Abstract
Background
Extubation failure (EF), defined as need for re-intubation within 24–72 h, is multifactorial. Factors predicting EF in adults generally are not useful in children.
Objective
To determine the factors associated with EF and to facilitate prediction of EF in mechanically ventilated infants and children less than 12 years of age.
Material and Methods
Design Prospective cohort study. Setting PICU and NICU of a multispecialty tertiary care institute. Patients All consecutive newborns, infants and children, who remained on the ventilator for more than 12 h, were included. Patients with upper airway obstruction, neuromuscular disorders, complex anatomic malformations, accidental extubation, tracheostomy or death before extubation were excluded. Methods The pre-extubation clinical, laboratory and ventilatory parameters were collected for 92 cases over a one and half year period. The EF rate was calculated for each variable using STATA 9. All the treating physicians were blinded to the data collection procedure.
Measurements and Results
Demographics were comparable between the extubation success and EF groups. Respiratory failure was the main cause requiring ventilation (46.74 %, 95 % CI 0.37–0.57) as well as EF (30.23 %, 95 % CI 0.08–0.23). 76.92 % (95 % CI 0.58–0.89) of patients that failed extubation had alterations in respiratory effort, 38.46 % (95 % CI 0.22–0.57) each had either poor or increased respiratory effort. Poor cough reflex (p = 0.001), thick endotracheal secretions (p = 0.02), failed spontaneous breathing trial (SBT) (p = 0.001) and higher rapid shallow breathing index (RSBI) (p = 0.001) were found to be associated with EF.
Conclusions
Paediatric EF is multifactorial. Increased or poor respiratory effort and failed SBT are potential factors in deciding re-intubation. Increased RSBI, poor cough reflex and thick.
Similar content being viewed by others
Background
After resolution of illness, mechanically ventilated patients are disconnected from the ventilator; extubation is the final step in this process. Extubation failure (EF) is defined as an inability to sustain spontaneous breathing and need for re-intubation within 24–72 h after extubation (Rothar and Epstein 2003). Prediction of EF is essential, as both delayed and failed extubation have detrimental consequences (Rothar and Epstein 2003). The incidence of EF varies between 2 to 47 % (Rothar and Epstein 2003; Kulkarni and Aggarwal 2008). It can be as high as 22 to 28 % in premature neonates (Khan et al. 1996).
A variety of patho-physiologic causes lead to EF. The prediction of EF is difficult (Rothar and Epstein 2003; Kulkarni and Aggarwal 2008). Newth et al. reported limited guidance on paediatric weaning and extubation from their literature review (Newth et al. 2009). Indices developed to predict weaning and extubation success (ES) are no better than clinical judgment (Newth et al. 2009). Similarly, Leclerc and Schindler observed that adult weaning predictors proposed by the Task Force of the American College of Chest Physicians have very poor predictive power in children (Yang and Tobin 1991; Leclerc et al. 2005; Schindler. 2005).
We undertook this study in newborns, infants and children to explore factors that may predict EF.
Methods
This prospective cohort study was conducted at PICU and NICU of a multispecialty tertiary care institute in India over a period of one and half years (December 2008 to May 2010). All consecutive infants and children less than 12 years of age, admitted and ventilated for more than 12 h were included in the study. Patients with upper airway obstruction, accidental extubation, tracheostomy, or death before extubation were excluded. The sample size was calculated using 95 % confidence interval, 10 % margin of error and 30 % estimated incidence of EF in the study area. We did a retrospective analysis of PICU register books to find out the incidence of EF in our PICU. We looked at the previous 2 years data and approximated the incidence of EF in our PICU to be around 30 %. We used the formula, n = t 2 x p (1−p) x 1/m 2, where ‘n’ is required sample size, ‘t’ is confidence level at 95 %, ‘p’ is estimated incidence of EF in the project area and ‘m’ is margin of error at 10 % (Calculating the sample size—IFAD). This gave us the minimum sample required for the study as 81 and we collected data for 92 patients. The research team was not involved directly in the clinical care. All decisions related to patient’s care were taken by the treating physician and they were blinded to data collection and analysis procedure.
The parameters that were collected were divided into: (1) Demographic data: Age, sex, weight, diagnosis on admission, indication for ventilation and duration of intubation, (2) Clinical parameters: Haemoglobin concentration, heart rate, spontaneous respiratory rate, blood pressure, peripheral oxygen saturation (SpO2), work of breathing, presence of cough reflex, amount and consistency of secretion, use of ionotropes, use of sedation and use of dexamethasone, (3) Blood gas parameters (venous gases): pH, partial pressure of carbon dioxide (PCO2), bicarbonate (HCO3 −), base excess (BE), lactate and (4) Ventilatory parameters: Ventilator mode, ventilator rate, peak inspiratory pressure (PIP), positive end-expiratory pressure (PEEP), fraction of inspired oxygen (FiO2), inspiratory time (Ti), expiratory time (Te), spontaneous breathing trial (SBT), rapid shallow breathing index (RSBI) and use of bubble CPAP following extubation. These parameters were measured pre-extubation, post-extubation (whenever necessary), and at the time of any re-intubation.
Derived parameters
Indices that incorporated more than one measurement of respiratory function i.e., mean airway pressure {MAP = [(PIP − PEEP) (Ti)/Ti + Te] + PEEP} and ventilator index {VI = [Ventilatory rate × (PIP − PEEP)*PCO2]/1000} were calculated.
Data analysis
EF rate was calculated for each variable using the statistical software STATA 9, version 17. Data was presented in frequency percentage with confidence intervals and mean (SD) and median (minimum–maximum). In the continuous parameters, average (mean/median) between the two groups was compared by using t test and Wilcoxan rank sum test. In the categorical variable, two groups were compared by using Chi-square and Fisher’s exact test. p value <0.05 was taken as significant.
Ethics, consent and permissions
Institute’s ethics committee approval was obtained and patients were recruited after written informed parental consent.
Consent to publish
Parental permissions were obtained for presentation of study results in conferences and also publication in journals.
Results
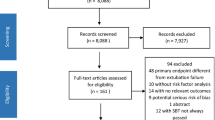
Two hundred and eleven infants and children were ventilated during the study period. 119 patients could not be included into the study (38 did not meet inclusion criteria, 46 died while on ventilator, 19 left hospital against medical advice and 16 patients referred out). 92 patients were included into the study; 66 patients successfully extubated (success rate 71.7 %, 95 % CI 0.62–0.79) whereas 26 patients failed extubation (failure rate 28.3 %, 95 % CI 0.21–0.38). The demographic data of the two groups are shown in Table 1.
Table 2 shows diagnosis of patients requiring mechanical ventilation. Most common indication of ventilation was pneumonia and sepsis accounting for 14 (15.2 %) cases each. In the neonatal category, most of babies were ventilated for hyaline membrane disease, birth asphyxia, meconium aspiration syndrome and congenital pneumonia, accounting for 11 (11.9 %), 8 (8.7 %), 7 (7.6 %) and 7 (7.6 %) cases respectively. Post-operative state accounted for 9 (9.8 %) cases, whereas 8 (8.7 %) cases were ventilated as a part of the management protocol in cases of refractory status epilepticus. Others being acute respiratory distress syndrome [3 (3.3 %) cases], acute bilirubin encephalopathy [1 (1.1 %) case], bronchiolitis [2 (2.2 %) cases], CNS bleed (vitamin K deficiency bleed) with seizures [1 (1.1 %) case], congenital diaphragmatic hernia (right) [1 (1.1 %) case], congenital heart disease with cardiac failure [1 (1.1 %) case], congenital pneumothorax [1 (1.1 %) case], refractory apnea of prematurity [1 (1.1 %) case], refractory status asthmaticus [2 (2.2 %) cases] and viral meningoencephalitis [1 (1.1 %) case].
Of the total 92 ventilated patients, 43 had pulmonary involvement (46.74 %, 95 % CI 0.27–0.57), 13 failed extubation (30.23 %, 95 % CI 0.18–0.45). 19 patients had central nervous system involvement (20.65 %, 95 % CI 0.14–0.31), four (21.05 %, 95 % CI 0.08–0.43) had EF. Multisystemic involvement (more than one system involvement) was seen in 18 patients (19.56 %, 95 % CI 0.13–0.29); four had EF (22.22 %, 95 % CI 0.1–0.45). Nine (9.78 %, 95 % CI 0.05–0.18) were ventilated post-operatively; three (33.33 %, 95 % CI 0.12–0.65) failed extubation. Three (3.26 %, 95 % CI 0.01–0.1) cases were ventilated for cardio–respiratory system involvement; two (66.67 %, 95 % CI 0.21–0.94) failed extubation. The patients were divided into various categories depending upon the involvement of system; multisystemic refers to more than two system involvement.
Out of the 26 cases with EF, 10 each 38.46 % (95 % CI 0.22–0.57) had either poor or increased respiratory effort; therefore alteration in the respiratory effort accounted for 76.38 % (95 % CI 0.58–0.89) of EF (two each had recurrence of seizures and post seizure respiratory arrest respectively, one developed pneumothorax and another one had recurrence of apnoea of prematurity).
We observed that poor cough reflex contributes to EF. In the EF group, one patient had good cough reflex and the rest 25 had poor cough reflex, whereas in the ES group, 33 had good cough reflex and the other 33 had poor cough reflex, p = 0.001. We also observed that thick secretions contributes to EF as well, 15 patients in the EF group and 21 patients in the ES group had thick secretions (p = 0.02), whereas the amount of secretion was not significant, p = 0.12. Another observation was—patients who passed 30 min SBT were successfully extubated (n = 48), whereas all patients who failed SBT and extubated subsequently had EF (n = 7); p = 0.001. Our study also showed that a higher RSBI was associated with EF, p = 0.001, 91 in ES vs 169 in EF Details in Table 3.
Please see Appendix 1 for definitions used in this study.
Discussion
We found that paediatric EF could be multifactorial. We considered a host of different factors which could possibly cause EF as observed in previous studies. We found that respiratory system involvement, failure to pass SBT and altered respiratory effort following extubation are major concerns towards EF as well as poor cough reflex and thick secretions. The major challenge we encountered was paucity of data from this part of the world on paediatric EF. Paediatric and neonatal EF is a very well addressed issue in the resourced countries unlike in the resource poor countries and therefore we think it would be unwise to compare our findings with the studies done previously in resourced countries. At the same time we would like to say, this is the first kind of study on EF from the entire Indian subcontinent including all age groups and variety of factors.
Several patient characteristics have been implicated in paediatric ES and EF (Rothar and Epstein 2003; Kulkarni and Aggarwal 2008). As compared to most neonatal and paediatric ICU’s in developed countries, we found a high EF rate, 28.3 % (95 % CI 0.21–0.38). Previous studies show incidences separately for neonatal and paediatric extubation failures. Our study population was a mixed one, but surprisingly we could not find any intergroup difference (0–1 m vs 1–12 m vs 1–12 year). Moreover, we could not find any data on neonatal or paediatric EF rates from Indian subcontinent to compare with.
In the current study, we noticed that 50 % of the children who failed extubation had a pre-morbid respiratory system involvement. EF amongst post-operative cases were possibly because of premature weaning and extubation by the treating team. Irrespective of the system involvement more than 3/4th (76.92 %) of the patients who failed extubation had a change in the respiratory effort (poor or increased). It has been shown in previously that paediatric EF is in part disease specific and pre-existing respiratory conditions predispose to re-intubation (Kurachek et al. 2003).
Prematurity, low birth weight, younger age, prolonged duration of ventilation, CPAP after extubation, use of inotropes, sedation and analgesia are known to contribute to EF, but our study failed to show any difference (Fontela et al. 2005; Hiremath et al. 2009; Epstein 2002a, 2002b; Dimitriou et al. 2002; Stawicki. 2007). Post-extubation nasopharyngeal bubble CPAP (Kaur et al. 2008) was used mostly for cases with respiratory system involvement. Likewise, poor oxygenation is an established risk factor for re-intubation. We could not look into A-a gradient, PaO2/FiO2 ratio and oxygenation index although it was planned. In our ICU setting most clinicians prefer venous blood gases (VBG) for determining acid–base status of the body as pH, PCO2, HCO3 and BE are comparable in both venous and arterial blood gases (Ahmet et al. 2006; Chu et al. 2003) and also relatively easy to perform in resource poor setting. We, in this study could not find any difference in terms of blood gas and ventilatory parameters in the two groups.
In the current study, we found that failure of SBT has a strong correlation with EF (p value 0.001). As a measure of extubation readiness, our ITUs have a policy of performing a 30 min SBT in in our NICU and PICU patients; the same protocol as that for a 2 h SBT, as suggested by Randolph et al. was followed but with adaptable modification (Curley et al. 2006; Venkataraman 2006; Thiagarajan et al. 1999). When found to have no clinical need for increased ventilatory need in the previous 12 h, spontaneously breathing, good effort of breathing, good tidal volume (VT) and SpO2 >95 %, child was started on SBT after stopping feeds and titrating sedation to minimum or stopped at least 4 h beforehand. Ventilator settings changed to flow triggered CPAP–PSV mode with FiO2 0.5, positive end expiratory pressure (PEEP) 5 cm H2O and pressure support (PS) as per the size of endotracheal tube (ETT); 10 cm H2O if ETT 3–3.5 mm; 8 cm H2O if ETT 4–4.5 mm; and 6 cm H2O if ETT ≥5 mm). SpO2, exhaled VT and respiratory rate (RR) monitored and if found to be in the target range at the end of 30 min, child was declared to pass SBT and prepared for extubation, otherwise patients were put back on the same pre-test ventilator settings. [Targets: SpO2 ≥95 %, exhaled VT ≥5 ml/kg and RR-<6 month, 20–60/min; 6 month–2years, 15–45/min; 2–5years, 15–40 and >5years, 10–35/min].
In our study, SBT was performed in 64 study patients. 48 patients passed SBT and all of them were successfully extubated. In EF group, nine patients passed SBT and extubated but eventually they failed extubation; whereas seven patients were extubated despite they failed SBT and all of them failed extubation. Farius and Kamlin also observed a similar association in their study (Paret et al. 1998; Kamlin et al. 2006). We also observed RSBI as a potentially useful index that can predict EF—the higher the value, the higher the chances of EF (ES 91 vs EF 169, p = 0.005). Previously, role of RSBI was described as controversial. Some researchers concluded it as a good indicator (Paret et al. 1998; Baumeister et al. 1997) whereas some labelled RSBI as a poor predictor (Farias et al. 2002; Venkataraman et al. 2000; Leclerc et al. 2002). We also found that poor cough reflex and thick secretion are potential risk factors for EF (p value 0.001 and 0.022 respectively); we could not find any relationship with secretion volume and EF (p value 0.122), though we could not exactly quantify secretion volume due to operational problems. These were, although been described as risk factors for EF in previous studies (Epstein 2002a, 2002b).
In the current study, we could not observe any difference in the use of pre extubation use of dexamethasone in preventing EF (p = 0.632). Previously, adult studies have proven the benefit of pre extubation steroid use in preventing EF, but paediatric studies have shown mixed results (McCaffrey et al. 2009; Sinha et al. 2010; Matthew. 2008; Khemani et al. 2008).
Limitation of the study
The consistency and amount of endotracheal secretions were subjective. Arterial blood gases would be a better parameter to use than venous blood gases (VBG). VBG was chosen as the pragmatic alternative.
The study of more specific ventilation variables like CROP (compliance, rate, oxygenation and pressure) (Paret et al. 1998; Farias et al. 2002; Venkataraman et al. 2000). Simplified weaning index (SWI), compliance of respiratory system (CRS), rapid shallow breathing occlusion pressure (ROP) (Kulkarni and Aggarwal 2008; Dimitriou et al. 2002; Paret et al. 1998). Maximal inspiratory pressure during an occlusion test (Pimax), modified tension timed index (TTI) (Matthew. 2008; Noizet et al. 2005; Jabour et al. 1991) and dead space to tidal volume ratio (VD/VT) (Harikumar et al. 2009) would have strengthened the study. As the measurement of these variables requires expertise and special instruments we were unable to perform the same.
Conclusion
Paediatric EF may be multifactorial and in part disease specific. The measurement of respiratory effort and SBT could be vital parameters in deciding re-intubation. In addition, increased RSBI, poor cough reflex and thick secretions may augment prediction of EF. This study also forms the basis for future studies on this topic. We believe this study would attract neonatologist, paediatricians and intensivists especially from resource limited countries for further discussions and research on EF.
References
Ahmet AK, Ogun CO, Bayir A, Kayas SA, Koylu R (2006) Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohaku J Exp Med 210:285–290
Baumeister BL, El-Khatib M, Smith PG, Blumer JL (1997) Evaluation of predictors of weaning from mechanical ventilation in paediatric patients. Pediatr Pulmonol 24(5):344–352
International Fund for Agricultural Development. Calculating the sample size. http://www.ifad.org/gender/tools/hfs/anthropometry/ant_3.htm. Accessed 1 Dec 2015
Chu YC, Chen CZ, Lee CH, Chen CW, Chang HY, Hsiue TR (2003) Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. J Formos Med Assoc 102:539–543
Curley MAQ, Arnold JH, Thompson JE (2006) Clinical trial design: effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care 21:23–32
Dimitriou G, Greenough A, Endo A, Cherian S, Rafferty GF (2002) Prediction of extubation failure in preterm infants. Arch Dis Child Fetal Neonatal Ed 86:F32–F35
Epstein SK (2002a) Decision to extubate. Intensive Care Med 28:535–546
Epstein SK (2002b) Extubation. Respir Care 47:483–492
Farias JA, Alía I, Retta A, Olazarri F, Fernández A, Esteban A, Palacios K, Di Nunzio L, Fernández G, Bordón A, Berrondo C, G Sheehan (2002) An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med 28:752–757
Fontela PS, Piva JP, Garcia PC, Bered PL, Zilles K (2005) Risk factors for extubation failure in mechanically ventilated paediatric patients. Pediatr Crit Care Med 6:166–170
Harikumar G, Egberongbe Y, Nadel S, Wheatley E, Moxham J, Greenough A, Rafferty GF (2009) Tension-time index as a predictor of extubation outcome in ventilated children. Am J Respir Crit Care Med 180:982–988
Hiremath GM, Mukhopadhyay K, Narang A (2009) Clinical risk factors associated with extubation failure in ventilated neonates. Indian Pediatr 46:887
Jabour ER, Rabil DM, Truwit JD, Rochester DF (1991) Evaluation of a new weaning index based on ventilatory endurance and the efficiency of gas exchange. Am Rev Respir Dis 144:531–537
Kamlin COF, Davis PG, Morley CJ (2006) Predicting successful extubation of very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 91(3):F180–F183
Kaur C, Sema A, Beri RS, Puliyel JMA (2008) Simple circuit to deliver bubbling CPAP. Indian Pediatr 45:312–314
Khan N, Brown A, Venkataraman ST (1996) Predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med 24(9):1568–1579
Khemani RG, Randolph A, Markovitz B. Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Update of Cochrane Database Syst Rev 2008;(2):CD001000. Cochrane Database Syst Rev 2009;(3):CD001000
Kulkarni A, Aggarwal V (2008) Extubation failure in intensive care unit: predictors and management. Indian J Crit Care Med 12:1–9
Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, Takano J, Easterling L, Scanlon M, Musa N, Brilli RJ, Wells D, Park GS, Penfil S, Bysani KG, Nares MA, Lowrie L, Billow M, Chiochetti E, Lindgren B (2003) Extubation failure in paediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med 31:2657–2664
Leclerc F, Lecine T, Riou Y, Grandbastien B, Noizet O, Dorkenoo A, Leteurtre S (2002) Nated infants and childrens multi-parameter indices of weaning from mechanical ventilation in children. Rev Mal Respir 19:53–61
Leclerc F, Noizet O, Sadik A, Grandbastien B, Riou Y, Dorkenoo A, Fourier C, Cremer R, Leteurtre S (2005) Does taking endurance into account improve the prediction of weaning outcome in mechanically ventilated children? Crit Care 9:R798–R807
Matthew JL (2008) Role of parenteral steroids to prevent extubation failure in ventilated children. Indian Pediatr 45:484
McCaffrey J, Farrell C, Whiting P, Dan A, Bagshaw SM, Delaney AP (2009) Corticosteroids to prevent extubation failure: a systematic review and meta-analysis. Intensive Care Med 35:977–986
Newth CJL, Venkataraman S, Willson F, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJS, Carcillo JA, Nicholson CE (2009) Weaning and extubation readiness in paediatric patients. Pediatr Crit Care Med 10:1–11
Noizet O, Leclerc F, Sadik A, Grandbastien B, Riou Y, Dorkenoo A, Fourier C, Cremer R, Leteurtre S (2005) Does taking endurance into account improve the prediction of weaning outcome in mechanically ventilated children? Crit Care 9:R798–R807
Paret G, Ziv T, Barzilai A, Ben-Abraham R, Vardi A, Manisterski Y, Barzilay Z (1998) Ventilation index and outcome in children with acute respiratory distress syndrome. Pediatr Pulmonol 26:125–128
Rothar RC, Epstein SK (2003) Extubation failure: magnitude of the problem, impact on outcomes and prevention. Curr Opin Crit Care 9(1):59–66
Schindler MB (2005) Prediction of ventilation weaning outcome: children are not little adults. Crit Care 9(6):651–652. doi:10.1186/cc3950
Sinha A, Jayashree M, Singhi S (2010) Aerosolized Lepinephrine vs budesonide for post-extubation stridor: a randomized controlled trial. Indian Pediatr 47:317–322
Stawicki SP (2007) ICU CORNER—Sedation scales: Very useful, very underused. OPUS 12 Scientist Vol. 1, No. 2
Thiagarajan RR, Bratton SL, Martin LD, Brogan TV, Taylor D (1999) Predictors of successful extubation in children. Am J Respir Crit Care Med 160:1562–1566
Venkataraman ST (2006) Mechanical ventilation and respiratory care. In: Fuhrman BP, Zimmerman JJ (eds) Pediatric Critical Care, 3rd edn. Mosby Elsevier, Philadelphia (HTML version)
Venkataraman ST, Khan N, Brown A (2000) Validation of predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med 28(8):2991–2996
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324:1445–1450
Authors’ contributions
The concept and design of the study was by NK. Data collection was carried out by BS with inputs from NK. Statistical analysis was planned and executed by VS. Analysis and interpretation of data was by NK and BS. Review of literature and initial drafting by NK and BS. All authors contributed to the critical review and revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
JMP, Head of Paediatrics and Neonatology, St Stephens Hospital, New Delhi, India for providing extensive support in carrying out the work. All participating patients and families. All nurses at the Pediatric and Neonatal Intensive Care units at St Stephens Hospital.
Competing interests
The authors declare that they have no competing interests.
Disclaimers
None.
Source of support (in the form of grants, equipment or drugs)
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Definitions of parameters that are being used in the study
Measurement of cough reflex
Patient’s cough reflex was assessed and graded as good or poor. As per the unit policy, a good cough reflex is one where patient produces vigorous cough with none to very minimal stimulation (e.g., during nebulisations or during suctioning of oro-pharyngeal secretions); otherwise the reflex is a poor one.
Measurement of amount of tracheal secretion
A note of amount of tracheal secretion in the previous 24 h before extubation was made and quantified as minimal or plenty depending upon requirement of tracheal suctioning by open suctioning system. Our paediatric and neonatal intensive care unit has a policy of tracheal suctioning once in every 6 h. If frequent suctioning of trachea (e.g., many times in an hour or hourly) was demanded because of secretions causing respiratory compromise in terms of oxygenation and ventilation, then the tracheal secretions were said to be ‘plenty’; otherwise tracheal secretions were labelled as minimal.
Measurement of consistency of tracheal secretion
The unit has a policy of labelling the tracheal secretions as thick or thin depending upon its stickiness to the suction catheter. Secretions were said to be thick when these blocks the suction catheter and were difficult to remove from the catheter lumen by simple measures like simply suctioning distilled water.
Definition and measurement of respiratory effort
We used Paediatric Advanced Life Support (PALS), American Heart Association, 2005 guidelines to assess respiratory effort of patients by observing the spontaneous respiratory rate, breathing pattern (deep or shallow breathing), use of accessory muscles of respiration, peripheral circulation (pulse rate and volume and/or change in colour) and SpO2. We defined poor respiratory effort as having bradypnea, bradycardia, pallor or cyanosis, desaturation, shallow breathing pattern and poor use of accessory muscles of respiration. Whereas increased respiratory effort was described as tachypnea, bradycardia or tachycardia, cyanosis, desaturation, deep or shallow breathing and excessive use of accessory muscles of respiration. Age and sex specific charts for respiratory rate and heart rate (as given in PALS guideline) were used to determine tachypnea/bradypnea or tachycardia/bradycardia.
Nasopharyngeal CPAP
It was delivered using a modified bubble CPAP machine developed at our unit (Kaur et al. 2008).
Extubation failure
In our study, we defined EF as need for re-intubation within 72 h of extubation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saikia, B., Kumar, N. & Sreenivas, V. Prediction of extubation failure in newborns, infants and children: brief report of a prospective (blinded) cohort study at a tertiary care paediatric centre in India. SpringerPlus 4, 827 (2015). https://doi.org/10.1186/s40064-015-1607-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-015-1607-1