Abstract
Purpose
Presence of circulating tumor cells (CTCs) is associated with impaired clinical outcome in several solid cancers. Limited data are available on the significance of CTCs in gynaecological malignancies. The aims of the present study were to evaluate the dynamics of CTCs in patients with ovarian, fallopian tube and peritoneal cancer during chemotherapy and to assess their clinical relevance.
Methods
43 patients with ovarian, fallopian tube and peritoneal cancer were included into this prospective study. Patients received chemotherapy according to national guidelines. CTC analysis was performed using the CellSearch system prior to chemotherapy, after three and six cycles.
Results
In 26% of the patients, ≥ 1CTC per 7.5 ml of blood was detected at baseline (17% of patients with de novo disease, compared to 35% in recurrent patients). Presence of CTCs did not correlate with other factors. After three cycles of therapy, CTC positivity rate declined to 4.8%. After six cycles, no patient showed persistent CTCs. Patients with ≥ 1 CTC at baseline had significantly shorter overall survival and progression-free survival compared to CTC-negative patients (OS: median 3.1 months vs. not reached, p = 0.006, PFS: median 3.1 vs. 23.1 months, p = 0.005). When only the subgroup with newly diagnosed cancer was considered, the association between CTC status and survival was not significant (OS: mean 17.4 vs. 29.0 months, p = 0.192, PFS: 14.3 vs. 26.9 months, p = 0.085). Presence of ≥ 1 CTC after three cycles predicted shorter OS in the entire patient cohort (p < 0.001).
Conclusions
Hematogenous tumor cell dissemination is a common phenomenon in ovarian, fallopian tube and peritoneal cancer. CTC status before start of systemic therapy correlates with clinical outcome. Chemotherapy leads to a rapid decline in CTC counts; further research is needed to evaluate the clinical value of CTC monitoring after therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the second most common gynaecological cancer and accounts for more deaths than any other cancer of the female reproductive system [1]. Despite optimal multivisceral cytoreductive surgery and standard platinum-based first-line chemotherapy, the majority of patients will suffer from a relapse within the first 2–3 years. Therefore, improved strategies to identify patients at risk for recurrence are urgently needed. In this context, blood-based biomarkers such as circulating tumor cells (CTCs) have emerged as a promising candidate.
Hematogenous dissemination of cancer cells shed by the primary tumor is a common phenomenon observed in several solid malignancies [2,3,4]. While blood-borne disease spread leading to development of distant metastases frequently occurs in entities such as breast, prostate and lung cancer, gynaecological tumors are more likely to show continuous spread within the abdominal cavity. Interestingly, based on clinical studies, isolated tumor cells can be detected in blood and bone marrow samples of patients with ovarian cancer with similar positivity rates as in breast cancer [5,6,7]. In a large pooled analysis of 495 patients with primary ovarian cancer disseminated tumor cells in bone marrow were detected in 27% of patients and predicted significantly shorter overall survival (OS) [6]. Since blood sampling is less invasive and allows serial measurements, the focus of translational research has shifted from disseminated tumor cells to CTCs in peripheral blood. Presence of two or more CTCs have already been shown to be associated with an unfavourable prognosis in relapsed ovarian cancer [7].
The aim of the present study was (1) to evaluate the prognostic relevance of CTCs at time of diagnosis and (2) to examine the dynamics of CTCs during chemotherapy in patients with ovarian, fallopian tube and peritoneal cancer.
Methods
43 patients from two certified Gynaecological Cancer Centers were enrolled in this prospective, open-label, non-randomized study. 34 patients were diagnosed with ovarian cancer, five with fallopian tube cancer and four with primary peritoneal cancer. Patients were scheduled to receive chemotherapy in the first-line (n = 23) or higher-line (n = 20) setting. Further inclusion criteria were: age 18 years and older, and diagnosis of primary or relapsed ovarian, fallopian tube or peritoneal cancer. Blood samples were collected before start of a new line of chemotherapy chosen according to national and institutional standards as well as after three and six cycles of therapy. Response to therapy was evaluated according to institutional guidelines, mostly by CT scan and CA125 determination. Informed consent was obtained from all individual participants included in the study.
Detection of CTCs
CTCs were detected using the CellSearch™ system (formerly Veridex LLC, NJ, USA, now Menarini Silicon Biosystems, Italy). Briefly, 7.5 ml peripheral blood were collected into CellSave Tubes and processed according to manufacturer’s instructions. The assay consists of an immunomagnetic enrichment step employing immunomagnetic beads coated with anti-epithelial cell adhesion molecule (EpCAM) antibody, followed by staining with several antibodies. A circulating tumor cell is defined as a CD45-negative cytokeratin-positive cell with a DAPI-stained nucleus. In the current study, CTC-positive patients were defined as those with at least one tumor cell per 7.5 ml blood.
Statistical analysis
Chi-squared test were used to evaluate the relationship between CTC detection and clinical-pathological factors. In the survival analysis, following primary end points were considered: (1) death and (2) progression. Survival intervals were measured from the time of blood sampling to the time of death or of the first clinical, histological or radiographic diagnosis of progression. We constructed Kaplan–Meier curves and used the log-rank test to assess the univariate significance of the parameters. Cox regression analysis was used for multivariate analysis. All reported p-values are two-sided. p values ≤ 0.05 were considered significant. Statistical analysis was performed by SPSS (SPSS Inc., Chicago, IL, USA). The analysis was performed according to the REporting recommendations for tumor MARKer prognostic studies (REMARK) criteria on reporting of biomarkers [8]. The primary question was the prognostic impact of CTCs in the entire patient cohort.
Results
Patients’ characteristics
Clinical–pathological data of 43 patients enrolled in the study are summarized in Tables 1 and 2. Blood sample was collected at time of first diagnosis of malignant disease in 53% of patients, in the remaining 47% of cases at time of recurrent or progressive disease. The majority of patients had ovarian cancer (79%), followed by fallopian tube (12%) and peritoneal cancer (9%). Previous therapies received by patients with recurrent/progressive disease are shown in Table 3. Details regarding therapy administered during study are shown in Tables 3 and 4. Among patients with primary disease, all but one received primary debulking surgery and were scheduled for adjuvant systemic treatment in accordance with current national treatment guidelines. In one case (Patient 40, Table 4) with advanced disease and tumor rest > 2 cm, the patient refused further blood sampling and received neoadjuvant systemic therapy followed by secondary laparotomy with hyperthermic intraperitoneal chemotherapy (HIPEC) at another hospital. The BRCA status of the tumor has been assessed in 10 patients with recurrent/progressive disease and revealed a somatic BRCA1 mutation in one case. The remaining nine patients had BRCA-negative tumors.
Correlation of CTCs with clinical-pathological data
In 26% of patients at least one CTC per 7.5 ml of peripheral blood was detected at baseline (range 0–76, mean 2.84). Presence of CTC at time of diagnosis was not associated with the tumor origin and established prognostic factors such as tumor stage or nodal status. CTC status did not correlate with macroscopic tumor rest. At least one CTC was detected in 17% of patients with de novo disease, compared to 35% in recurrent patients, however this difference was not statistically significant (p = 0.187). After three cycles of systemic therapy, the CTC positivity rate declined to 4.8%; all patients with primary cancer were CTC-negative at this time point. After six cycles of therapy, no patient showed persistent CTCs.
Survival analysis
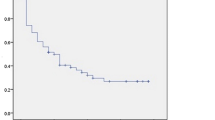
After a median follow-up of 25 months (range 3–36 months), 18 patients died. Patients with at least one detectable CTC at baseline had significantly shorter OS compared to CTC-negative patients (mean OS 12.3 [95% CI 4.4–20.1] vs. 24.6 [19.7–29.4] months, median 3.1 [0.0–12.0] vs. not reached; p = 0.006, Fig. 1, Table 5). When only the subgroup with newly diagnosed cancer was considered, the association between CTC status and survival was not significant (mean 17.4 [1.7–33.1] vs. 29.0 [24.3–33.7] months, p = 0.192, Fig. 2, Table 5). Presence of at least one CTC after three cycles of systemic treatment predicted shorter OS in the entire patient cohort (mean OS 11.1 vs. 31.2 months, p < 0.001). In the entire cohort, CTC-positive patients at baseline had median progression-free survival of 3.1 months, compared to 23.1 months in CTC-negative patients (p = 0.005, Fig. 1, Table 5). In the multivariate analysis including CTC status, disease setting, histology and tumor rest, only the presence of CTCs significantly predicted reduced OS, while residual tumor and CTC status were the only independent factors predicting PFS (Table 6).
Discussion
In the present study, we demonstrated that hematogenous dissemination is a common phenomenon in patients with ovarian, fallopian tube and primary peritoneal cancer. Using the CellSearch assay, CTCs were detected in one-fourth of enrolled patients. While this finding might be at first surprising—giving the preference of these tumor entities for local tumor growth within abdominal cavity—it confirms our previously published data on the presence of disseminated tumor cells in bone marrow of patients with gynaecological malignancies [6]. In this prospective multicentre trial including 495 patients with primary ovarian cancer we reported a prevalence rate of disseminated tumor cells of 27%. Similar positivity rates were observed by others (Table 7) [9,10,11,12,13]. Since bone marrow sampling involves an invasive procedure, the research focus has shifted to examination of peripheral blood over the last two decades and an increasing body of evidence on CTCs in the blood of ovarian cancer patients is available. The largest study to date was conducted in relapsed ovarian cancer. Poveda et al. detected CTCs using the same assay as in our study (CellSearch) and reported a significantly reduced progression-free and overall survival in CTC-positive patients [7]. Interestingly, in contrast to other trials, Poveda et al. defined CTC-positivity as presence of two or more CTCs per 7.5 ml blood, so patients with one CTC were qualified as CTC-negative. Setting a specific cut-off value in case of CTC-based trials is common in other entities. For instance, in metastatic breast cancer several clinical trials used 5 CTCs per 7.5 ml blood as a threshold to differentiate between patients with favourable and unfavourable outcome [14,15,16], whereas 3 CTCs have been shown to be a more suitable cut-off value in metastatic colorectal cancer [17].
While the prognostic relevance of hematogenous tumor cell dissemination was confirmed in large trials in entities such as breast cancer, data regarding ovarian cancer are still limited. In the present study presence of at least one CTC was associated with worse PFS and OS in the entire cohort. When patients with primary and relapsed cancer were considered as separate subgroups, the correlation was not significant. However, this trial was not powered for subgroup analysis. Similar results have been reported by several other studies. Positive CTC status, defined as ≥ 2 CTCs per 7.5 ml blood, predicted shorter progression-free survival and OS in patients with relapsed ovarian cancer [7]. An association with OS or disease-free survival has been reported by two smaller studies conducted in primary ovarian cancer as well [9, 18]. However, a correlation with survival has not been shown by others, so far [10, 19]. Evidence is clearer with respect to tumor cell detection in the bone marrow: in the pooled analysis of individual patients data from three centers presence of disseminated tumor cells significantly predicted reduced survival [6]. Several hypotheses were discussed as to the biological fate of the single tumor cells. While we cannot exclude the possibility that CTCs and disseminated tumor cells are solely an epiphenomenon of current tumor load, the available data suggest that their role is beyond being just a by-product without their own clinical relevance. Since patients with ovarian carcinoma rarely develop secondary bone metastases, bone marrow seems to serve as a temporary “compartment” for disseminated tumor cells, where they might stay dormant for prolonged periods of time [20, 21]. Subsequently, they might be able to leave their homing site and cause metastatic growth or locoregional recurrence [6]. Hypothetically, these single cells might also be able to re-populate the abdominal cavity, where they encounter a microenvironment suitable to support ovarian cancer growth.
Possibly, not only the presence of CTCs, but their expression profiles may predict the clinical potential. Zhang et al. examined blood samples from 109 patients with newly diagnosed ovarian cancer using Multiplex-RT-PCR based on the detection of six cancer-related genes [22]. While this assay yielded very high CTC detection rates of 90%, the survival analysis showed that only EpCAM positivity of CTCs predicted shorter OS. Interestingly, the CellSearch system, used in our study, includes an enrichment step based on anti-EpCAM antibodies. For that reason, CTCs detected by this assay are more likely to express EpCAM that those detected by other methods (Table 4).
Although the majority of patients with primary ovarian carcinoma initially responds to (neo)adjuvant platinum-based chemotherapy, most will relapse following the state-of-the-art treatment [23]. Therefore, strategies for identification of patients at high risk for relapse early during first-line therapy are urgently needed. In our study, the CTC positivity rate declined rapidly during treatment and no patient showed CTCs at the end of sixth cycle of chemotherapy. In the study by Zhang et al., CTC counts decreased during adjuvant and neoadjuvant therapy as well [22]. Interestingly, peripheral blood was obtained both before and 7–14 days after surgery and a rapid increase in CTC counts has been observed between these two time points. Since the baseline blood sample in our study was collected after surgery, we do not know whether such a decline could be observed using the CellSearch detection system as well. In contrast, Aktas et al. evaluated blood samples from primary ovarian cancer patients obtained before surgery in 86 and/or after adjuvant chemotherapy in 70 cases using the RT-PCR-based AdnaTest and found higher CTC positivity rate after chemotherapy (27% vs. 19%, respectively) [9]. Positive CTC status correlated with shorter OS, independent of the time point of blood sampling (p = 0.0054 before surgery and p = 0.047 after chemotherapy).
Conclusions
In this prospective translational study, we show that hematogenous tumor cell dissemination is a common phenomenon in ovarian, fallopian tube and primary peritoneal carcinoma and is not restricted to patients with high-grade or node-positive disease. With regard to the clinical relevance of this phenomenon, CTC detection before start of adjuvant treatment significantly predicted shorter OS and PFS. However, since CTC counts declined rapidly during systemic therapy, this approach does not seem likely to identify patients at particular risk of relapse. Future research is required to fully understand the potential of CTC detection and characterization in patients with these tumor entities.
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CTC:
-
Circulating tumor cell
- DDFS:
-
Distant disease-free survival
- DFS:
-
Disease-free survival
- DTC:
-
Disseminated tumor cell
- EpCAM:
-
Epithelial cell adhesion molecule
- ICC:
-
Immunocytochemistry
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- REMARK:
-
REporting recommendations for tumor MARKer prognostic studies
References
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68:284–296. https://doi.org/10.3322/caac.21456
Janni WJ, Rack B, Terstappen LW, Pierga JY, Taran FA, Fehm T, Hall C, de Groot MR, Bidard FC, Friedl TW et al (2016) Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res 22:2583–2593. https://doi.org/10.1158/1078-0432.CCR-15-1603
Hille C, Pantel K (2018) Prostate cancer: circulating tumour cells in prostate cancer. Nat Rev Urol 15:265–266. https://doi.org/10.1038/nrurol.2018.25
Kapeleris J, Kulasinghe A, Warkiani ME, Vela I, Kenny L, O'Byrne K, Punyadeera C (2018) The prognostic role of circulating tumor cells (CTCs) in lung cancer. Front Oncol 8:311. https://doi.org/10.3389/fonc.2018.00311
Banys M, Solomayer EF, Becker S, Krawczyk N, Gardanis K, Staebler A, Neubauer H, Wallwiener D, Fehm T (2009) Disseminated tumor cells in bone marrow may affect prognosis of patients with gynecologic malignancies. Int J Gynecol Cancer 19:948–952. https://doi.org/10.1111/IGC.0b013e3181a23c4c
Fehm T, Banys M, Rack B, Janni W, Marth C, Blassl C, Hartkopf A, Trope C, Kimmig R, Krawczyk N et al (2013) Pooled analysis of the prognostic relevance of disseminated tumor cells in the bone marrow of patients with ovarian cancer. Int J Gynecol Cancer 23:839–845. https://doi.org/10.1097/IGC.0b013e3182907109
Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P, Monk BJ (2011) Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol 122:567–572. https://doi.org/10.1016/j.ygyno.2011.05.028
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93:387–391. https://doi.org/10.1038/sj.bjc.6602678
Aktas B, Kasimir-Bauer S, Heubner M, Kimmig R, Wimberger P (2011) Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int J Gynecol Cancer 21:822–830. https://doi.org/10.1097/IGC.0b013e318216cb91
Marth C, Kisic J, Kaern J, Trope C, Fodstad O (2002) Circulating tumor cells in the peripheral blood and bone marrow of patients with ovarian carcinoma do not predict prognosis. Cancer 94:707–712
Schindlbeck C, Hantschmann P, Zerzer M, Jahns B, Rjosk D, Janni W, Rack B, Sommer H, Friese K (2007) Prognostic impact of KI67, p53, human epithelial growth factor receptor 2, topoisomerase IIalpha, epidermal growth factor receptor, and nm23 expression of ovarian carcinomas and disseminated tumor cells in the bone marrow. Int J Gynecol Cancer 17:1047–1055. https://doi.org/10.1111/j.1525-1438.2007.00920.x
Braun S, Schindlbeck C, Hepp F, Janni W, Kentenich C, Riethmuller G, Pantel K (2001) Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. J Clin Oncol 19:368–375
Chebouti I, Blassl C, Wimberger P, Neubauer H, Fehm T, Kimmig R, Kasimir-Bauer S (2016) Analysis of disseminated tumor cells before and after platinum based chemotherapy in primary ovarian cancer. Do stem cell like cells predict prognosis? Oncotarget 7:26454–26464. https://doi.org/10.18632/oncotarget.8524
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23:1420–1430. https://doi.org/10.1200/JCO.2005.08.140
Bidard FC, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabieres C, Janni W, Messina C, Paoletti C, Muller V, Hayes DF et al (2013) Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev 32:179–188. https://doi.org/10.1007/s10555-012-9398-0
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O'Rourke MA, Lew DL et al (2014) Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 32:3483–3489. https://doi.org/10.1200/JCO.2014.56.2561
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC et al (2009) Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 20:1223–1229. https://doi.org/10.1093/annonc/mdn786
Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML (2009) Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol 112:185–191. https://doi.org/10.1016/j.ygyno.2008.09.021
Sehouli J, Konsgen D, Nimpsch R, Stengel D, Oskay G, Mustea A, Oertel J, Lichtenegger W (2003) Prognostic significance of epithelial cells in the blood of patients with gynaecological malignancies. Anticancer Res 23:4133–4140
Banys M, Hartkopf AD, Krawczyk N, Kaiser T, Meier-Stiegen F, Fehm T, Neubauer H (2012) Dormancy in breast cancer. Breast Cancer 4:183–191. https://doi.org/10.2147/BCTT.S26431
Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S (1987) Distant metastases in epithelial ovarian carcinoma. Cancer 60:1561–1566
Zhang X, Li H, Yu X, Li S, Lei Z, Li C, Zhang Q, Han Q, Li Y, Zhang K et al (2018) Analysis of circulating tumor cells in ovarian cancer and their clinical value as a biomarker. Cell Physiol Biochem 48:1983–1994. https://doi.org/10.1159/000492521
Chien J, Kuang R, Landen C, Shridhar V (2013) Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front Oncol 3:251. https://doi.org/10.3389/fonc.2013.00251
Acknowledgements
The authors wish to thank the Stiftung Gynäkologische Onkologie (Hamburg, Germany) for supporting this study.
Funding
The study was supported by a research grant from Stiftung Gynäkologische Onkologie, Hamburg, Germany. The funding body had no role in study design or collection, analysis, and interpretation of data nor in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
MBP: Protocol and project development, patient recruitment, data collection, data management, data analysis, statistical analysis, manuscript writing. TF: Protocol and project development, CTC detection, manuscript editing. HN: Protocol and project development, CTC detection, manuscript editing. PP: patient recruitment, data collection, data management, manuscript editing. FMS: CTC detection, manuscript editing. NK: CTC detection, manuscript editing. CW: patient recruitment, data collection, data management. AK: patient recruitment, data collection, data management. GG: Protocol and project development, patient recruitment, data collection, data management, data analysis, statistical analysis, manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethical approval
All procedures performed in this study were in accordance with the ethical standard of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local ethical committees of participation institutions (September, 1st 2015; approval number: PV5061, Ethikkommission der Ärztekammer Hamburg).
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banys-Paluchowski, M., Fehm, T., Neubauer, H. et al. Clinical relevance of circulating tumor cells in ovarian, fallopian tube and peritoneal cancer. Arch Gynecol Obstet 301, 1027–1035 (2020). https://doi.org/10.1007/s00404-020-05477-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05477-7