Abstract
Background
Due to common bean derives lower nitrogen (N) from symbiotic N2-fixation, it requires N either from inorganic fertilizer or soil N. Field experiments were conducted at four locations to evaluate the effect of Rhizobium leguminosarum bv. phaseoli inoculation on agronomic efficiency of N of common bean var. Dursitu major growing areas of eastern Ethiopia.
Methods
Six levels of inorganic N (0, 20, 40, 60, 80 and 100 kg N ha−1) and two inoculation treatments (uninoculated and inoculated) were factorially combined and laid out in randomized completely block design, replicated three times.
Results
AE-N, nodule number per plant (NN) and nodule dry weight per plant (NDW) decreased with N rates of application beyond 20 kg N ha−1. The highest AE-Ns at Babillae, Fedis and Haramaya sites were obtained from 20 kg N ha−1 applied with Rhizobium inoculation while 40 kg N ha−1 supplied with Rhizobium inoculation at Hirna site. Regardless of experimental sites, inoculation improved AE-N. A positive relationship between AE-N and NDW was also observed in all experimental sites. Significant increase in grain yield with increasing rates of N application was also observed.
Conclusion
Hence, it can be concluded that inoculation is recommendable to increases the efficient utilization of applied Mineral N.
Similar content being viewed by others
Background
Common bean (Phaseolus vulgaris L.) is the most important food legume worldwide, providing the main source of protein for more than 300 million people, supplying about 20% of the protein intake per person (CIAT 2001; Broughton et al. 2003). Despite the fact that some grain yield gain recorded over the last decades, common bean production is still very low in many regions of sub-Saharan African (SSA). Average dry bean yield in most developing countries are very much less than that of potential, indicating that common bean production could be improved by increasing yields per unit land area (Yan et al. 1995). In Ethiopia, common bean is one of the major grain legumes cultivated, with its production centred in small farmers’ fields where the use of N fertilizer is limited and average yields are low, usually less than 1 ton ha−1 (CSA 2013). While under experimental condition, the productivity of common bean at experimental site has obtained up to 4,517 kg ha−1 (IAR 1997, 1998; Assefa et al. 2006). The main causes of low productivity at farmer fields are a poor technology level, utilization of low agricultural input and cropping in low fertility soils, especially with low N content (Haile 1990; Beebe et al. 2013). Low soil fertility is one of the most important yield-limiting factors in most of the bean-producing regions of Ethiopia (Tana and Fininsa 2006). Among essential plant nutrients, nitrogen (N) is the most important limiting nutrient for crop production in the tropics, including Ethiopia (Sanchez 1976). In Latin American, the yield losses due to N deficiency has been recorded up to 45% when compared to the N fertilized plants (Thung and Rao 1999). This indicates the need for inorganic N application and/or a search for more effective rhizobial strains to enhance the growth and grain yield of common bean.
Use of nitrogenous fertilizers by smallholder farmers to increase crop production in SSA has been limited due to unaffordable by many subsistent farmers. Beside the fact that common bean recovered usually less than 50% of the applied N fertilizer (dos Santos and Fageria 2007, 2008). These conditions have therefore necessitated an approach to crop production that emphasizes biological N2 fixation (BNF), with programs for rhizobial strain selection with superior symbiotic performance at a low cost. However, common bean is a promiscuous legume able to form symbioses with many rhizobial species and this nature result in poor N2 fixing plant compared to other grain legumes (Hardarson et al. 1993). N2 fixation in common bean can be increased through inoculation with certain highly efficient strains of Rhizobium leguminosarum biovar phaseoli (Hungria et al. 2000). Hungria et al. (2003) found comparable yield of common bean at 60 kg N ha−1 and Rhizobium tropici inoculated plants. Mulas et al. (2011) observed that inoculation of effective rhizobia attenuated intrinsic soil characteristics through the production of plant growth promoting properties beside N2 fixation (Atzorn et al. 1988; Ahemad and Khan 2011; Stajkovic et al. 2011). Studies, however, indicated that the performance of Rhizobium inoculation alone in the field conditions is not satisfactory (Graham 1981; Buttery et al. 1987). This indicates the need of some mineral N application even though fertilized with N owing to poor nodulation and lack of responses to inoculation under field conditions (Graham et al. 2003). Studies indicated that low rates of inorganic N application have been shown to enhance nodule formation and function but are not sufficient to achieve maximum yields (da Silva et al. 1993; Hungria et al. 2003). These authors also indicate that low levels of N fertilizer associated with the inoculation with selected rhizobial strains, can stimulate plant growth, N2 fixation and grain yield. Under these circumstances, inoculation trials must emphasize not only the benefits of bean inoculation, but also of the combination of that practice with N fertilization, in order to achieve a decrease in mineral N input whilst still obtaining maximum yields. However, there is little information on the effect of inoculation of elite Rhizobium sp. on use efficiency of N by common bean. Therefore the objectives of this experiment were to evaluate the effect of inoculation of elite Rhizobium sp. on use efficiency of N, nodulation and productivity of common bean in four representative experimental sites of Eastern Ethiopia.
Methods
Study areas
Four major common bean growing areas of eastern Ethiopia were selected to determine the effect of Rhizobium on use efficiency of N, nodulation and yield of common bean. The experimental fields are located in the Hirna [N09°13.157″ and E041°06.488″ at an altitude of 1,808 m above sea level (m.a.s.l.)], Fedis (N09°06.941″ and E042°04.835″ at an altitude of 1,669 m.a.s.l.), Babillae (N09°13.234″ and E042°19.407″ at 1,669 m.a.s.l.) and Haramaya (N09°24.954″ and E042°02.037″ at an altitude of 2,020 m.a.s.l.) agricultural research centers. These experiments were conducted during 2012 cropping season.
The soils had not been inoculated before with rhizobia strain nodulating common bean. Soil samples were collected in each experimental site by taking 25 cores (diameter 2.5 cm) from the top 20 cm depth in a grid pattern covering the entire filed site after removing debris and the top 1 cm of soil. The pooled sample was thoroughly mixed and a subsample of 1 kg soil was taken. The sub-sampled soils were air-dried, crushed, and passed through a 2-mm sieve prior to physical and chemical analysis. Details of physical and chemical characteristics of the soil of experimental sites are given in Table 1.
The population density of indigenous rhizobia nodulating common bean in the soils was determined by a plant infection technique (Vincent 1970). The seeds of common bean var. Dursitu were surface sterilized and germinated in petri dish that contained moist filter paper. One seedling was placed on pouches that contained Jensen’s N-free nutrient solution. Each seedling was inoculated with 1.0 ml of soil solution 2 weeks afterwards. Nodulation was examined 21 days after inoculation. The total numbers of nodulated seedling were converted into most probable number of indigenous rhizobia nodulating common bean g−1 of soil.
Sources of seeds and Rhizobium strain
A common bean var. Dursitu was supplied by Lowland pulses research project, Haramaya University, Ethiopia. Variety was selected based on their yield, their maturity time and recentness of year of release. Strain of Rhizbium spp. (HUPvR-16) was obtained from bio fertilizer research and production project, Haramaya University.
Inoculums preparation
Agar slope of strain of Rhizobium was supplied by Soil microbiology research laboratory, Haramaya University. For purification, the isolate was preliminarily cultured in yeast extract mannitol agar medium (YEMA) (10 g mannitol, 1 g yeast-extract, 1 g KH2P04, 0.1 g NaCl, and 0.2 g MgS04·7H20 per liter, pH 6.8) and incubated at 28°C for 5 days. The pure colony of the isolate was later transferred to YEM broth medium with gentle shaking at 120 rpm for 5 days. By this procedure, the Rhizobium culture reached the middle or late logarithmic phase, and cell density in the culture was estimated by measuring optical density (OD) using spectrophotometer at 540 nm. Rhizobium inoculant was prepared by mixing 30 g of sterilised decomposed filter-mud with 15 ml of broth cultures of the appropriate Rhizobium strain in polyethylene bags. The moisture content of the inocula was 35% (w/w). After incubating the inoculated filer-mud for 2 weeks at 28°C, the count of the Rhizobium was 1 × 109 g−1 carrier material. Populations of rhizobia in the inoculants were determined by duplicate plate counts (Vincent 1970).
Experimental design
Field trials were conducted in order to investigate the effects of dual application of inorganic N fertilizer with different rate with and without inoculations of Rhizobium strain on Agronomic efficiency of N of common bean. The treatments of this experiment t were obtained by factorially combined six levels of inorganic N (0, 20, 40, 60, 80 and 100 kg N ha−1) and two inoculation treatments (inoculated and uninoculated). Then, the treatments were arranged in split plot with randomized complete block design (RCBD) with three replications. N rates of application were assigned as main plot treatment. Rhizobium treatments were applied as sub-plot treatments. N fertilizer in each level was divided into two equal parts; the first part of the N (20 kg N ha−1) was applied along the furrow by hand and incorporated before planting time, and the remaining parts were used in the flowering stages.
The area was moldboard-plowed and disked before planting. The size of each main and sub plots were 3 × 5 m2 and 3 × 2 m2, respectively. Phosphorus (P) was applied at 20 kg P ha−1 as triple superphosphate uniformly before planting all plots. There were five rows per plot and the spacing was 40 cm between rows, 10 cm between plants, 1 m between sub plots and 1.5 between main plots. Disinfected seeds of common bean were planted after they were moistened with a 20% solution of sucrose and then inoculated (7 g inoculant per kg seed) with Rhizobium. Two seeds were sown per hill. After germination, the plants were thinned to one seedling per hill to obtain about 30 plants per row. Weeds were controlled over the growth period with hand hoeing. At late flowering and early pod setting stage (R3-stage), five plants from the central rows of each plot were randomly chosen and harvested from central rows to record number of nodule plant−1 (NN), nodule dry weight plant−1 (NDW) and shoot dry weight plant−1 (SDW). Furthermore, shoots of the plants were dried and later ground to pass a 0.5 cm sieve. Total N determinations were done by the Kjeldahl method of Bremner (1965). At physiological maturity stage on October 30, yield and yield attributed of common bean were recorded. Total dry biomass yield (TBY) and grain yield (GY) at 13% moisture content were determined. Agronomic efficiency of N (AE-N) was calculated by using the following formula (Fageria et al. 2013).
where \( {\text{G}}_{\text{f}} \) is the grain yield of the N fertilized plot, \( {\text{G}}_{\text{u}} \) is the grain yield of the unfertilized plot, and Na is the quantity of N applied.
Data analysis
Data were submitted to analysis of variance (SAS Institute 1999). Statistically significant differences between means were also determined by the LSD test (SAS Institute 1999).
Results and discussion
Agronomic efficiency of N
Table 2 shows a significant effect of inoculation of Rhizobium leguminosarum bv. viciae in conjunction with N rates of application on AE-N by common bean at P < 0.05. Regardless of the experimental sites and inoculation treatments, AE-N was significantly decreased with increasing rates of N application. Similar finding was previously reported on common bean by Fageria et al. (2014), as stated in Mitscherlich’s law of diminishing yield. Reduction of AE-N could be due to the amount of grain yield of common bean increases reduced with increasing rates of N application (Rebeschini et al. 2014), consequently leading to lower AE-N at higher rate of N application. Similarly, the nutrient use efficiency usually decreased with increasing nutrient amount added (Dobermann and Cassman 2004). Salvagiotti et al. (2008) also found the largest AE-N at N rates less than 50 kg N ha−1.
In all experimental sites, the Rhizobium inoculated treatment recorded higher AE-N than those obtained corresponding rates of N without inoculation (Figures 1, 2). Similarly, Fagaria et al. reported higher N use efficiency in 50 mgN treated with Rhizobium inoculation as compared to that obtained from 200 mgN treatment). On top of fixing N from atmosphere, many species of rhizobia can secrete growth hormone and thereby improving root growth (Antoun et al. 1998; Canbolat et al. 2006; Remans et al. 2008; Ahemad and Khan 2012) and nutrients uptake. This could also be due to increases the plant access to soil nutrients and, improving P availability through solubilizing unavailable P (Zaidi et al. 2009) and thus enhancing the N use efficiency of common bean. In addition, Camerini et al. (2008) found that inoculation of Rhizobium sp. enhanced the Indol-acetic acid (IAA) biosynthetic pathway thereby produced 60 times more IAA in its nodule than the wild counterpart. Kumar et al. (2011) found that plant growth promoting rhizobia improved grain yield of fenugreek by 36%.
The present study also indicated the positive effect of Rhizobium inoculation on AE-N at low rates of N application (i.e., less than 40 kg N ha−1) (Figure 3). Figure 2 also displays that the rates of N beyond 20 kg N ha−1 applied with Rhizobium inoculation displayed the non-significant effect on AE-N. Several studies indicated 20 kg N ha−1 N application is the starter dose which enhances productivity and nodulation of common bean (Tsai et al. 1993b; Daba and Haile 2000; Hungria et al. 2003).
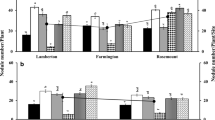
At Babillae site, the highest AE-N (10.16 and 11.6 kg kg−1) from inoculated and uninoculated treatments was obtained at 40 and 20 kg N ha−1, respectively. At Fedis site, 20 kg N ha−1 resulted in the highest AE-N (9.4 and 19.15 kg kg−1) along uninoculated and inoculated treatments, respectively. Similarly at Haramaya site, 20 kg N ha−1 application gave the highest AE-N (8.7 and 24.2 kg kg−1) along uninoculated and inoculated treatments, respectively. At Hirna site, 40 kg N ha−1 gave the highest AE-N (9.49 and 14.70 kg kg−1) among uninoculated and inoculated treatments, respectively. This indicates the need of inoculation to boost grain yield at Fedis site. This also indicates the need of lower rate of N when inoculated common bean with effective Rhizobium sp. for the highest AE-N. All study locations, the AE-N obtained from uninoculated treatment were ranged from 3.9 to 11.8 kg kg−1 which was previously reported for common bean by Fageria et al. (2013). They found the range of AE-N between 3.9 and 11.8 kg kg−1.
The highest AE-N of common bean at Babillae, Fedis and Haramaya were 15.29, 19.15 and 24.20 kg kg−1, respectively, all obtained from Rhizobium inoculation in conjunction with 20 kg N ha−1 application. The highest AE-N (14.70) from Hirna site was recorded from Rhizobium inoculation with 40 kg N ha−1 application. The lowest AE-N observed at Babillae and Hirna were 6.44 and 2.52 kg kg−1, respectively, from combined application of inoculation and 80 kg N ha−1. The lowest AE-N (2.48 and 3.50 kg kg−1) at 80 and 100 kg N ha−1, were obtained from Fedis and Haramaya sites, respectively.
Agronomic efficiency of N and nodulation
The regression analysis indicated significant and polynomial association between AE-N and increasing rates of N application solely and in combination with Rhizobium inoculation. Concave upward (R2 = 0.372 at P ≤ 0.05) and downward (R2 = 0.440 at P ≤ 0.05) associations between the AE-N and N rates of application at Babillae site were observed in inoculated and uninoculated treatment, respectively (Figure 4a). This indicates the need of inoculation with low N application to increase AE-N at this experimental site. Negative and polynomial association between the AE-N and N rates of application were observed at Fedis site, with coefficient of determination of R2 = 0.819 and R2 = 0.898 in Rhizobium inoculated and uninoculated treatments, respectively, at P < 0.05 (Figure 4b). In Haramaya site, negative and polynomial association between AE-N and N rates of application with higher coefficient of determination (R2 = 0.906) was observed in Rhizobium inoculated treatment (Figure 4c). Negative polynomial and linear association AE-N and N rates of application at Hirna site were observed in Rhizobium inoculated and uninoculated treatments, respectively. (Figure 4d). This may indicate the native rhizobia could have been competent and effective N2 fixation than the inoculated Rhizobium isolate.
It is also noted that the positive association between AE-N and NDW was observed in all experimental sites irrespective of inoculation treatments. Both inoculation treatments, the non-significant association between AE-N and NDW was observed at Babillae site (Figure 5a). At Fedis site, positive and significant association between AE-N and NDW was observed, with coefficient of determination of R2 = 0.812 and R2 = 0.504 in inoculated and uninoculated treatments, respectively (Figure 5b). Concave downward polynomial association was observed in Rhizobium inoculated treatments, whereas in uninoculated treatment exhibited concave upward polynomial association. At Haramaya site, significant and polynomial association between AE-N and NDW with coefficient of determination of R2 = 0.701 and R2 = 0.742 were observed in Rhizobium inoculated and uninoculated treatment, respectively (Figure 5c). These associations were concave upward and downward in inoculated and uninoculated treatments, respectively. This indicates that the highest AE-N was recorded at lower NDW value with inoculated treatment than uninoculated treatment. This could be due to the highly effective and competitiveness of inoculated isolate than those present in the indigenous soils as previously confirmed by Willams and Phillips (1983) and; Asad et al. (1991). Significant and concave downward association between AE-N and NDW in Hirna site was observed only in uninoculated treatments, but the association in inoculated treatment was insignificant (Figure 5d).
Nodulation
A significant variation in NN and NDW along increasing rates of N application alone and in combination with Rhizobium inoculation was observed (Tables 3, 4). Regardless of inoculation treatments, NN and NDW were decreased with increasing rates of N fertilizer application in all experimental sites. Similarly Brockwell et al. (1989) demonstrated that nodulation was diminished at high N application irrespective of rates of inoculation. Rebeschini et al. (2014) observed reduction of nodule number and dry weight with increasing rates of N application. Beside this, several findings also indicated reduction of nodule formation and development by inorganic N application (Chemining’wa et al. 2004; Gentile et al. 2006; Otieno et al. 2009; Shamseldin and Moawad 2010). This reduction of nodulation could arise from reducing the flavonoids and isoflavonoids by the plant roots into the soils (Waterer and Vessey 1993).
At Babile site, statistically higher NN and NDW was produced at 20 kg N ha−1 alone and in combination with inoculation of Rhizobium. This study, however indicated the non-significant difference of NDW obtained from 20 kg N ha−1 and the control treatment (without N application). The highest NN produced at Babile site was 161.67 at 20 kg N ha−1 with Rhizobium inoculation. At Fedis site, significantly higher NN and NDW were observed at 20 and 40 kg N ha−1 applications along uninoculated treatment. Along Rhizobium inoculation treatment, the control treatment induced the highest NN and NDW. Likewise, at Haramaya site, 20 kg N ha−1 application without inoculation resulted in significantly higher NN and NDW than the other treatments. Along Rhizobium inoculated, the control treatment produced the highest NN, although this NN was statistically similar with those NN produced at 20 and 40 kg N ha−1. At Haramaya site, 20 kg N ha−1 produced significantly higher NDW than those treatment of N application greater than 20 kg N ha−1. These results indicate that the inoculated isolate could fulfill the N needs of the plant in addition to the native soil total N at Fedis and Haramaya site as previously observed by Mnasri et al. (2007). The result obtained in these three sites agreed with previously reported by Daba and Haile (2000) who found that starter N with Rhizobium inoculation recommended to improve nodulation and yield of common bean in eastern Ethiopia. The application of a small amount of fertilizer N (15–30 kg N ha−1) enhances nodulation of different legume crops (da Silva et al. 1993; Tsai et al. 1993b; Hungria et al. 2003). The better performance of common bean in nodulation in inoculated treatments might be related with plant growth promoting activities of inoculated isolate, beside N2 fixation (Atzorn et al. 1988). They also found that plant growth hormones biosynthesis genes are closely linked to the genes for nodulation and N fixation. Wani et al. (2007) found that plant growth promoting rhizobia improved the nodule number by 23%.
The highest NN at Fedis and Haramaya were 146.67 and 282.00, respectively, at 20 kg N ha−1 application. Combined application of 20 kg N ha−1 and Rhizobium inoculation produced the higher NDW at Babillae and Haramaya, indicating the needs of Rhizobium inoculation and starter N to improve the nodule formation and development at these sites. Sole application of 20 kg N ha−1 resulted in the highest NDW at Fedis site, implying that this site could have higher native rhizobia nodulating common bean, thus inhibiting the effectiveness of inoculated isolate (Giller et al. 1998).
Regardless of the inoculation treatments, the highest NN and NDW at Hirna site were observed at the control treatments. The highest NN (216.67) at Hirna site was obtained from the control treatments with no inoculation of Rhizobium sp. This may indicate that soil had sufficient and effective number of rhizobia nodulating common bean (Theuri et al. 2006) and sufficient inherent soil N until the plant start fix N. Similar finding was reported by Chemining’wa et al. (2007) who found reduction in nodule number and nodule dry of common bean when added starter N fertilizer and improvement of these traits when inoculated Rhizobium alone.
Total biomass yield
TBY of common bean exhibited significant differences along increasing rates of N application alone and in combination with Rhizobium inoculation, excluding TBY at Haramaya site along inoculation treatment (Table 5). Generally, TBY was significantly improved with increasing rates of N application solely and in combination with Rhizobium inoculation. Similar trends of TBY improvement was previously observed by Herridge and Rose (2000) who found that enhancement of shoot biomass has been achieved through increasing N availability from N2 fixation. Fageria et al. (2006) found that N application improves the leaf growth, leaf area duration, and photosynthetic rate per unit leaf area, consequently improving the total biomass of the crop. In all experimental sites, 100 kg N ha−1 gave significantly higher TBY, compared to the control treatments regardless of inoculation treatments, indicating the need of N application for common bean production. In both inoculation treatments, 40 kg N ha−1 and above rates of N application produced statistically similar TBY at Babillae site. At Fedis site, statistically similar TBY were also observed in all N treatments excluding the control, in both inoculation treatments. Rates of N application above 40 kg N ha−1 resulted in statistically similar TBY along uninoculated treatment at Haramaya site. While along inoculated treatment, TBY exhibited the non-significant difference with increasing rates of N application, excluding the unfertilized treatment. This may show the presence of high number of rhizobia nodulating common bean and higher total inorganic N in this soil (Thies et al. 1991; Tsai et al. 1993a; Gan et al. 2009). This might causes ineffectiveness of inoculated Rhizobium sp. (Li et al. 2009) and less response to N application (Mulvaney et al. 2001). Regardless of N rates of application, all N rates of application gave significantly higher TBY than the control in both Rhizobium inoculation treatments at Hirna site.
The highest TBY (3,648.1 kg ha−1) at Babillae was produced at 100 kg N ha−1 applied with Rhizobium inoculation. This TBY was 387.3 kg ha−1 over that obtained from 100 kg N ha−1 alone. Similarly, at 100 kg N ha−1 supplied with Rhizobium sp. inoculation had the highest TBY (4,740.7 kg ha−1) at Fedis site. At Haramaya site, however, the highest TBY (6,474.1 kg ha−1) was obtained from 100 kg N ha−1 applied alone, indicating the presence of competitive and/or effective rhizobia in Haramaya soil. While at Hirna site, 100 kg N ha−1 with Rhizobium inoculation had the highest TBY (8,000.0 kg ha−1) which was 988.4 kg ha−1 over that obtained from 100 kg N ha−1 alone. This indicates that common bean cultivated at this site highly responded to Rhizobium inoculation in comparison to uninoculated treatment, although the soil had higher soil N and native rhizobia nodulating common bean. The lowest TBY at Babillae, Fedis, Haramaya and Hirna sites were 1,787.0, 2,077.0, 48.19.8 and 5,051.3 kg ha−1 respectively. The control treatment without inoculation gave the lowest TBY at Fedis, Haramaya and Hirna sites. While at Babillae site, the control treatment with inoculation resulted in the lowest TBY, indicating the non-responsiveness of common bean for inoculation without starter N.
Grain yield
It is notice that there was a significant effect of N rates of application solely and in combination with Rhizobium inoculation on GY of common bean at P ≤ 0.05 (Table 6). Generally, GY of common bean was significantly increased with increasing rates of N application solely and along with Rhizobium inoculation. Similar response of common bean for N fertilizer was previously observed by Vargas et al. (2000). This shows that the N obtained from biological N fixation and the experimental soil was inadequate to minimize the yield gaps and increase the common bean production. This finding is in line with previous report of Küçük (2011) who found that significant improvement of grain yield of common bean was observed at 40 kg N ha−1 alone and in combination with Rhizobium inoculation. Rhizobium inoculated with 100 kg N ha−1 gave significantly higher GY than the other treatments at Babillae site. This indicates the synergetic effect of inoculation to utilize efficiently the applied inorganic N fertilizer. Along uninoculated treatment, at Fedis site, 100 kg N ha−1 resulted in significantly higher GY compared to the other N treatments. While among Rhizobium inoculation treatments, 100 kg N ha−1 gave the highest GY, but had no significant difference in GY N rated between 40 and 100 kg N ha−1. This indicates the need of low rates of N application to boost the grain yield of common bean when applied with inoculation as compared to N fertilizer alone.
N rated between 20 and 100 kg N ha−1 was exhibited non-significant effect on GY at Haramaya sites. In both inoculation treatments, the result indicated increases in GY with increasing rates of N application, although N rates of application reduced NN and NDW. Similar finding was observed on soybean by Osborne and Riedell (2011). At Hirna site, a significant increase in GY with increasing rates of N application without inoculation was observed. While along Rhizobium inoculation treatment, a significant improvement of GY was observed up to 40 kg N ha−1 but the rates above 40 kg N ha−1 exhibited a significant reduction of GY. The presence of competitive and efficient indigenous rhizobia (Thies et al. 1991) and high soil total N (Gan et al. 2009) could be the causes of need of low N application when applied in conjuction with inoculation.
The highest GY (2,089.54 kg ha−1) at Babillae site was obtained from 100 kg N ha−1 applied with Rhizobium inoculation. This GY was 259.45 kg ha−1 over those obtained from 100 kg N ha−1 alone. Similarly, at Fedis site, the highest GY (1,653.89 kg ha−1) was produced from 100 kg N ha−1 applied with Rhizobium inoculation. Similar finding on chickpea has been previously reported by Namvar et al. (2011) who found that inoculation together with N application gave better yield of chickpea than those obtained from N applied without inoculation. The highest GY (2,475.28 kg ha−1) at Haramaya site was obtained from sole application of 100 kg N ha−1. Similarly, improvement of common bean seed yield has been previously observed by Soratto et al. (2004) and Pelegrin et al. (2009). They found that the highest GY of common bean was obtained at 130 and 182 kg N ha−1 of N application in different tillage practices. Rhizobium inoculation applied with 40 kg N ha−1 gave the highest GY (2,441.57 kg ha−1) at Hirna site. Similarly, Hungria et al. (2003) found the highest common bean production at low rates of N applied with Rhizobium inoculation. On the other hand, the lowest GY at Babillae, Fedis and Haramaya were 996.39, 1,005.37 and 2,029.91 kg ha−1 obtained from Rhizobium inoculated without N application. This indicates that Rhizobium inoculation without N application in these experimental sites is insufficient to increase common bean yield even though, it was improved significantly the NN and NDW. However, the lowest GY (1,767.29 kg ha−1) at Hirna site was produced at the control treatment without inoculation implies that the native rhizobia are not effective in N2 fixation.
Plant total tissue N
In Table 7 indicates a significant effect of N rates of application solely and along with Rhizobium inoculation on PTTN at P ≤ 0.05. At Babillae site, 20 kg N ha−1 gave significantly higher PTTN, compared to the other N rates of application along uninoculated treatments. While along Rhizobium inoculated treatment, 80 kg N ha−1 had the highest PTTN, although this PTTN had no significant difference with those PTTN obtained from other N treatments. The highest PTTN at Fedis site along uninoculated treatment was recorded at 100 kg N ha−1. While along inoculated treatments, the PTTN exhibited the non-significant difference along increasing rates of N application. This implies that the presence of higher soil N causes the ineffectiveness of inoculated Rhizobium sp., despite the fact that inoculation improved nodulation. At Haramaya site, decreases in PTTN with increasing rates of N application were recorded regardless of inoculation treatments. This may indicate the presence of competitive and effective indigenous rhizobia nodulating common bean. in this site, the highest PTTN were obtained from 40 kg N ha−1 alone and 20 kg N ha−1 applied with inoculation. On the other hand, low PTTN at high N application could be related with the inhibitory effect of N application on nodulation and N2 fixation of common bean in this soil, as has already been indicated by Herridge and Brockwell (1988). Decrease in plant tissue N of Onobrychis viciifolia was also observed with increasing rates of N application beyond 40 kg N ha−1 (Tufenkci et al. 2006). Excessive application of N had a detrimental effect, possibly through reducing nitrogenase activities (Tsai et al. 1993b; Saxena et al. 1996) and thus reducing the symbiotic N2 fixation (Voisin et al. 2002). Sanginga et al. (1988) observed that N fertilizer depressed N fixation by 56%. At Hirna site, slight increase in PTTN with increasing rates of N application was observed. Accordingly, the highest PTTN was obtained at 100 kg N ha−1 alone and 80 kg N ha−1 conjunction with inoculation. Enhancement of PTTN with increasing N application in high P containing soil has been previously reported by Ankomah et al. (1996).
Conclusion
The result of this experiment indicated that inoculation of elite isolate of Rhizobium leguminosarum bv. Phaseoli improves the use efficiency of N by common bean in all experimental locations. Similarly the AE-N of common bean showed direct and positive relationship with nodule dry eight common beans, indicating the importance of nodulation and the consecutive output of this biological process on to improve efficient utilization of applied mineral N fertilizer. Accordingly, the starter amount of N fertilizer is recommended for all experimental sites to get the highest AE-N of common bean. Beside this, the soil inherent soil fertility status and indigenous rhizobia nodulating common bean affects effectiveness of sole application of N fertilizer and in combination with Rhizobium inoculation on the productivity of common bean. Rhizobium inoculation reduced the need of exogenous N application to get maximum common bean yield in the study sites.
References
Ahemad M, Khan MS (2011) Ecotoxicological assessment of pesticides towards the plant growth promoting activities of Lentil (Lens esculentus)-specific Rhizobium sp. strain MRL3. Ecotoxicology 20:661–669
Ahemad M, Khan MS (2012) Ecological assessment of biotoxicity of pesticides towards plant growth promoting activities of pea (Pisum sativum)-specific Rhizobium sp. strain MRP1. Emirates J Food Agric 24:334–343
Ankomah AB, Zapata F, Hardarson G, Danso SKA (1996) Yield, nodulation, and N2 fixation by cowpea cultivars at different phosphorus levels. Biol Fertil Soils 22:10–15
Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204:57–67
Asad S, Malik KA, Hafeez FY (1991) Competition between inoculated and indigenous Rhizobium/Bradyrhizobium spp. strains for nodulation of grain and fodder legumes in Pakistan. Biol Fertil Soils 12:107–111
Assefa T, Assefa H, Kimani P (2006) Development of improved Haricot bean germplasm for the mid-and low-altitude sub-humid agro-ecologies of Ethiopia. In: Ali K, Ahmed S, Beniwal S, Kenneni G, Malhotra RS, Makkouk K, Halila MH (eds) Food and forage legumes of Ethiopia: Progress and Prospects, Proceedings of the Workshop on food and forage legumes 22–26 September 2003, Addis Ababa, Ethiopia. EIAR and ICARDA, Sponser. International Center for Agriculture Research in the Dry Areas (ICARDA), Aleppo, Syria
Atzorn R, Crozier A, Wheeler CT, Sandberg G (1988) Production of gibberellins and indole-3-acetic acid by Rhizobium phaseoli in relation to nodulation of Phaseolus vulgaris roots. Planta 175:532–538
Beebe SE, Rao IM, Blair MW, AcostaGallegos JA (2013) Phenotyping common beans for adaptation to drought. Frontiers in Plant Physiology 4:1–20
Bremner JM (1965) Inorganic forms of nitrogen. In: Black CA et al (eds) Methods of soil analysis, Part 2, 2nd ed., agron. Monogr. 9. ASA and SSSA, Madison
Brockwell J, Gault RR, Morthorpe LJ, Peoples MB, Turner GL, Bergersen FJ (1989) Effects of soil nitrogen status and rate of inoculation on the establishment of populations of Bradyrhizobium japonicum and on the nodulation of soybeans. Aust J Agric Res 40:753–762
Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55–128
Buttery BR, Park SJ, Findlay WI (1987) Growth and yield of white bean (Phaseolus vulgaris L.) in response to nitrogen, phosphorus and potassium fertilizer and to inoculation with Rhizobium. Can J Plant Sci 67:425–432
Camerini S, Senatore B, Lonardo E, Imperlini E, Bianco C, Moschetti G et al (2008) Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch Microbiol 190:67–77
Canbolat MY, Bilen SC, Akmakc R, Sahin F, Aydın A (2006) Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol Fertil Soils 42:350–357
Centro Internacional de Agricultura Tropical (CIAT) (2001) Plant genetic resources: beans (Internet). http://www.ciat.cgiar.org/pgr/beans.htm. Accessed 10 Mar 2015
Chemining’wa GN, Muthomi JW, Obudho EO (2004) Effect of rhizobia inoculation and urea application on nodulation and dry matter accumulation of green manure legume at Katumani and Kabete sites of Kenya. Legume Res Project Newsl (11):13–17
Chemining’wa GN, Muthomi JW, Theuri SWM (2007) Effect of rhizobia and starter on nodulation, shoot dry biomass and yield of Grains legumes. Asian J Plant Sci 6(7):1113–1118
CSA (2013) Report on area and production for major crops in 2012/2013. Central Statistical Authority (CSA), Addis Ababa
da Silva PM, Tsai SM, Bonetti R (1993) Response to inoculation and N fertilization for increased yield and biological nitrogen fixation of common bean (Phaseolus vulgaris L.). Plant Soil 152:123–130
Daba S, Haile M (2000) Effects of rhizobial inoculant and nitrogen fertilizer on yield and nodulation of common bean. J Plant Nutr 23(5):581–591
Dobermann A, Cassman KG (2004) Environmental dimensions of fertilizer N: what can be done to increase nitrogen use efficiency and ensure global food security? In: Mosier AR et al (eds) Agriculture and the nitrogen cycle: assessing the impacts of fertilizer use on food production and the environment. SCOPE 65. Island Press, Washington D.C., pp 261–278
dos Santos AB, Fageria NK (2007) Nitrogen fertilizer management for efficient use by dry bean in tropical lowland. Pesquisa Agropecuaria Brasileira 42:1237–1248
dos Santos AB, Fageria NK (2008) Physiological characteristics of common bean in tropical varzea soils as affected by rate and nitrogen management. Ciência e Agrotecnologia 32:23–31
Fageria NK, Baligar VC, Clark RB (2006) Physiology of crop production. Haworth Press, New York
Fageria NK, Melo LC, de Oliveira J (2013) Nitrogen use efficiency in dry bean genotypes. J Plant Nutr 36:2179–2190
Fageria NK, Melo LC, Ferreira EPB, Oliveira JP, Knupp M (2014) Dry matter, grain yield, and yield components of dry bean as influenced by nitrogen fertilization and rhizobia. Commun Soil Sci Plant Anal 45:111–125
Gan YT, Warkentin TD, McDonald CL, Zentner RP, Vandenberg A (2009) Seed yield and yield stability of chickpea in response to cropping systems and soil fertility in northern latitudes. Agron J 101:1113–1122
Gentile F, Wall LG, Huss-Danell K (2006) Effects of Phosphorus and Nitrogen on nodulation are seen already at the stage of early cortical cell divisions in Alnus Incana. Ann Bot 98:309–315
Giller KE, Amijee F, Brodrick SJ, Edje OT (1998) Environmental constraints to nodulation and nitrogen fixation of Phaseolus vulgaris L. in Tanzania. II. Response to N and P fertilizers and inoculation with Rhizobium. Afr Crop Sci J 6:171–178
Graham PH (1981) Some problems of nodulation and symbiotic nitrogen fixation in Phaseolus vulgaris L.: a review. Field crops Res 4:93–112
Graham PH, Rosas JC, de Jensen CE, Peralta E, Tlusty B, Acosta-Gallegos J et al (2003) Addressing edaphic constraints to bean production: the bean/Cowpea CRSP project in perspective. Field Crops Res 82:179–192
Haile M (1990) Preminary studies of biological nitrogen fixation by haricotbean on two soil types in Hararghe, Ethiopia. In: Proceedings of Second Regional Workshop on bean Research in Eastern Africa, Nairobi, Kenya, pp 97–109
Hardarson G, Bliss FA, Cigales-Riveri MR, Henson RA, Kipe-Nolt JA, Longeri L et al (1993) Genotypic variation in biological nitrogen fixation by common bean. Plant and Soil 152:59–70
Herridge DF, Brockwell J (1988) Contributions of fixed nitrogen and soil nitrate to the nitrogen economy of irrigated soybean. Soil Biol Biochem 20:711–717
Herridge D, Rose I (2000) Breeding for enhanced nitrogen fixation in crop legumes. Field Crops Res 65:229–248
Hungria M, Andrade DS, Chueire LMO, Probanza A, Guttierrez-Maero FJ, Megias M (2000) Isolation and characterization of new efficient and competitive bean (Phaseolus vulgaris L.) rhizobia from Brazil. Soil Biol Biochem 32:1515–1528
Hungria M, Campo RJ, Mendes IC (2003) Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol Fertil Soils 39:88–93
IAR (1997) Melkassa Agricultural Research Center Progress Report for the period 1995/96. Institute of agricultural Research (IAR), Melkassa
IAR (1998) Melkassa Agrictural Research Center Progress Report for the period 1997/98. Institute of Agricultural Research (IAR), Melkassa
Küçük Ç (2011) Inoculation with Rhizobium spp. in kidney bean (Phaseolus vulgaris L.) varieties. Agriculture 98:49–56
Kumar H, Dubey RC, Maheshwari DK (2011) Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.). Crop Prot 30:1396–1403
Li YY, Yu CB, Cheng X, Li CJ, Sun JH, Zhang F et al (2009) Intercropping alleviates the inhibitory effect of N fertilization on nodulation and symbiotic N fixation of faba bean. Plant Soil 323:295–308
Mnasri B, Tajini F, Trabelsi M, Aouani ME, Mhamdi R (2007) Rhizobium gallicum as an efficient symbiont for bean cultivation. Agron Sustain Dev 27:331–336
Mulas D, García-Fraile P, Carro L, Ramírez-Bahena MH, Velázquez E, González-Andrés F (2011) Distribution and efficiency of Rhizobium leguminosarum strains nodulating Phaseolus vulgaris in Northern Spanish soils: selection of native strains that replace conventional N fertilization. Soil Biol Biochem 43:2283–2293
Mulvaney RL, Khan SA, Hoeft RG, Brown HM (2001) A soil organic nitrogen fraction that reduces the need for nitrogen fertilization. Soil Sci Soc Am J 65:1164–1172
Namvar A, Sharifi RS, Khandan T (2011) Growth analysis and yield of chickpea (Cicer arietinum L.) in relation to organic and inorganic nitrogen fertilization. EKOLOGIJA 57(3):97–108
Osborne SL, Riedell WE (2011) Impact of low rates of nitrogen applied at planting on soybean nitrogen fixation. J Plant Nutr 34:436–448
Otieno PE, Muthomi JW, Chemining’wa GN, Nderitu JH (2009) Effect of rhizobia inoculation, farm yard manure and nitrogen fertilizer on nodulation and yield of food grain legumes. J Biol Sci 9(4):326–332
Pelegrin R, Mercante FM, Otsubo IMN, Otsubo AA (2009) Response of common bean crop to nitrogen fertilization and rhizobium inoculation. Revista Brasileira de Ciência do Solo 33:219–226
Rebeschini AC, Mazzuchelli RCL, Araujo ASF, Araujo FF (2014) Nitrogen application and inoculation with Rhizobium tropici on common bean in the fall/winter. Afr J Agric Res 9(42):3156–3163
Remans R, Beebe S, Blair M, Manrique G, Tovar E, Rao I et al (2008) Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.). Plant Soil 302:149–161
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A (2008) Nitrogen uptake, fixation and response to fertilizer N in soybeans. Field Crops Res 108:1–13
Sanchez PA (1976) Properties and management of soils in the tropics. Wiley, New York
Sanginga N, Mulongoy K, Ayanaba A (1988) Nodulation and growth of Leucaena leucocephala (Lam.) de Wit as affected by inoculation and N fertilizer. Plant Soil 112:129–135
SAS (1999) SAS Procedures Guide, Version 8. SAS Institute Inc., Cary, NC
Saxena AK, Rathi SK, Tilak KVBR (1996) Selection and evaluation of nitrate-tolerant strains of Rhizobium leguminosarum biovar viceae specific to the lentil. Biol Fertil Soils 22:126–130
Shamseldin A, Moawad H (2010) Inhibition of nitrogenase enzyme and completely of nodulation in common bean (Phaseolus vulgaris L.) at high levels of available nitrogen. American-Eurasian J Agric Environ Sci 7(1):75–79
Soratto RP, Carvalho MAC, Orivaldo ARF (2004) Chlorophyll content and grain yield of common bean as affected by nitrogen fertilization. Pesquisa Agropecuária Brasileira 39:895–901
Stajkovic O, Delic D, Josic D, Kuzmanovic Ð, Rasulic N, Knezevic-Vukcevic J (2011) Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Roman Biotechnol Lett 16:5919–5926
Tana T, Fininsa C (2006) Cropping systems and soil fertility research on Harcot bean (Phaseolus vulgaris L.) in eastern Ethiopia. A review. In: Kemal Ali, Seid Ahmed, Surendra Beniwal, Gemechu Kenneni, Rajendra S. Malhotra, Khaled Makkouk (eds) Food and forage legumes of Ethiopia: Progress and Prospects, Proceedings of the Workshop on food and forage legumes 22–26 September 2003, Addis Ababa, Ethiopia. EIAR and ICARDA, Sponser. International center for Agriculture Research in the Dry Areas (ICARDA), Aleppo, Syria, pp. 167–171
Theuri SWM, Chemining’wa GN, Muthomi JW (2006) The abundance of indigenous rhizobia nodulating cowpea and common bean in central Kenyan soils. In: Proceedings of the 10th KARI Biennial Scientific Conference, 13–17 November 2006, Nairobi, Kenya
Thies JE, Singleton PW, Bohlool BB (1991) Influence of the size of indigenous rhizobial population on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol 57:19–28
Thung M, Rao IM (1999) Integrated management of abiotic stresses. In: Singh SP (ed) Common bean improvement in the twenty-first century. Kluwer, Dordrecht, pp 331–370
Tsai SM, Bonetti R, Agbala SM, Rossetto R (1993a) Minimizing the effect of mineral nitrogen on biological nitrogen fixation in common bean by increasing nutrient levels. Plant Soil 152:131–138
Tsai SM, Da Silva PM, Cabezas WL, Bonetti R (1993b) Variability in nitrogen fixation of common bean (Phaseolus vulgaris L.) intercropped with maize. Plant Soil 152:93–101
Tufenkci S, Erman M, Sonmez F (2006) Effects of phosphorus and nitrogen applications and Rhizobium inoculation on the yield and nutrient uptake of sainfoin (Onobrychis viciifolia L.) under irrigated conditions in Turkey. N Z J Agric Res 49:101–105
Vargas MAT, Mendes IC, Hungria M (2000) Response of field-grown bean (Phaseolus vulgaris L.) to Rhizobium inoculation and nitrogen fertilization in two Cerrados soils. Biol Fertil Soils 32:228–233
Vincent JM (1970) A manual for the practical study of root-nodule bacteria (IBP handbook no 15). Blackwe1l, Oxford
Voisin A, Salon C, Munier-Jolain NG, Ney B (2002) Quantitative effects of soil nitrate, growth potential and phenology on symbiotic nitrogen fixation of pea (Pisum sativum L.). Plant Soil 243:31–42
Wani PA, Khan MS, Zaidi A (2007) Co inoculation of nitrogen fixing and phosphate solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron Hung 55:315–323
Waterer JG, Vessey JK (1993) Effect of low static nitrate concentrations on mineral nitrogen uptake, nodulation, and nitrogen fixation in field pea. J Plant Nutr 16:1775–1789
Willams LE, Phillips DA (1983) Increased soybean productivity with a Rhizobium japonicum mutant. Crop Sci 23:246–250
Yan V, Lynch JP, Beebe SE (1995) Genetic variation for phosphorus efficiency of common bean in contrasting soil types: I vegetative response. Crop Sci 35:1086–1093
Zaidi A, Khan MS, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung 56:263–284
Authors’ contributions
Both of us participated starting from the development of the research idea, writing proposal and competing research grant and development of this manuscripts. But, mine was more participated in the management and collection of data from the field experiment that is way; I am the first author of this manuscript. Both authors read and approved the final manuscript.
Acknowledgements
This study received the financial support from Ethiopia Institute of Agricultural Research under the project ‘National Biofertilizer Development Project’. We also thank Girmaye Mekonnen, Berhanu Mengistu and Dejene Ayenew for the field experimental support and Dr. Mashilla Dejene for editorial support while writing the manuscript.
Compliance with ethical guidelines
Competing interests This research was funded by two governmental higher institutes. Haramaya University and Ethiopia Institute of Agricultural Research were provided and fully funded and covered the cost of this research. We, as author and principal investigator of this research, acknowledged and say thanks for those institutes supported this project. Both authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Argaw, A., Akuma, A. Rhizobium leguminosarum bv. viciae sp. inoculation improves the agronomic efficiency of N of common bean (Phaseolus vulgaris L.). Environ Syst Res 4, 11 (2015). https://doi.org/10.1186/s40068-015-0036-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-015-0036-z