Abstract
Purpose
To determine whether corticosteroids reduce the rate of extubation failure in intensive care patients of all age groups.
Methods
Medline, EMBASE, the Cochrane Central Register of Controlled Trials, bibliographies of relevant articles, selected conference abstracts and unpublished trial databases were searched. Randomised clinical trials (RCTs) evaluating corticosteroids for the purpose of preventing extubation failure in mechanically ventilated, critically ill patients of all ages were included. Two authors independently assessed the validity of included studies and extracted data regarding characteristics of the studies and the rates of reintubation and manifestations of laryngeal oedema.
Results
Fourteen RCTs including 2,600 participants were included. The mean duration of ventilation prior to attempted extubation ranged from 3 to 21 days. There was a reduction in reintubation with the use of corticosteroids, with a pooled odds ratio (OR) of 0.56 (95% CI; 0.41–0.77, P < 0.0005). The effect of corticosteroids tended to be more pronounced in studies when used at least 12 h prior to attempted extubation (OR 0.41, 95% CI; 0.26–0.64). The results were consistent across neonatal, paediatric and adult populations. There was also a reduction in laryngeal oedema in participants receiving corticosteroids, with a pooled OR of 0.36 (95% CI 0.27–0.49, P < 0.0005).
Conclusions
Corticosteroids reduce laryngeal oedema and importantly reduce the incidence of extubation failure in critically ill patients of all ages.
Similar content being viewed by others
Introduction
Extubation failure, defined as the need for reintubation shortly after removal of an endotracheal tube, is a significant problem in critical care. Extubation failure is associated with an increased duration of mechanical ventilation, higher risk for nosocomial pneumonia, prolonged intensive care and hospital length of stay [1–3] and is independently associated with increased mortality [1–4]. While there are numerous causes for extubation failure [5], laryngeal oedema, a common complication of prolonged intubation [6, 7], either causes or contributes to extubation failure in many cases [2, 8].
Corticosteroids have been investigated as a measure to alleviate laryngeal oedema and therefore reduce the rate of extubation failure [9]. Theoretically, corticosteroids can reduce the inflammatory response and decrease oedema [10]. Indeed, early randomised studies have shown decreases in subjective manifestations of laryngeal oedema when corticosteroids were used prior to extubation [11–14]. However, no studies have been adequately powered to determine whether the use of corticosteroids influence more clinically important outcome measures, in particular rates of extubation failure.
Therefore, we performed a systematic review and meta-analysis to investigate whether corticosteroids reduce the rate of extubation failure in critically ill patients of all ages.
Methods
Search strategy
Two authors independently conducted an electronic search of the MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials databases. Search terms were individualised for each database. Search terms for corticosteroids (steroids OR glucocorticoids OR corticosteroids) were combined with terms for “extubation” and with sensitive RCT filters [15, 16]. All databases were searched from inception until 22 June 2008. The electronic search strategies are available in Appendix 1. The search was limited to studies conducted in humans and no language restriction was placed on the search. We also searched conference abstracts from American Thoracic Society (2005–2008), the American College of Chest Physicians (2005–2007) and the European Society for Intensive Care Medicine (2005–2007), as well as the meta-register of controlled trials, including the medical editors’ trials amnesty register [17], along with the bibliographies of included studies and relevant review articles [18].
Inclusion criteria
Studies were considered eligible for inclusion if they reported a prospective randomised clinical trial that compared any corticosteroid with placebo or standard care, given to intubated patients in intensive care, for the purpose of reducing laryngeal oedema and preventing extubation failure. Two authors independently assessed each potentially eligible study for inclusion. Disagreement regarding the inclusion of studies was resolved by discussion with referral to a third reviewer if required.
Data abstraction
Two authors independently abstracted data from all reports. Data were abstracted regarding the population of participants included in the studies, regimen of corticosteroid used, duration of corticosteroid treatment and the duration of ventilation prior to attempts at extubation. We recorded the rates of reintubation from any cause. We also recorded the rates of manifestation of laryngeal oedema, such as stridor; however, this was recorded in the included studies. When available, we recorded data regarding mortality, ICU and hospital length of stay, total duration of ventilation, as well as rates of hyperglycaemia and infection.
Validity assessment
All included studies were assessed for validity by two authors, with disputes resolved by discussion. A component approach was utilised [19]. Each report was assessed for the adequacy of allocation concealment, blinding and the performance of an intention-to-treat analysis. Studies were considered to have adequate blinding when the control group received a placebo, rendering the participants, the health care workers and the outcome assessors blinded to treatment allocation. We also assessed whether specific criteria were used to define that participants were ready for extubation, and the need for re-intubation and whether a cuff-leak test was used prior to attempts at extubation [20].
Quantitative synthesis
Agreement on the inclusion of studies was assessed using the kappa (κ) statistic. The potential for publication bias was assessed by visual inspection of the funnel plot and the statistical test described by Egger [21]. Statistical heterogeneity was assessed by the χ 2 statistic and the I 2 statistic, with an I 2 value of >50% indicating at least moderate heterogeneity [22]. The rates of reintubation were pooled using the fixed-effect method of Mantel and Haenszel to produce a pooled odds ratio (OR) [23, 24]. Estimates of the number needed to treat (NNT) were obtained by applying the pooled estimate of the OR to the pooled baseline event rate. Sensitivity analysis was performed using a random effects model. Cumulative doses of corticosteroid were calculated as mg equivalents of dexamethasone; 1 mg dexamethasone = 5 mg prednisolone, = 5 mg methylprednisolone, = 25 mg hydrocortisone. To explore possible sources of heterogeneity, studies conducted in neonatal, paediatric and adult populations were pooled separately, and studies that used the corticosteroid for less than 12 h were pooled separately from those that gave at least 12 h of treatment. To test for between-group heterogeneity, differences between subgroups were assessed using meta-regression to examine for an interaction between subgroups and overall treatment effect. All analyses were conducted using STATA 10.0 (Statacorp, College Station, Tx).
Results
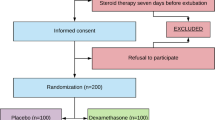
The search retrieved a total of 370 references. After application of the inclusion criteria, 14 studies, including a total of 2,600 participants, were included in this review [11–14, 25–33]. The flow of studies and reasons for exclusion are shown in Fig. 1. Agreement between the two reviewers regarding the inclusion of studies was reached in 45/46 cases (κ = 0.95).
The characteristics of the included studies are shown in Table 1. There were three studies of neonates, four studies in paediatric populations and, seven in adults. The results of the validity assessment are shown in Table 2. Two studies included participants with known laryngeal oedema [13, 33]; the remaining studies included participants at high risk for laryngeal oedema due to prolonged duration of intubation. A range of different types and doses corticosteroids was used in the studies in adult populations.
A total of 13 of the 14 studies, including 2,541 participants, reported at least one episode of reintubation [11–14, 25–27, 29–33] (Fig. 2). One study in neonates [28] assessed for reintubation; however, there were no episodes of reintubation in either the intervention or control groups. There was no suggestion of bias on inspection of the funnel plot (see Appendix 2), and this was confirmed by the quantitative analysis (Egger’s statistic = −0.74, P = 0.31). There was no evidence of statistical heterogeneity (χ 2 P = 0.08), and the I 2 = 38%. The estimate of the pooled OR for reintubation was 0.56 (95%CI 0.41–0.77, P < 0.0005), indicating a significant reduction in the rate of reintubation with the use of corticosteroids. The pooled estimate of the baseline rate of reintubation was 8.2%. Utilising this baseline event rate, the estimate of the NNT to prevent one episode of reintubation was 29, this equates to 35 reintubations prevented per 1,000 extubations (95%CI 18–47).
Sensitivity and subgroup analysis
When the results were pooled using a random effects model, the estimate of the pooled OR was 0.54 (95%CI 0.33–0.90, P = 0.018). The results of sensitivity analysis based on predefined validity appraisal and trial level covariates are shown in Table 3. There tended to be a greater effect of corticosteroids and less heterogeneity in studies with duration of therapy of greater than 12 h prior to extubation and in studies with adequate allocation concealment, although the tests for between group heterogeneity were not statistically significant.
Effect of corticosteroids on manifestations of laryngeal oedema
There were nine studies that reported subjective manifestations of laryngeal oedema. There was a significant degree of heterogeneity, with the χ 2 <0.005 and the I 2 = 71%. There was a reduction in the subjective manifestations of laryngeal oedema with the estimate of the pooled OR of 0.36 (95% CI 0.27–0.49, P < 0.0005).
Effect of corticosteroids on other outcomes
The effect of corticosteroids on the other outcomes was infrequently reported, as were potential adverse effects of corticosteroids. A summary of these outcomes is shown in Table 4.
Overall strength and quality of the evidence
The overall strength and quality of the evidence is summarised according to the GRADE recommendations [34] in Table 5.
Discussion
We conducted a systematic review and meta-analysis to investigate the effect of corticosteroids on the rates of reintubation in critically ill patients of all ages. We found that corticosteroids significantly reduce the rate of reintubation for patients in intensive care undergoing mechanical ventilation. These results are consistent across neonatal, paediatric and adult populations, and robust to the model used to pool the data. The effect of steroids may be more pronounced when the treatment is commenced at least 12 h prior to extubation. We also found that the use of corticosteroids was associated with a significant reduction in clinically evident manifestations of laryngeal oedema such as stridor.
There are a number of strengths to our systematic review and meta-analysis. By following current standards for the conduct and reporting of meta-analyses [35], systematic bias should be minimised. The thoroughness of the search has identified unpublished [32] and previously unrecognised studies [33, 36]. This review is also strengthened by the choice of a robust, objective, clinically important primary outcome. There are also some limitations of this study. In common with all meta-analyses, the strength of the conclusions that can be drawn relies on the validity of the studies included in the review. While the majority of the studies described methods to maintain allocation concealment and blinding, only one study fulfilled all of the validity criteria in this review, thus limiting the strength of the conclusions. The populations included in the studies covered by this review were mechanically ventilated for prolonged periods; therefore, the results of this review should not be extrapolated to patients who require only a short duration of intubation.
The results of this meta-analysis are consistent with those of the largest randomised trial in this area [12], a finding that adds credence to the results. The finding that corticosteroids reduce the rates of reintubation and stridor in a number of populations in different intensive care settings, even allowing for different corticosteroid regimens, adds to the generalisability of the findings. A recent review published in the Cochrane Database of Systematic Reviews [18], that utilised reintubation due to severe stridor and airway obstruction as the primary outcome, did not find a significant reduction in rates of reintubation, although there were non-significant trends supporting the use of corticosteroids to prevent reintubation and the incidence of stridor. Compared to this current review, the Cochrane review contains fewer studies and fewer events, therefore reducing its power to detect a clinically important treatment effect. The primary outcome used in the Cochrane review, reintubation attributable to severe stridor and airway obstruction, is also prone to ascertainment bias. It may be difficult to determine whether the laryngeal oedema is the primary cause for reintubation, or a contributing factor to the requirement for reintubation. We contend that all-cause reintubation is more clinically important and more robust, and offers greater value to clinicians and patients alike. Previous reviews have examined the effect of corticosteroids on the prevention of reintubation in adult populations [37]. This study extends these results to paediatric and neonatal populations.
The reduction in the rate of reintubation found in this analysis was accompanied by a significant reduction in the subjective manifestations of laryngeal oedema, such as stridor. This suggests that a reduction in laryngeal oedema associated with the use of corticosteroids is responsible for at least part of the reduction in the rate of reintubation. There are also a number of other possible mechanisms by which corticosteroids may reduce the rate of extubation failure. Corticosteroids have been shown to have a beneficial effect on patients with pneumonia [38] and have also been shown to improve the rate of ventilator weaning in patients with relative adrenal insufficiency [39]. It is also possible that corticosteroids may produce their action by influencing other factors that are known to be associated with extubation failure [40]. Future studies need to consider incorporating these factors into their study designs, to further elucidate other possible mechanisms of action of corticosteroids with respect to the prevention of reintubation.
For clinicians caring for intubated critically ill patients, the results of this systematic review and meta-analysis offer some guidance. For patients similar to those included in the studies in this review, i.e. those who have been intubated for more than 3 days, who are at significant risk for reintubation, or who are not already receiving corticosteroids, corticosteroid therapy should be considered at least 12 h prior to extubation. In neonatal and paediatric populations, dexamethasone at doses of 0.25–0.5 mg/kg (or equivalent) would be appropriate. In adult populations, the type and dose of corticosteroid used in the studies included in this review were more varied. Given this variation, it would be prudent to follow the dosing given in the largest high-quality RCT [12].
Further research in this area is still required. In particular, as the overall rate of reintubation is low, further studies aimed at defining a population most likely to benefit from corticosteroids in preventing reintubation are warranted [41]. Patients with difficult or traumatic intubations or who have required multiple intubations may constitute high-risk groups who are more likely to benefit from this therapy. Future studies should consider utilising the cuff-leak test to define a group of patients at greater risk of extubation failure secondary to laryngeal oedema. It should be noted that, while a longer course of therapy appeared to confer additional benefit, patients who received a longer course of treatment also received a higher cumulative dose of corticosteroid. Defining an optimal regimen of therapy with regards to the type of corticosteroid used, dose and timing warrants further study. Future studies should report other important outcomes such as total duration of ventilation, intensive care and hospital length of stay and all-cause mortality.
This systematic review and meta-analysis demonstrated that corticosteroids, when used in patients in intensive care undergoing prolonged endotracheal intubation, significantly reduce the rate of extubation failure and also reduce the manifestation of laryngeal oedema. As extubation failure is associated with significant morbidity and mortality, clinicians should consider the use corticosteroids in patients at high risk of extubation failure.
References
Epstein SK, Ciubotaru RL, Wong JB (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112:186–192
Gowardman JR, Huntington D, Whiting J (2006) The effect of extubation failure on outcome in a multidisciplinary Australian intensive care unit. Crit Care Resusc 8:328–333
Seymour CW, Martinez A, Christie JD, Fuchs BD (2004) The outcome of extubation failure in a community hospital intensive care unit: a cohort study. Crit Care 8:R322–R327
Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, Macias S, Allegue JM, Blanco J, Carriedo D, Leon M, de la Cal MA, Taboada F, Gonzalez de Velasco J, Palazon E, Carrizosa F, Tomas R, Suarez J, Goldwasser RS (1997) Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 156:459–465
Rothaar RC, Epstein SK (2003) Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care 9:59–66
Colice GL, Stukel TA, Dain B (1989) Laryngeal complications of prolonged intubation. Chest 96:877–884
Whited RE (1984) A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope 94:367–377
El Solh AA, Bhat A, Gunen H, Berbary E (2004) Extubation failure in the elderly. Respir Med 98:661–668
Meade MO, Guyatt GH, Cook DJ, Sinuff T, Butler R (2001) Trials of corticosteroids to prevent postextubation airway complications. Chest 120:464S–468S
Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med 353:1711–1723
Cheng KC, Hou CC, Huang HC, Lin SC, Zhang H (2006) Intravenous injection of methylprednisolone reduces the incidence of postextubation stridor in intensive care unit patients. Crit Care Med 34:1345–1350
Francois B, Bellissant E, Gissot V, Desachy A, Normand S, Boulain T, Brenet O, Preux PM, Vignon P (2007) 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet 369:1083–1089
Harel Y, Vardi A, Quigley R, Brink LW, Manning SC, Carmody TJ, Levin DL (1997) Extubation failure due to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. Int J Pediatr Otorhinolaryngol 39:147–158
Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW (1991) Dexamethasone in the prevention of postextubation stridor in children. J Pediatr 118:289–294
Wong SS, Wilczynski NL, Haynes RB (2006) Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 94:41–47
Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR (2005) Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 330:1179
MetaRegister of controlled trials (2008) Medical Editors’ Trial Amnesty. Cochrane Library, Oxford
Markovitz BP, Randolph AG, Khemani RG (2008) Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Cochrane Database Syst Rev:CD001000
Juni P, Altman DG, Egger M (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323:42–46
Fisher MM, Raper RF (1992) The ‘cuff-leak’ test for extubation. Anaesthesia 47:10–12
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Villar J, Mackey ME, Carroli G, Donner A (2001) Meta-analyses in systematic reviews of randomized controlled trials in perinatal medicine: comparison of fixed and random effects models. Stat Med 20:3635–3647
Deeks JJ (2002) Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 21:1575–1600
Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP (1996) Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med 24:1666–1669
Couser RJ, Ferrara TB, Falde B, Johnson K, Schilling CG, Hoekstra RE (1992) Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. J Pediatr 121:591–596
Darmon JY, Rauss A, Dreyfuss D, Bleichner G, Elkharrat D, Schlemmer B, Tenaillon A, Brun-Buisson C, Huet Y (1992) Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone. A placebo-controlled, double-blind, multicenter study. Anesthesiology 77:245–251
Ferrara TB, Georgieff MK, Ebert J, Fisher JB (1989) Routine use of dexamethasone for the prevention of postextubation respiratory distress. J Perinatol 9:287–290
Gaussorgues P, Boyer F, Piperno D, Gerard M, Leger P, Robert D (1987) Laryngeal oedema after extubation. Can it be prevented by corticosteroids? PRESSE MED 16:1531–1532
Ho LI, Harn HJ, Lien TC, Hu PY, Wang JH (1996) Postextubation laryngeal edema in adults. Risk factor evaluation and prevention by hydrocortisone. Intensive Care Med 22:933–936
Lee CH, Peng MJ, Wu CL (2007) Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care 11:R72
Shih CM, Chen W, Tu CY, Chen HJ, Lee JC, Tsai WK, Hsu WH (2007) Multiple injections of hydrocortisone for the prevention of post-extubation stridor in acute respiratory failure. Poster 102 in: Proceedings of Conference of the American Thoracic Society, San Francisco, May 2007
Tibballs J, Shann FA, Landau LI (1992) Placebo-controlled trial of prednisolone in children intubated for croup. Lancet 340:745–748
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW Jr, Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 354:1896–1900
Courtney SE, Weber KR, Siervogel RM, Spohn WA, Guo S, Malin SW, Bender CV (1992) Effects of dexamethasone on pulmonary function following extubation. J Perinatol 12:246–251
Fan T, Wang G, Mao B, Xiong Z, Zhang Y, Liu X, Wang L, Yang S (2008) Prophylactic administration of parenteral steroids for preventing airway complications after extubation in adults: meta-analysis of randomised placebo controlled trials. BMJ 337:a1841
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU (2005) Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 171:242–248
Huang CJ, Lin HC (2006) Association between adrenal insufficiency and ventilator weaning. Am J Respir Crit Care Med 173:276–280
Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguia C, Gonzalez M, Hill NS, Nava S, D’Empaire G, Anzueto A (2006) Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 130:1664–1671
Bagshaw SM, Delaney A, Farrell C, Drummond J, Brindley PG (2008) Steroids to prevent post-extubation airway obstruction in adult critically ill patients. Can J Anaesth 55:382–385
Acknowledgments
The authors would like to thank Dr Ed Litton and Dr Jan Wiegand for their assistance with the translation of French and German language articles.
Conflict of interest statement
All authors declare that they have no conflict of interest including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: search strategies
PUBMED
((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading]) AND (extubation) AND ((steroids) OR (glucocorticoids)).
CENTRAL
-
1
glucocorticoids.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] (2582)
-
2
steroids.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] (3372)
-
3
corticosteroids.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] (3555)
-
4
1 or 2 or 3 (8285)
-
5
extubation.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] (1385)
-
6
4 and 5 (26)
EMBASE
-
1
RANDOMIZED CONTROLLED TRIAL.pt. (0)
-
2
CONTROLLED CLINICAL TRIAL.pt. (0)
-
3
RANDOMIZED CONTROLLED TRIALS.sh. (0)
-
4
RANDOM ALLOCATION.sh. (0)
-
5
DOUBLE BLIND METHOD.sh. (0)
-
6
SINGLE BLIND METHOD.sh. (0)
-
7
1 or 2 or 3 or 4 or 5 or 6 (0)
-
8
(ANIMALS not HUMAN).sh. (0)
-
9
7 not 8 (0)
-
10
CLINICAL TRIAL.pt. (0)
-
11
exp CLINICAL TRIALS/ (493,448)
-
12
(clin$ adj25 trial$).ti,ab. (131,765)
-
13
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. (90,501)\
-
14
PLACEBOS.sh. (0)
-
15
placebo$.ti,ab. (102,691)
-
16
random$.ti,ab. (356,558)
-
17
RESEARCH DESIGN.sh. (0)
-
18
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (782,546)
-
19
18 not 8 (782,546)
-
20
19 not 9 (782,546)
-
21
COMPARATIVE STUDY.sh. (101,880)
-
22
exp EVALUATION STUDIES/ (50,880)
-
23
FOLLOW UP STUDIES.sh. (0)
-
24
PROSPECTIVE STUDIES.sh. (0)
-
25
(control$ or prospectiv$ or volunteer$).ti,ab. (1,611,246)
-
26
21 or 22 or 23 or 24 or 25 (1,722,125)
-
27
26 not 8 (1,722,125)
-
28
27 not (9 or 20) (1,414,085)
-
29
9 or 20 or 28 (2,196,631)
-
30
exp CORTICOSTEROD/ or corticosteroids.mp. or exp CORTICOSTEROID THERAPY/ (361,933)
-
31
exp CORTICOSTEROID/ or corticosteroids.mp. or exp CORTICOSTEROID THERAPY/ (361,933)
-
32
glucocorticoid.mp. or exp GLUCOCORTICOID/ (286,723)
-
33
steroids.mp. or exp Steroid/ (567,141)
-
34
30 or 31 or 32 or 33 (576,495)
-
35
extubation.mp. or EXTUBATION/ (5,588)
-
36
29 and 34 and 35 (228)
-
37
from 36 keep 1–199 (199)
-
38
from 36 keep 200–228 (29)
Appendix 2: corticosteroids to prevent reintubation. Funnel plot with pseudo 95% confidence limits

Rights and permissions
About this article
Cite this article
McCaffrey, J., Farrell, C., Whiting, P. et al. Corticosteroids to prevent extubation failure: a systematic review and meta-analysis. Intensive Care Med 35, 977–986 (2009). https://doi.org/10.1007/s00134-009-1473-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1473-9