):549–58. https://doi.org/10.1097/SLA.0000000000003035.
Sun YF, Wang PX, Cheng JW, et al. Postoperative circulating tumor cells: an early predictor of extrahepatic metastases in patients with hepatocellular carcinoma undergoing curative surgical resection. Cancer Cytopathol. 2020;128(10):733–45. https://doi.org/10.1002/cncy.22304.
Lei Y, Wang X, Sun H, et al. Association of Preoperative NANOG-positive circulating tumor cell levels with recurrence of hepatocellular carcinoma. Front Oncol. 2021;11:601668. https://doi.org/10.3389/fonc.2021.601668.
Basso U, Facchinetti A, Rossi E, et al. Prognostic role of circulating tumor cells in metastatic renal cell carcinoma: a large, multicenter. Prospect Trial Oncologist. 2021;26(9):740–50. https://doi.org/10.1002/onco.13842.
Zhang P, Wang Z, Yang X, Gao K, Li M, Chong T. The significance of detection of circulating tumor cells and Beclin1 in peripheral blood of patients with renal cell carcinoma. Crit Rev Eukaryot Gene Expr. 2020;30(6):483–92. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2020036246.
Graf RP, Hullings M, Barnett ES, Carbone E, Dittamore R, Scher HI. Clinical utility of the nuclear-localized AR-V7 biomarker in circulating tumor cells in improving physician treatment choice in castration-resistant prostate Cancer. Eur Urol. 2020;77(2):170–7. https://doi.org/10.1016/j.eururo.2019.08.020.
Sperger JM, Emamekhoo H, McKay RR, et al. Prospective evaluation of clinical outcomes using a multiplex liquid biopsy targeting diverse resistance mechanisms in metastatic prostate Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(26):2926–37. https://doi.org/10.1200/JCO.21.00169.
Armstrong AJ, Luo J, Nanus DM, et al. Prospective multicenter study of circulating tumor cell AR-V7 and Taxane versus hormonal treatment outcomes in metastatic castration-resistant prostate Cancer. JCO Precis Oncol. 2020;4:PO.20.00200. https://doi.org/10.1200/PO.20.00200.
Schonhoft JD, Zhao JL, Jendrisak A, et al. Morphology-predicted large-scale transition number in circulating tumor cells identifies a chromosomal instability biomarker associated with poor outcome in castration-resistant prostate Cancer. Cancer Res. 2020;80(22):4892–903. https://doi.org/10.1158/0008-5472.CAN-20-1216.
Zapatero A, Gómez-Caamaño A, Cabeza Rodriguez MÁ, et al. Detection and dynamics of circulating tumor cells in patients with high-risk prostate cancer treated with radiotherapy and hormones: a prospective phase II study. Radiat Oncol Lond Engl. 2020;15(1):137. https://doi.org/10.1186/s13014-020-01577-5.
Banys-Paluchowski M, Fehm T, Neubauer H, et al. Clinical relevance of circulating tumor cells in ovarian, fallopian tube and peritoneal cancer. Arch Gynecol Obstet. 2020;301(4):1027–35. https://doi.org/10.1007/s00404-020-05477-7.
Hiltermann TJN, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol. 2012;23(11):2937–42. https://doi.org/10.1093/annonc/mds138.
Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease — latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16(7):409–24. https://doi.org/10.1038/s41571-019-0187-3.
Cohen SJ, Punt CJA, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal Cancer. J Clin Oncol. 2008;26(19):3213–21. https://doi.org/10.1200/JCO.2007.15.8923.
Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–14. https://doi.org/10.1016/S1470-2045(14)70069-5.
Lucci A, Hall CS, Patel SP, et al. Circulating tumor cells and early relapse in node-positive melanoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(8):1886–95. https://doi.org/10.1158/1078-0432.CCR-19-2670.
Zhang L, Ridgway LD, Wetzel MA, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5(180). https://doi.org/10.1126/scitranslmed.3005109.
Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung Cancer. Clin Cancer Res. 2009;15(22):6980–6. https://doi.org/10.1158/1078-0432.CCR-09-1095.
Cui Z, Su F, Li Y, Yang D. Circulating tumour cells as prognosis predictive markers of neoadjuvant chemotherapy-treated breast cancer patients. J Chemother. 2020;32(6):304–9. https://doi.org/10.1080/1120009X.2020.1774207.
Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1):349–61. https://doi.org/10.1016/j.cell.2011.11.025.
Ilie M, Hofman V, Long-Mira E, et al. “Sentinel” circulating tumor cells allow early diagnosis of lung Cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9(10). https://doi.org/10.1371/journal.pone.0111597.
Joosse SA, Souche FR, Babayan A, et al. Chromosomal aberrations associated with sequential steps of the metastatic Cascade in colorectal Cancer patients. Clin Chem. 2018;64(10):1505–12. https://doi.org/10.1373/clinchem.2018.289819.
Buscail E, Chiche L, Laurent C, et al. Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Mol Oncol. 2019;13(9):1811–26. https://doi.org/10.1002/1878-0261.12534.
Tzanikou E, Markou A, Politaki E, et al. PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: a direct comparison study. Mol Oncol. 2019;13(12):2515–30. https://doi.org/10.1002/1878-0261.12540.
Li Z, Wu Y, Yates ME, et al. Hotspot ESR1 mutations are multimodal and contextual modulators of breast Cancer metastasis. Cancer Res. 2022;82(7):1321–39. https://doi.org/10.1158/0008-5472.CAN-21-2576.
Fernandez SV, Bingham C, Fittipaldi P, et al. TP53 mutations detected in circulating tumor cells present in the blood of metastatic triple negative breast cancer patients. Breast Cancer Res BCR. 2014;16(5):445. https://doi.org/10.1186/s13058-014-0445-3.
Gerratana L, Davis AA, Polano M, et al. Understanding the organ tropism of metastatic breast cancer through the combination of liquid biopsy tools. Eur J Cancer Oxf Engl. 1990;2021(143):147–57. https://doi.org/10.1016/j.ejca.2020.11.005.
Fehm T, Becker S, Duerr-Stoerzer S, et al. Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res BCR. 2007;9(5):R74. https://doi.org/10.1186/bcr1783.
Jaeger BA, Neugebauer J, Andergassen U, et al. The HER2 phenotype of circulating tumor cells in HER2-positive early breast cancer: a translational research project of a prospective randomized phase III trial. PLoS One. 2017;12(6):e0173593. https://doi.org/10.1371/journal.pone.0173593.
Beije N, Sieuwerts AM, Kraan J, et al. Estrogen receptor mutations and splice variants determined in liquid biopsies from metastatic breast cancer patients. Mol Oncol. 2018;12(1):48–57. https://doi.org/10.1002/1878-0261.12147.
Mastoraki S, Strati A, Tzanikou E, et al. ESR1 methylation: a liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast Cancer receiving endocrine treatment. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(6):1500–10. https://doi.org/10.1158/1078-0432.CCR-17-1181.
Autio KA, Dreicer R, Anderson J, et al. Safety and efficacy of BIND-014, a Docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate Cancer: a phase 2 clinical trial. JAMA Oncol. 2018;4(10):1344–51. https://doi.org/10.1001/jamaoncol.2018.2168.
Boral D, Vishnoi M, Liu HN, et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat Commun. 2017;8(1):196. https://doi.org/10.1038/s41467-017-00196-1.
Klotz R, Thomas A, Teng T, et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Discov. 2020;10(1):86–103. https://doi.org/10.1158/2159-8290.CD-19-0384.
Nicolazzo C, Raimondi C, Mancini M, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6:31726. https://doi.org/10.1038/srep31726.
Hosokawa M, Hayata T, Fukuda Y, et al. Size-selective microcavity Array for rapid and efficient detection of circulating tumor cells. Anal Chem. 2010;82(15):6629–35. https://doi.org/10.1021/ac101222x.
Aceto N. Fluctuating numbers of circulating tumor cells in cancer patients and the meaning of zero counts. Oncotarget. 2019;10(28):2658–9. https://doi.org/10.18632/oncotarget.26850.
Page K, Shaw JA, Guttery DS. The liquid biopsy: towards standardisation in preparation for prime time. Lancet Oncol. 2019;20(6):758–60. https://doi.org/10.1016/S1470-2045(19)30310-9.
Ning D, Cui K, Liu M, et al. Comparison of CellSearch and Circulating Tumor Cells (CTC)-Biopsy Systems in Detecting Peripheral Blood Circulating Tumor Cells in Patients with Gastric Cancer. Med Sci Monit Int Med J Exp Clin Res. 2021;27:e926565. https://doi.org/10.12659/MSM.926565.
Zavridou M, Mastoraki S, Strati A, et al. Direct comparison of size-dependent versus EpCAM-dependent CTC enrichment at the gene expression and DNA methylation level in head and neck squamous cell carcinoma. Sci Rep. 2020;10(1):6551. https://doi.org/10.1038/s41598-020-63055-y.
Bartkowiak K, Koch C, Gärtner S, Andreas A, Gorges TM, Pantel K. In vitro modeling of Reoxygenation effects on mRNA and protein levels in hypoxic tumor cells upon entry into the bloodstream. Cells. 2020;9(5):1316. https://doi.org/10.3390/cells9051316.
Acknowledgements
BioRender.com was used to create the schematic.
Funding
The work was supported by the National Natural Science Foundation of China (No. 81972597, No. 81972453). The work was sponsored by Zheng Shu Medical Elite Scholarship Fund.
Author information
Authors and Affiliations
Contributions
JSW, JFY and WLB designed the review. JSW and CC researched the literature and drafted the manuscript. ZJH, XL, CYX, ZX, LZQ and ZJC edited the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ju, S., Chen, C., Zhang, J. et al. Detection of circulating tumor cells: opportunities and challenges. Biomark Res 10, 58 (2022). https://doi.org/10.1186/s40364-022-00403-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-022-00403-2