Abstract
Melanoma is a deadly skin cancer with high mortality, and its incidence is increasing every year. Although numerous anticancer drugs have been developed, these treatments have various side effects, such as skin rash, fatigue, diarrhea, cough, and muscle pain. Therefore, there is a need for research on novel anticancer drugs with low cytotoxicity and few side effects. In this study, we investigated whether afrormosin (7-hydroxy-4′,6-dimethoxyisoflavone), a member of the isoflavonoid family, could have the potential as a novel anticancer drug. Afrormosin decreased the viability of B16F10 melanoma cells in a time- and dose-dependent manner. We also found that the afrormosin-induced decrease in cell viability was caused by the reduction of cell proliferation through Go/G1 arrest and the induction of apoptosis in B16F10 melanoma cells. Furthermore, afrormosin decreased the metastatic activity (cell invasion and migration) of B16F10 melanoma cells. At the molecular level, afrormosin reduced the levels of Bcl-2, an anti-apoptotic protein, and augmented the levels of Bax, a pro-apoptotic protein, and p53, a tumor suppressor. Additionally, procaspase-3 levels were reduced by afrormosin treatment. When we examined the signaling pathways affected by afrormosin, we found that the AKT/ERK pathways were inhibited and the p38/JNK pathway was activated by afrormosin. Collectively, these results suggest the potential anticancer effect of afrormosin, making it a prospective candidate for development as an anticancer drug.
Similar content being viewed by others
Introduction
The skin is an innate immune system that functions as the first defense barrier and protects the human body from various environmental attacks [1]. The skin is particularly vulnerable to exposure to various harmful substances, sun damage, and microbes [1]. A combination of genetic and environmental risk factors causes most skin cancers [2]. The three major types of skin cancer include melanoma, squamous cell carcinoma (SSC), and basal cell carcinoma (BBC). Melanoma is a lethal skin cancer whose incidence rate is increasing rapidly every year [3]. Although melanoma constitutes approximately 2% of skin cancers, it has a highly metastatic property that leads to the majority of deaths [4, 5]. Early diagnosis and rapid treatment are required to decrease the mortality rate of malignant melanoma; however, it is difficult to distinguish melanoma from spots, and early diagnosis is difficult because of its rapid spread [6,7,8]. Several chemotherapeutic drugs are available for the treatment of inoperable or metastatic melanoma. Dacarbazine and temozolomide have mainly been used to treat metastatic melanoma. Both dacarbazine and temozolomide suppress cancer cell division and trigger cancer cell death by methylating nucleic acids, thereby causing DNA damage [9, 10]. However, they damage some healthy cells and have common side effects such as skin rash, fatigue, diarrhea, cough, and muscle pain, which are the most urgent and significant dilemmas in cancer therapy [11]. Therefore, developing new anticancer treatment strategies is required to reduce side effects and improve the therapeutic effect for malignant melanoma.
Cancer is caused by the misregulation of the cell cycle and apoptotic processes. Therefore, available anticancer drugs exhibit cytotoxic and antiproliferative effects by inducing apoptosis or arresting the cell cycle [12]. The apoptotic processes are the main mechanism that cancer drugs cause the death of tumor cells [13]. The apoptosis pathway is achieved by regulating the expression levels of anti-apoptotic proteins (Bcl-2, Bcl-w, Bcl-xL, Mcl-1, and A1) and pro-apoptotic proteins (Bax, Bak, Bad, and Bok). Mechanisms regulating the proliferation, survival, and migration (metastasis) of cancer cells include the MAPK (mitogen-activated protein kinase) and PI3K (phosphoinositide 3-kinase)/AKT signaling pathways. The MAPK signaling pathway consists of c-Jun N-terminal kinases (JNK), extracellular signal-related kinases (ERK), and p38 proteins (p38) signaling pathways [14]. ERK signaling pathway has been known to be overactivated in over 80% of all melanomas. The carcinogenic effects of B-RAF and RAS genes are related to the overactivation of the MAPK pathway that leads to the unlimited growth of melanoma cells [15]. p38 has been shown to function as an anti-tumorigenic factor in cancer. For example, p38 activation triggers apoptosis when oxidative stress increases [16]. JNK is a MAPK family protein that is mainly activated by stress stimuli [17]. Many indispensable cellular processes, including cell growth, differentiation, and survival, are regulated by the PI3K/AKT pathway, which is induced by various extracellular stimuli, such as cytokines, hormones, and growth factors [18, 19]. AKT activation by PI3K is involved in many cellular processes (the proliferation, cell cycle, survival, and migration) by upregulating various transcription factors [20]. Abnormal mTOR activation has been observed in metastatic melanoma and several different types of cancer [21].
Currently, treatments for cancer have already been developed and used; however, cancer survival rates have not been significantly improved by chemically synthesized drugs, which have several problems, such as side effects and high cost. Therefore, cancer treatment will continue to be an unresolved problem in the future [22]. Novel strategies and chemotherapies are required to complement the current cancer treatments, and recently, drugs using phytochemicals have emerged as new anticancer agents. Phytochemicals are bioactive plant chemicals that have been reported to have various biological functions, including immunomodulatory properties, anti-inflammation, anticancer, antioxidant, and antibacterial effects. They also appear to be beneficial because of their wide availability and cost-effective properties [1]. Therefore, recently, research on phytochemicals as potential new anticancer agents that can overcome the side effects of existing chemotherapy has become more active, and drugs using phytochemicals such as paclitaxel and podophyllotoxin have already been used for cancer treatment [23]. Paclitaxel is a natural anticancer drug derived from Taxus brevifolia. Paclitaxel prevents cancer cell growth and induces cell cycle arrest by suppressing microtubule dynamics [24]. Podophyllotoxin is an aryltetralin-type lignan extracted from Podophyllum species that also induces apoptosis by preventing tubulin assembly [25].
Afrormosin, a member of the isoflavone family, is a 4'-methoxyisoflavone, which is substituted with two methoxy groups at the 6 and 4' positions and a hydroxyl group at the 7 position. Afrormosins are typically found in soybeans and other soy products. In addition, genistein and daidzein, as phytochemicals from soybeans, have been known to have anticancer effects. Genistein can regulate several intracellular signaling pathways and work as an anticancer agent for various cancers by inhibiting the proliferation of cancer cells [26]. Daidzein is also known to inhibit cell proliferation and induce apoptosis in various cancer cells [27]. Although there have been some reports on the biological activity of afrormosin [28, 29], its anticancer mechanism has not yet been studied. In this study, we investigated the anticancer mechanism of afrormosin in melanoma cells.
Materials and methods
Chemicals and reagent
Afrormosin was purchased from ChemFaces (Wuhan, China). Dulbecco’s Modified Eagle’s medium (DMEM) and Penicillin/streptomycin/glutamine (PSQ) were purchased from Hyclone (Marlborough, MA, USA) and Gibco (Waltham, MA, USA), respectively. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), trypan blue, collagen type I, paraformaldehyde, DMSO, and crystal violet were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). DPBS and trypsin (EDTA) solutions were purchased from WELGENE, Inc. (Gyeongsan, Korea). Carboxyfluorescein diacetate succinimidyl ester (CFSE) and propidium iodide (PI) were purchased from Invitrogen (Carlsbad, CA, USA). Ethanol was purchased from Emsure (Billerica, MA). Transwell inserts (8-µm pore) and fetal bovine serum (FBS) were purchased from Corning (Corning, NY, USA). Agar was purchased from Affymetrix (Santa Clara, CA). FITC-labeled annexin V was purchased from BD Pharmingen (San Diego, CA, USA). Primary antibodies [β-actin (Cat.#8457), Caspase-3 (Cat.#9662), Bcl-2 (Cat.#3498), Bax (Cat.#2772), phospho-p38 (Cat.#9216), phospho-ERK (Cat.#4370), phospho-AKT (Cat.#4060) and phospho-JNK (Cat.#9255)] were purchased from Cell Signaling Technology (Danvers, MA, USA) and anti-p53 (Cat.#MA5-12453) was purchased from Invitrogen (Carlsbad, CA, USA). Secondary antibodies (goat anti-mouse IgG (Cat.#5220–0341) and goat anti-rabbit IgG (Cat.#5220–0036) were purchased from SeraCare (Gaithersburg, MD, USA).
Cell culture
The melanoma cell line (B16F10 (mouse)) was a gift from Dr. Kwang Dong Kim (Gyeongsang National University, Jinju, Korea). Cells were cultured in DMEM (high glucose medium) supplemented with 10% FBS and 1% PSQ at 37 °C in a 5% CO2 incubator.
Cell viability and density
After 24 h of seeding B16F10 cells in a 48-well plate at a density of 2 × 103 cells/well, B16F10 cells were incubated with afrormosin (25, 50, and 100 µM) and incubated for 24, 48, and 72 h. Afterward, MTT (1 mg/mL) assay was performed. The absorbance was measured using a Varioskan LUX Multimode Microplate Reader (Thermo Fisher Scientific Co.) at 570 nm.
To assess cell density, B16F10 cells were plated in a 24-well plate at a 4 × 103 cells/well for 24 h, and then cells were incubated with 100 μM afrormosin for 72 h. Afterward, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature (RT) and stained with crystal violet (0.05%). The cell density was recorded using a microscope (IMT i-Solution Inc.).
Trypan blue exclusion assay
B16F10 cells were cultured in 24-well plates at 4 × 103 cells/well. The next day, cells were incubated with 25, 50, and 100 µM afrormosin for 24, 48, and 72 h. Living cells were counted using a hemocytometer after staining with trypan blue solution.
Apoptosis assay
After 24 h of seeding B16F10 cells in 6-well plates at 2 × 104 cells/well, the cells were cultured with 100 μM afrormosin for 48 h. For staining, the cells were incubated with FITC labeled Annexin V and propidium iodide (PI) for 15 min at RT. Apoptosis rates were analyzed by flow cytometry (BD FACSVerse™, BD Biosciences).
Colony formation assay
B16F10 cells were seeded in 6-well plates at 5 × 102 cells/well. After one day, the cells were incubated with 100 μM afrormosin at 37 °C with 5% CO2 for 72 h. The cells were incubated with 4% paraformaldehyde for 10 min at RT, and then further incubated with 0.05% crystal violet for 10 min at RT. Colonies were dissolved in methanol for 1 h. The degree of colony formation was analyzed with a microplate reader at 570 nm.
CFSE-based proliferation assay
After 24 h of seeding B16F10 cells in 6-well plates at 2 × 104 cells/well, the cells were stained with 3 μM carboxyfluorescein diacetate succinimidyl ester (CFSE). And then, the cells were treated with 100 μM afrormosin and incubated for 24, 48, and 72 h. The degree of proliferation was analyzed by fluorescence-activated cell sorting (FACS) machine (BD FACSVerse™, BD Biosciences).
Cell cylce assay
B16F10 cells were seeded in 6-well plates at 2 × 104 cells/well. On the next day, the cells were treated with 100 μM afrormosin and cultured for 72 h. After staining the cells with propidium iodide (PI) solution (10 μg/mL), DNA content was analyzed using fluorescence-activated cell sorting (FACS) machine (BD FACSVerse™, BD Biosciences).
Migration assay
For the wound healing assay, B16F10 cells were seeded in 6-well plates at 5 × 105 cells/well. On the next day, the cell monolayer was scratched with a sterile yellow tip, and then the cells were cultured in the medium containing 100 μM afrormosin for 24 h. Cell migration was recorded under a microscope, and the area of the wound gap was measured using ImageJ software.
Transwell migration of B16F10 cells was analyzed using a transwell insert (8-µm pore). Before seeding, serum starvation was performed for 24 h. After that, 1 × 105 cells were cultured in the upper region of the collagen-coated transwell insert for 24 h. Non-migration cells were removed from the upper part. The migrated cells were incubated with 4% paraformaldehyde for 10 min. Afterward, the cells were further incubated with 0.05% crystal violet solution and examined under a microscope (IMT i-Solution, Inc.).
Invasion assay
The bottom of a 6-well plate was filled with medium containing 0.6% agar, and the cells (5 × 103 cells/well) were seeded in medium containing 0.3% agar. The cells were incubated for 3 weeks at 37 °C. The size of cell colonies was analyzed using a microscope (IMT i-Solution, Inc.).
Western blot analysis
After 24 h of seeding B16F10 cells in a 60 mm culture dish at 105 cells/well, the cells were incubated with 100 µM afrormosin for 2 or 48 h. Cells were collected and lysed with lysis RIPA buffer (150 mM NaCl, 10 mM Tris pH 7.4, 1 mM EDTA (pH 8), 1% sodium deoxycholate, 1% Triton X-100, phosphatase inhibitors (30 mM NaF and 1.5 mM NaVO4), protease inhibitors (1 mM PMSF and 1 mg/mL each of aprotinin, leupeptin, and pepstatin A). The proteins were resolved by SDS-PAGE and transferred to a nitrocellulose (NC) membrane with the wet method. After blocking the NC membrane with 5% skimmed milk in Tris-buffered saline (TBS) buffer supplemented with 0.1% Tween-20 for 30 min, the NC membrane was incubated with primary antibodies for 4 h or overnight. Then, the membrane was washed with TBS buffer supplemented with 0.1% Tween-20 every 10 min for 30 min. Subsequently, the membrane was incubated with secondary antibodies (horseradish peroxidase (HRP)-conjugated IgG) for 1 h. The membrane was then washed with TBS buffer supplemented with 0.1% Tween-20 every 10 min for 30 min. Finally, the membranes were incubated with an ECL solution, and the signals were detected using a Chemidoc (iBright™ CL1500, Invitrogen). Signal intensities were further analyzed via ImageJ software.
Statistical analysis
Data are represented as three independent replicate experiments. The results are presented as mean ± standard deviation (SD). The difference between groups was analyzed using Student’s t-test. Statistical significance is indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
Result
The effects of afrormosin on the viability of B16F10 melanoma cells
First, to evaluate the effect of afrormosin on the viability of B16F10 cells, an MTT assay was performed at various concentrations of afrormosin (25, 50, and 100 μM) for 24, 48, and 72 h. As shown in Fig. 1A and B, afrormosin significantly inhibited cell viability time- and dose-dependently. In the trypan blue exclusion assay, afrormosin reduced cell viability in a dose-dependent manner (Fig. 1C). Crystal violet assay was executed to assess cell density (Fig. 1D). The density of B16F10 cells was lower in afrormosin-treated cells than in control cells. These results confirmed that afrormosin reduced cell viability.
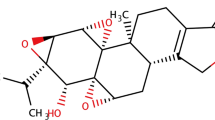
The effects of afrormosin on the viability of B16F10 melanoma cells. A Chemical structure of afrormosin. B The effect of afrormosin on the viability of B16F10 cells using the MTT assay. C Trypan blue exclusion assay. D Cell density was determined using crystal violet staining. *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with the control group. The data are presented as the mean ± SD; n = 3
Afrormosin induces apoptosis in B16F10 melanoma cells
To investigate whether the reduction in viability of B16F10 cells by afrormosin was due to the induction of apoptosis, we measured the type of afrormosin-induced cell death using Annexin V/PI double staining. (Fig. 2A). In cell viability analysis (Fig. 1B), cell viability at 48 h was similar to that at 72 h. Therefore, we performed the apoptosis analysis at 48 h. As a result of treatment with 100 μM afrormosin for 48 h, the induction of apoptosis was approximately 2 times higher than that of the control group. (Fig. 2B). This suggests that afrormosin could have anticancer effects by inducing apoptosis in malignant melanoma B16F10 cells.
The effects of afrormosin on apoptosis of B16F10 melanoma cells. The apoptotic rate of B16F10 cells was measured by flow cytometry. A The FITC-positive population indicates the apoptotic rate. B The proportion of apoptosis is represented by the bar graph. The apoptotic rate of the cells, following treatment with 100 μM afrormosin for 48 h, is 2 times higher than that of the control. **p < 0.01 when compared with the control group. The data are presented as the mean ± SD; n = 3
Effects of afrormosin on the proliferation of B16F10 melanoma cells
A subsequent study was conducted to ascertain the effect of afroromosin on the proliferation of B16F10 cells. The effect on B16F10 cell proliferation was validated using a colony formation assay under the same conditions (Fig. 3A–C). When B16F10 cells were cultured at a low density and treated with 100 μM afrormosin for 72 h, the colony size was reduced compared to that of the control (Fig. 3B), but there was no significant difference in the number of colonies (Fig. 3C). A CFSE-based proliferation assay was performed to analyze the effect of afrormosin on the proliferation of B16F10 cells (Fig. 3D). After CFSE fluorescent labeling, B16F10 cells were incubated with 100 μM afrormosin and cultured for 24, 48, and 72 h. The extent of cell proliferation was monitored by flow cytometry. Compared to the control cells, cells treated with afrormosin showed higher fluorescence intensity. This suggests that afrormosin reduces the proliferation of B16F10 cells.
The effects of afrormosin on cell proliferation in B16F10 cells. A The colony formation assay was performed after treating cells with 100 μM afrormosin for 7 days and staining using crystal violet. B The bar graph shows the percentage of OD values. C The bar graph shows the percentage of the number of colony units. D The CFSE-based proliferation is analyzed using flow cytometry. ***p < 0.001 when compared with the control group. The data are presented as the mean ± SD; n = 3
Afrormosin causes cell cycle arrest in the G0/G1 phase in B16F10 melanoma cells
To determine whether the effect of afrormosin on the proliferation of B16F10 cells was the result of cell cycle inhibition, an experiment was conducted to confirm the cell cycle using flow cytometry. As shown in Fig. 4A–C, when afrormosin-treated group for 72 h was compared to the control, the afrormosin-treated group showed increased cells in the G0/G1 population and decreased cells in the G2/M population. In addition, subG1 groups that are considered as the apoptotic population were increased by afrormosin treatment. Therefore, we suggest that afrormosin induces apoptosis in B16F10 cells and causes cell cycle arrest in the G0/G1 phase.
The effects of afrormosin on cell cycle in B16F10 melanoma cells. The cell cycle was measured by flow cytometry. A The histogram shows the cell cycle phase of B16F10 cells following treatment with 100 μM afrormosin for 72 h. B, C The bar graph shows the percentage of each cell cycle phase. **p < 0.01 and ***p < 0.001 when compared with the control group. The data are presented as the means ± SD; n = 3
Effects of afrormosin on the migration of B16F10 melanoma cells
Next, we investigated whether afrormosin affected cell movement. The wound healing assay involves scratching the cell layer and verifying cell movement in two dimensions while maintaining cell-to-cell junctions. To minimize the effect of cell proliferation on cell migration, a wound healing assay was performed with DMEM containing1% FBS. The wound gap in cells treated with 100 μM afrormosin did not heal after 24 h (Fig. 5A and B). In contrast, the wound gap was completely closed in the control cells (Fig. 5A and B). This suggests that afrormosin inhibits the migration of B16F10 cells. In addition, the migratory effect of afrormosin was verified using a transwell migration analysis. We found that afrormosin inhibited chemotactic cell migration through the membrane (Fig. 5C).
The effects of afrormosin on the inhibition of migration in B16F10 melanoma cells. The wound healing assay was performed to confirm cell mobility following 100 μM afrormosin treatment for 24 h after scratching. A The cell movement was captured using microscopy. B The bar graph represents the percentage of the wound gap in B16F10 melanoma cells. C A transwell migration assay was performed to confirm cell mobility. The migrated cells were captured using a microscope. **p < 0.01 and ***p < 0.001 when compared with the control group. The data are presented as the mean ± SD; n = 3
Effects of afrormosin on the invasion of B16F10 melanoma cells
Next, we checked the colony-forming cell invasion ability using soft agar assay, a well-established in vitro system for proving malignant transformation in cells. Cells with anchorage-independent capacities transform and continue to grow, forming colonies of various sizes. The size of colonies observed under the microscope is shown in Fig. 6A. The results showed that afrormosin inhibited colony size (Fig. 6B), indicating that afrormosin can effectively suppress cell invasion in vitro.
The effects of afrormosin on inhibition of migration of B16F10 melanoma cells. The soft agar colony forming assay was performed to confirm anchorage-independent growth, followed by 100 μM afrormosin treatment for 3 weeks. A The cell size of the colony was captured using microscopy. B The bar graph represents the percentage of the size of the colony of B16F10 melanoma cells. *p < 0.05 when compared with the control group. The data are presented as the mean ± SD; n = 3. Scale bars: 100 μm
Afrormosin modulates the apoptosis signaling pathway
Western blot analysis of apoptotic proteins was performed to reveal the mechanisms of apoptotic processes. We confirmed that the expression of BAX, a pro-apoptotic protein related to apoptosis, increased, and the expression of the anti-apoptotic protein, Bcl-2, decreased (Fig. 7A and B). Furthermore, we confirmed that the expression of p53 (a tumor suppressor) was increased, and caspase-3 was activated (Fig. 7A and B). Therefore, these results indicated that afrormosin could induce apoptosis by inhibiting anti-apoptotic proteins (Bcl-2) and activating pro-apoptotic proteins/apoptotic mediators (BAX, caspase-3, and p53).
Signaling apoptosis pathway in B16F10 melanoma cells following treatment with afrormosin. Afrormosin modulates the apoptosis signaling pathway in B16F10 cells. A After treatment with afrormosin for 48 h, the expression of the pro-apoptotic protein BAX increased and the expression of the anti-apoptotic protein Bcl-2 decreased. After treatment with afrormosin for 48 h, the expression level of p53 increased and the protein level of procaspase-3 decreased. B The analysis of the protein level by quantitation. *p < 0.05 and **p < 0.01when compared with the control group. The data are presented as the mean ± SD; n = 3
Afrormosin modulates the MAPK and PI3K/AKT pathways
AKT and MAPK signaling pathways play essential roles in cell survival and proliferation. We investigated the effects of afrormosin on the MAPK and AKT signaling pathways in B16F10 cells. While afrormosin decreased the phosphorylation of ERK and AKT, it augmented the activation of p38 and JNK as cellular stress mediators (Fig. 8A and B). These results indicated that afrormosin regulates apoptosis and cell proliferation through the MAPK and AKT signaling pathway in B16F10 melanoma cells.
Signaling pathway of PI3K/AKT and MAPK in B16F10 cells following treatment with afrormosin. Afrormosin modulates the MAPK signaling pathway in B16F10 cells. A After treatment of B16F10 cells with afrormosin for 2 h, phosphorylation levels of ERK and AKT decreased and phosphorylation levels of p38 and JNK increased. B Analysis of protein level by quantitation. *p < 0.05 and **p < 0.01 when compared with the control group. The data are presented as the mean ± SD; n = 3
Discussion
Skin cancers occur due to various reasons, such as environmental and genetic risk factors. Melanoma has a high mortality rate owing to rapid metastasis, even though it has the lowest incidence among the three types of skin cancer [2, 5, 30]. The number of patients with melanoma is increasing every year, and new treatments are needed. Drugs for melanoma treatments, such as dacarbazine and temozolomide, are being used, but they have problems such as drug tolerance and side effects [11]. Accordingly, it is necessary to develop novel anticancer drugs that can overcome the limitations of existing anticancer drugs. Recently, phytochemicals with relatively fewer side effects and lower cost compared to existing chemotherapy drugs have emerged as new anticancer drug candidates [23]. Paclitaxel and podophyllotoxin are phytochemical drugs used to treat melanomas. Paclitaxel is a natural compound derived from Taxus brevifolia that promotes tubulin assembly into microtubules, thereby blocking cancer cell growth, mitosis, and cell cycle progression [24]. Podophyllotoxin is a natural compound obtained from podophyllum species and has anticancer activities that prevent tubulin from assembling into microtubules and inducing apoptosis [25]. Isoflavones are known to have anticancer, anti-inflammation, and antioxidant properties [31]. Among the isoflavone-based phytochemicals, genistein and daidzein are known to have anticancer effects. The anticancer mechanism of afrormosin, an isoflavone, has not yet been reported. Here, we found that afrormosin inhibited cell proliferation by mediating G0/G1 arrest, inducing apoptosis by regulating pro/anti-apoptotic proteins, and decreasing the metastatic activity (cell migration/invasion) of melanoma. Furthermore, we identified that the anticancer effects of afrormosin could be mediated by the regulation of the MAPK and AKT signaling pathways.
When we investigated the effect of afrormosin on the viability of B16F10 cells, we identified that afrormosin reduced the viability of B16F10 cells in a time- and dose-dependent manner. Afrormosin induced apoptosis and inhibited cell proliferation by inducing G0/G1 arrest. These indicated that afrormosin could reduce B16F10 cell viability under the influence of both apoptosis induction and cell proliferation inhibition. Moreover, afrormosin decreased the invasion and migration of the B16F10 cells. In terms of apoptosis, afrormosin reduced the level of anti-apoptotic protein Bcl2, augmented the level of pro-apoptotic protein BAX, increased the level of tumor suppressor protein p53, and activated caspase3, an important mediator of apoptosis. The MAPK pathway regulates various biological functions such as differentiation, apoptosis, proliferation, invasion, migration, and stress responses. The JNK/p38 pathway primarily involves cellular stress and apoptosis, and the ERK signaling pathway regulates cell proliferation and growth [32]. The PI3K/AKT pathway also controls cell proliferation, growth, cell cycle, angiogenesis, and motility [33]. Afrormosin decreased the phosphorylation of ERK and AKT and augmented the phosphorylation of JNK and p38. These results suggest that afrormosin may exert anticancer effects by activating the JNK/p38 pathway to induce apoptosis and inhibit proliferation by downregulating the ERK and AKT pathways. In addition, isoflavones, as diphenolic compounds, have estrogen-like chemical structures. They can interact with estrogen receptor alpha (ERα) and beta (ERβ) to regulate various biological functions [34]. Therefore, it needs to investigate next whether afrormosin, a member of the isoflavone family, can mediate anticancer effects through ER.
In conclusion, we found that afrormosin activated the JNK/p38 pathway in B16F10 cells to induce apoptosis and downregulate the ERK and AKT pathways to induce G0/G1 arrest and inhibit proliferation (Fig. 9). These results imply that the phytochemical afrormosin has the potential to become a new alternative drug with fewer side effects through the regulation of apoptosis and proliferation in the future for the treatment of malignant melanoma.
Availability of data and materials
The datasets that support the finding of this study are available from the corresponding author on reasonable request.
References
Ng CY, Yen H, Hsiao HY, Su SC (2018) Phytochemicals in skin cancer prevention and treatment: an updated review. Int J Mol Sci. https://doi.org/10.3390/ijms19040941
Watson M, Holman DM, Maguire-Eisen M (2016) Ultraviolet radiation exposure and its impact on skin cancer risk. Semin Oncol Nurs 32:241–254. https://doi.org/10.1016/j.soncn.2016.05.005
Danciu C, Soica C, Antal D, Alexa E, Pavel IZ, Ghiulai R, Ardelean F, Babuta RM, Popescu A, Dehelean CA (2018) Natural compounds in the chemoprevention of malignant melanoma. Anticancer Agents Med Chem 18:631–644. https://doi.org/10.2174/1871520617666171121142522
Linares MA, Zakaria A, Nizran P (2015) Skin cancer. Prim Care 42:645–659. https://doi.org/10.1016/j.pop.2015.07.006
Turner N, Ware O, Bosenberg M (2018) Genetics of metastasis: melanoma and other cancers. Clin Exp Metastasis 35:379–391. https://doi.org/10.1007/s10585-018-9893-y
Corneli P, Zalaudek I, Magaton Rizzi G, di Meo N (2018) Improving the early diagnosis of early nodular melanoma: can we do better? Expert Rev Anticancer Ther 18:1007–1012. https://doi.org/10.1080/14737140.2018.1507822
Davis LE, Shalin SC, Tackett AJ (2019) Current state of melanoma diagnosis and treatment. Cancer Biol Ther 20:1366–1379. https://doi.org/10.1080/15384047.2019.1640032
Rigel DS, Russak J, Friedman R (2010) The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin 60:301–316. https://doi.org/10.3322/caac.20074
Jiang G, Li RH, Sun C, Liu YQ, Zheng JN (2014) Dacarbazine combined targeted therapy versus dacarbazine alone in patients with malignant melanoma: a meta-analysis. PLoS ONE 9:e111920. https://doi.org/10.1371/journal.pone.0111920
Quirbt I, Verma S, Petrella T, Bak K, Charette M (2007) Members of the melanoma disease site group of cancer care ontario’s program in evidence-based, C. Temozolomide for the treatment of metastatic melanoma. Curr Oncol 14:27–33. https://doi.org/10.3747/co.2007.98
Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO, Harguindey S et al (2015) Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int 15:71. https://doi.org/10.1186/s12935-015-0221-1
Thomadaki H, Tsiapalis CM, Scorilas A (2005) Polyadenylate polymerase modulations in human epithelioid cervix and breast cancer cell lines, treated with etoposide or cordycepin, follow cell cycle rather than apoptosis induction. Biol Chem 386:471–480. https://doi.org/10.1515/BC.2005.056
Debatin KM (2004) Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother 53:153–159. https://doi.org/10.1007/s00262-003-0474-8
Knight T, Irving JA (2014) Ras/Raf/MEK/ERK pathway activation in childhood acute lymphoblastic leukemia and its therapeutic targeting. Front Oncol 4:160. https://doi.org/10.3389/fonc.2014.00160
Wang AX, Qi XY (2013) Targeting RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB Life 65:748–758. https://doi.org/10.1002/iub.1193
Martinez-Limon A, Joaquin M, Caballero M, Posas F, de Nadal E (2020) The p38 pathway: from biology to cancer therapy. Int J Mol Sci. https://doi.org/10.3390/ijms21061913
Pua LJW, Mai CW, Chung FF, Khoo AS, Leong CO, Lim WM, Hii LW (2022) Functional roles of JNK and p38 MAPK signaling in nasopharyngeal carcinoma. Int J Mol Sci. https://doi.org/10.3390/ijms23031108
Khan KH, Yap TA, Yan L, Cunningham D (2013) Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer 32:253–265. https://doi.org/10.5732/cjc.013.10057
Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X (2019) Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer 18:26. https://doi.org/10.1186/s12943-019-0954-x
Alzahrani AS (2019) PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol 59:125–132. https://doi.org/10.1016/j.semcancer.2019.07.009
Wang B, Zhang W, Zhang G, Kwong L, Lu H, Tan J, Sadek N, Xiao M, Zhang J, Labrie M et al (2021) Targeting mTOR signaling overcomes acquired resistance to combined BRAF and MEK inhibition in BRAF-mutant melanoma. Oncogene 40:5590–5599. https://doi.org/10.1038/s41388-021-01911-5
Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O (2019) Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol 10:1614. https://doi.org/10.3389/fphar.2019.01614
Singh S, Sharma B, Kanwar SS, Kumar A (2016) Lead phytochemicals for anticancer drug development. Front Plant Sci 7:1667. https://doi.org/10.3389/fpls.2016.01667
Zhu L, Chen L (2019) Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 24:40. https://doi.org/10.1186/s11658-019-0164-y
Ardalani H, Avan A, Ghayour-Mobarhan M (2017) Podophyllotoxin: a novel potential natural anticancer agent. Avicenna J Phytomed 7:285–294
Tuli HS, Tuorkey MJ, Thakral F, Sak K, Kumar M, Sharma AK, Sharma U, Jain A, Aggarwal V, Bishayee A (2019) Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol 10:1336. https://doi.org/10.3389/fphar.2019.01336
Chan KKL, Siu MKY, Jiang YX, Wang JJ, Leung THY, Ngan HYS (2018) Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int 18:65. https://doi.org/10.1186/s12935-018-0559-2
Konoshima T, Kokumai M, Kozuka M, Tokuda H, Nishino H, Iwashima A (1992) Anti-tumor-promoting activities of afromosin and soyasaponin I isolated from Wistaria brachybotrys. J Nat Prod 55:1776–1778. https://doi.org/10.1021/np50090a011
Konoshima T, Okamoto E, Kozuka M, Nishino H, Tokuda H, Tanabe M (1988) Studies on inhibitors of skin tumor promotion, III. Inhibitory effects of isoflavonoids from Wisteria brachybotrys on Epstein-Barr virus activation. J Nat Prod 51:1266–1270. https://doi.org/10.1021/np50060a038
Braeuer RR, Watson IR, Wu CJ, Mobley AK, Kamiya T, Shoshan E, Bar-Eli M (2014) Why is melanoma so metastatic? Pigment Cell Melanoma Res 27:19–36. https://doi.org/10.1111/pcmr.12172
Yu J, Bi X, Yu B, Chen D (2016) Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. https://doi.org/10.3390/nu8060361
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL (2020) ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 19:1997–2007. https://doi.org/10.3892/etm.2020.8454
Lau MT, Leung PC (2012) The PI3K/Akt/mTOR signaling pathway mediates insulin-like growth factor 1-induced E-cadherin down-regulation and cell proliferation in ovarian cancer cells. Cancer Lett 326:191–198. https://doi.org/10.1016/j.canlet.2012.08.016
Messina MJ, Wood CE (2008) Soy isoflavones, estrogen therapy, and breast cancer risk: analysis and commentary. Nutr J 7:17. https://doi.org/10.1186/1475-2891-7-17
Acknowledgements
Not applicable.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Grant Number: 2020R1F1A1070844) and the Korea Polar Research Institute (KOPRI) Grant funded by the Ministry of Oceans and Fisheries (KOPRI Project No. * PE21900).
Author information
Authors and Affiliations
Contributions
HK, MH, S-AS, and JA performed the experiments. HK and CSL wrote the manuscript with guidance from CSL, M-JA, JHL, and HHP provided intellectual contribution to this study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H., Han, M., Shin, SA. et al. Afrormosin exerts an anticancer effect via MAPK and AKT signaling pathways in B16F10 cells. Appl Biol Chem 65, 71 (2022). https://doi.org/10.1186/s13765-022-00743-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00743-5