Abstract
Synucleinopathies are a group of neurodegenerative disorders characterized by pathologic aggregates of neural and glial α-synuclein (α-syn) in the form of Lewy bodies (LBs), Lewy neurites, and cytoplasmic inclusions in both neurons and glia. Two major classes of synucleinopathies are LB disease and multiple system atrophy. LB diseases include Parkinson’s disease (PD), PD with dementia, and dementia with LBs. All are increasing in prevalence. Effective diagnostics, disease-modifying therapies, and therapeutic monitoring are urgently needed. Diagnostics capable of differentiating LB diseases are based on signs and symptoms which might overlap. To date, no specific diagnostic test exists despite disease-specific pathologies. Diagnostics are aided by brain imaging and cerebrospinal fluid evaluations, but more accessible biomarkers remain in need. Mechanisms of α-syn evolution to pathologic oligomers and insoluble fibrils can provide one of a spectrum of biomarkers to link complex neural pathways to effective therapies. With these in mind, we review promising biomarkers linked to effective disease-modifying interventions.
Similar content being viewed by others
Introduction
Synucleinopathies are neurodegenerative diseases that share the presence of α-synuclein (α-syn) aggregates in neurons and glia, and are found as Lewy bodies (LBs), Lewy neurites (LNs), and neuronal and glial cytoplasmic inclusions [1]. The disease spectrum includes Parkinson’s disease (PD), PD dementia (PDD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA) [2]. The three main α-synucleinopathies are PD, DLB, and MSA [3]. The most common α-synucleinopathy is PD [4], while the others are less common, differential disease diagnoses among them are clouded. This is made ever more difficult as PD affects up to 2% of the population above 60 years of age. Yearly, 90,000 people in the United States are newly diagnosed with PD to yield a prevalence of 10 million people worldwide [5, 6]. Globally, PD-associated disability and death are rising faster than other neurological disorders. In the past quarter century, PD prevalence has doubled and continued to rise with global population aging. According to the World Health Organization (WHO), more than 8.5 million individuals were afflicted with PD. In 2019, PD has resulted in 5.8 million disability-adjusted life years (DALYs); an 81% increase since 2000 [7]. Most concerning rests in epidemiological studies which indicate that up to 40% of PD patients also have dementia in which disease incidence rates are increasing 4–6 times compared to those without PD [8,9,10,11]. At least 75% of patients who live with PD for more than 10 years will develop dementia [12, 13].
Lewy body dementia (LBD) describes neurodegenerative disorders characterized by the pathological aggregation of α-syn into LBs in the brain [14]. Two well-known subtypes of LBD include DLB and PDD. While DLB and PDD clinical pathologies overlap, they are differentiated by the chronology of symptoms [15]. Patients diagnosed with Parkinsonism prior to the development of cognitive impairments are classified as having PDD while those who develop cognitive impairment prior to or within 1 year of Parkinsonism are classified as having DLB [16]. DLB is the second most common form of dementia after Alzheimer’s disease (AD) and is 20% of the total case numbers [17]. PDD is a second type of DLB, but both have unique disease courses [18]. The incidence and prevalence of PDD and DLB vary greatly in clinical studies and population-based cohorts [19]. In Minnesota alone, from 1991 to 2005, the incidence rate of DLB and PDD was 31.6 and 23, respectively, per 100,000 person-years [20]. Other studies reviewing LBD subtypes in the United States showed 0.02% and 4.4–5.4% amongst Floridians and all dementia cases in Medicare beneficiaries, respectively [21, 22]. A recent study showed that in the United States from 2010 to 2016, the incidence and prevalence of LBD among Medicare beneficiaries ranged from 0.18–0.21% to 0.83–0.9%, respectively. The costs of treating LBD were $18,309 for the pre-diagnosis and $29,174 and $22,814 at years 1 and 5 after diagnosis [23]. Comparisons between studies are challenging because of divergent study designs, patient populations, and disease time course.
The accurate diagnosis of these synucleinopathies and timely and cost-effective follow-ups as well as therapeutic response monitoring represent great needs. These include monitoring of treatment regimens and facilitating patients’ enrollment into clinical studies. Moreover, misdiagnosis can lead to suboptimal treatment, unnecessary care, and costly follow-ups to confirm diagnoses [24, 25]. The poor diagnostic accuracy for synucleinopathies in the absence of pathology-specific biomarkers increases the risks of confounding clinical trial inclusions and accuracies. Accurate, easily accessible, and cost-effective diagnostic biomarkers permit accurate clinical use for evaluating disease progression and disease-modifying therapies (DMTs). Optimal time windows exist during disease progression when DMTs may be most effective. Targeting key pathological hallmarks, such as α-syn misfolding and aggregation in synucleinopathies, will likely be a first step toward more effective therapies with improved and earlier interventional modalities before disease continues toward irreversible neurodegeneration [26, 27]. Therefore, biomarkers detecting disease pathology before the onset of disabling symptoms are needed.
This review is divided into three main sections reflective of the scenario occurring in the medical and pharmaceutical fields. Herein, we summarize the traditional signs and symptoms used in the clinic to establish a LB diagnosis. Next, the underlying disease mechanisms are presented, followed by prospects for improving diagnostics and treatment interventions in the future. These needs are discussed in context of biomarkers available for diagnostic and prognostic purposes. Last, we present more translational biomarkers that track disease and therapeutic responses.
Current available diagnostics
Some neurodegenerative diseases are characterized by abnormal aggregation and accumulation of pathologically altered proteins that are specific to disease groups and are typically designated as proteinopathies. Simultaneously, they involve dysfunctions of a range of cellular mechanisms, including mitochondrial or lysosomal dysfunction, oxidative stress, and inflammation caused primarily by glial cell activation that ultimately results in neuronal degeneration [28]. These mechanisms are common for several neurodegenerative diseases and may vary with degrees of neurodegeneration.
Neurodegenerative diseases employ multiple categories of diagnostics such as clinical signs and symptoms, neuroimaging acquisitions, genetic markers, and biological and biochemical indicators in cerebrospinal fluid (CSF), blood, or tissues (Fig. 1). In addition to transcriptomic studies, other “omic” technologies such as proteomics and metabolomics, both of which study functional molecules (proteins and neurotransmitter metabolites) that are potentially involved in neurodegenerative processes, are increasingly being used [29, 30]. A main objective is to uncover a signature profile, i.e., combinations of biomarkers specific to a specific disease or a group of diseases that share common pathologies or processes.
LB disease signs, symptoms, diagnostics, and disease pathobiology. Disease signs and symptoms: Motor and non-motor symptoms are both included as operative processes leading to significant disability. Motor symptoms include bradykinesia, tremors, rigidity, and postural instability. The non-motor sings include, but are not limited to, depression, anxiety, hyposmia, and constipation. Neuroimaging methods: Tomographic acquisitions for neurodegenerative diseases include positron emission tomography (PET) scanning using 18F-fluorodeoxyglucose (18F-FDG) and 11C-IMA107 for DLB [31] and PD [32]. Pathology: The pathologies of synucleinopathies such as PD [33] and DLB [34] show depigmentation in the midbrain substantia nigra, Lewy neurites, and Lewy bodies (black arrows). The figure was created with BioRender.com. Images taken from publications or web pages were referenced in the figure caption
Clinical signs and symptoms
For all synucleinopathies, α-syn accumulation in LBs with associated loss of substantia nigra pars compacta (SNpc) dopaminergic neurons and dopamine neurotransmitter heralds motor and non-motor symptoms. These are significant sources of each patient’s clinical disabilities. Cardinal motor symptoms of PD include bradykinesia, tremors, rigidity, and postural instability. Bradykinesia presents as slowness of movement interrupted by halts in movement [35]. Another indicator for PD is a unilateral tremor that occurs while the afflicted limb is at rest and can manifest in legs, lips, and chin [36]. Rigidity in the flexion and extension of limbs is another characteristic of PD. Posture instability is due to the inability of maintaining equilibrium in a static state, such as sitting or standing, or instability in the transition from static state to a moving state [37]. Two forms of PD can be distinguished clinically with tremor or axial form depending on the predominance of symptoms. Motor symptoms begin insidiously and progress gradually over time [38]. In addition, the spectrum of symptoms for PD has expanded to non-motor symptoms, which often precede the onset of motor symptoms by 5–10 years [39]. These non-motor symptoms can range from mood changes to alterations in sleep habits [40]. PD patients have also experienced olfactory dysfunctions [41]. In a longitudinal study, PD patients with higher United Parkinson’s Disease Rating Scale (UPDRS) scores showed higher levels of olfactory dysfunction compared to PD patients who had lower UPDRS scores [42]. Rapid-eye-movement (REM) sleep behavior disorder (RBD) has also been seen as a pre-clinical indicator for PD [43]. Constipation is also associated with PD, which underscores the importance of the gut-brain axis due to the hypothesis that PD etiology may originate in the gut and migrate to the brain [44].
One of the most common disabling non-motor features in PD is dementia [45]. PDD and DLB are two disorders which encompass Parkinsonian-related dementias. The temporal onset of dementia is a major discriminating factor between PDD and DLB and it is termed the “1-year rule”. In DLB, onset of cognitive decline precedes presentation of PD symptoms by 1 year or less. While in PDD, cognitive decline typically develops within 1 year of PD diagnosis [46]. Thus, the temporal onset of dementia and Parkinsonism for PD, PDD, and DLB could provide beneficial importance for the differential diagnoses of those disorders (Table 1). Additional clinical factors have been associated with PDD such as older age of PD onset, male sex preponderance, hallucinations, increased severity of motor symptoms and bradykinesia, akinetic-dominant Parkinsonism, axial impairment, and depression [13, 47, 48]. Further, RBD, posterior-cortical dysfunction, cardiovascular autonomic dysfunction, color discrimination ability, and gait dysfunction are strong predictors of development of PDD in PD patients [49, 50], whereas in DLB, cognitive decline is not accompanied by PD symptoms and typically precedes PD diagnosis [46]. The cardinal features of DLB include dementia, fluctuating cognition, visual hallucinations, RBD, and Parkinsonism [16]. Motor symptoms may be absent in up to 25% of autopsy confirmed DLB patients [51]. While DLB patients share many symptoms with PD, cognitive impairment including deficits in attention, executive function, and visuospatial ability, precede the onset of motor symptoms [18, 52]. A consensus for differentiating LB diseases were developed from four studies of a DLB consortium from 1996 to 2017 [16], and established a set of criteria for diagnosing PDD and DLB [53]. Dementia associated with DLB or PDD is defined as a progressive cognitive impairment that interferes with daily life activities and normal work-related and social occupations [54]. Screening patients for dementia should be performed according to the Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and neurophysiological assessments and combined for initial diagnosis [55]. Reviews elsewhere provide criteria for PDD diagnosis [56, 57], and revised criteria according to DLB Consortium for DLB diagnosis [16, 58].
Neuroimaging
Intensive research using neuroimaging techniques allowed assessment of structural and functional alterations of neurodegenerative patients and enhanced the criteria for differential diagnoses for DLB, PD, PDD, and AD [59]. In PD, different imaging techniques can verify the validity of a PD diagnosis from the motor and non-motor symptoms. Magnetic resonance imaging (MRI) scans provide deeper insight into the progression of PD and differentiate PD cases from other neurodegenerative diseases. The onset of PD leads not only to neuronal changes within the brain, but also large-scale physical changes such as atrophy in many cortical and subcortical areas along with overall decreases in brain volume [60] and increased frontal lobe volumes [61]. Structural MRI, including T1- and T2-weighted imaging, is used to study patterns of brain atrophy as an estimate of regional neurodegeneration. MRI measures of gray matter volumes and cortical thickness often exhibit low intra-individual variability over time, therefore small differences in atrophy rates can be detected in longitudinal clinical trials [62]. With measurement of other structural characteristics associated with PD, MRI provides an instrument capable of capturing small spatial differences within strictly defined areas of the brain. Moreover, thin section T2 and proton density spin echo images have been able to discriminate between PD and MSA with 88% sensitivity and 89% specificity [63]. Another MIR focus is iron mapping. Iron content is known to be regionally increased in the substantia nigra of PD patients compared to normal healthy controls [64]. This gives us the ability to create a reference for diseased brains compared to normal brains. A meta-analysis study showed that MRI measures of iron content in PD patients across multiple studies provided a reliable marker for PD that correlated with the severity of motor symptoms [65]. Unfortunately, information about loss of cell populations or cellular structures is not yet possible with MRI. However, for several neurodegenerative diseases, the blood–brain barrier (BBB) integrity is disrupted and can be evaluated by certain MRI techniques, including dynamic contrast-enhanced and dynamic susceptibility contrast MRI, which depend on a more permeable BBB and leakage of gadolinium-based contrast agents into the brain [66].
Many avenues have assessed specific synaptic connections between the dopaminergic neurons for PD diagnosis and progression [67]. Loss of the dopamine transporter (DAT) in the substantia nigra has gained much attention as a possible biomarker for PD. Positron emission tomography (PET) imaging can be used to determine the density of dopaminergic nerve terminals in the basal ganglia which is typically reduced in PD, MSA, progressive supranuclear palsy (PSP), and corticobasal degeneration [68]. Recently, PET ligands binding to the synaptic vesicle protein 2A (SV2A) were found to detect regionally decreased synaptic density in PD and AD [69, 70]. 18F-FDG-PET has been used for diagnostic workup of several neurodegenerative diseases, in part, to certain patterns of regional hypometabolism. For example, frontal and anterior temporal hypometabolism are typically observed in the behavioral variant of frontotemporal dementia (FTD) [71]. 11C-dihydrotetrabenazine (11C-DTBZ) is another PET ligand for vesicular monoamine type 2 transporter (VMAT2) within presynaptic dopaminergic neurons. 11C-DTBZ PET imaging with correlational tractography showed diminished nigrostriatal tract integrity with lower 11C-DTBZ distribution in PD patients, which suggested nigrostriatal axonal dysfunction [72]. These imaging methods are often used in studies to evaluate the potential neuroprotective effects of treatments on dopaminergic neurons. Another popular imaging probe is 123I-ioflupane detected by single-photon emission computed tomography (SPECT). The combination is known as dopamine transporter scan (DaTscan) [32]. This type of imaging allows better differentiation between PD and other types of Parkinsonism. Alternative nuclear medicine techniques have also been applied along with subsequent radiolabeled tracers. One such example is the 18F-FE-PE2I PET imaging, which has proven comparable sensitivity to mainstream 123I-ioflupane SPECT imaging [73, 74].
Another promising MRI method is diffusion tensor imaging (DTI) which is used for measuring fractional anisotropy and microstructural indices of brain white matter in different neurodegenerative diseases [75]. Lower than normal fractional anisotropy and higher than normal diffusivity is associated with loss of microstructural integrity and neurodegeneration. Previous DTI studies in PD demonstrated abnormal fractional anisotropy in multiple white matter regions as well as in the dopaminergic nuclei [76]. DTI has also shown promise in discerning PD patients from healthy subjects. In a meta-analysis of 39 studies, mean diffusivity and fractional anisotropy found differences in the substantia nigra, corpus callosum, and cingulate and temporal cortices of PD patients [77]. In addition, DTI has been used to differentiate PD patients from PDD patients by assessing microstructural changes in the nucleus basalis of Meynert (NBM). The NBM of PDD patients revealed increased mean diffusivity and reduced gray matter volume compared to PD patients without cognitive impairment. This microstructural change in the NBM was shown to precede gray matter volumetric loss suggesting an early biomarker for PDD [78]. Furthermore, in PDD, the combination of DTI with resting state functional MRI (fMRI) showed diminished functional connectivity of the posterior cingulate-right medial temporal lobe as well as microstructural damage to the left hippocampus [79]. This suggests that using a combination of imaging techniques could provide predictive markers of PDD. Other imaging studies have found that cortical thinning of the frontal, right precentral, and anterior cingulate cortex in combination with gray matter atrophy are predictive of cognitive decline in PD [80, 81].
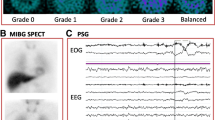
123Iodine-meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy is another imaging tool used initially to assess sympathetic denervation, density, and function in organs richly innervated by sympathetic nervous system such as the postganglionic presynaptic of the cardiac sympathetic nerve endings. The radiolabeled 123I-MIBG (a norepinephrine analogue) is taken up by postganglionic postsynaptic nerve endings and its uptake was found to be correlated to adrenergic innervation and integrity of substantia nigra neurons [82]. 123I-MIBG was found to differentiate between DLB and AD and predict the prognosis of DLB, due to the correlation of postganglionic neurons associated with DLB. The technique was included in DLB consortium criteria as an indicative biomarker due to the high sensitivity to discriminate between DLB and AD via reduction of cardiac uptake of 123I-MIBG by DLB patients, but not AD patients [83].
Disease pathobiology for diagnostic/therapeutic development
Genetics
Research performed in the past two decades identified multiple autosomal recessive and dominant genes associated with familial PD. Duplicate or triplicate mutations in α-syn gene (SNCA) cause dominant inherited forms of PD [84, 85]. Additionally, genome-wide association studies (GWAS) have uncovered variations in at least two of the familial PD genes (SNCA and leucine-rich repeat kinase 2; LRRK2). These have proven to be significant risk factors for sporadic PD [86,87,88,89]. Missense mutations in SNCA were identified in familial PD (A53T, A30P, E46K, and H50Q) [90, 91] as well as in sporadic PD (A18T and A29S) [92]. Six mutations in LRRK2 were identified as disease-causing: G2019S, R1441C/G/H, Y1699C, and I2020T in familial PD [93, 94]. G2019S and R1441C mutations are responsible for up to 30% of familial PD in select populations, and up to 10% and 2.5%, respectively, in sporadic PD [95, 96]. Moreover, studies identified associations between PD and both PARK-designated genes (SNCA, PRKN, UCHL1, PINK1, DJ-1, LRRK2, ATP13A2, GIGYF2, HTRA2, PLA2G6, FBX07, VPS35, EIF4G1, DNAJC6, SYNJ1, DNAJC13, and VPS13C) and non-PARK-designated genes (BST1, CCDCC2/HIP1R, DGKQ/GAK, GBAMAPT, MCCC1/LAMP3, STK39, SYT11/ RAB25, GAK, MAPT, GBA, NAT2, INOS2A, GAK, HLA-DRA, and APOE) [97].
Associations between impaired protein and mitochondrial homeostasis and the development and progression of PD were shown. This notably includes oxidative stress acting as an important link between the pathogenic events. In addition to abnormal protein overproduction and aggregation, impaired degradation pathways, such as lysosomal dysfunctions and autophagy contribute to PD [98, 99]. Autophagy serves to remove aggregated misfolded proteins and dysfunctional organelles to clear pathologic components and prevent toxicity and subsequent cell death. Multiple studies suggest that aggregation of α-syn is consequent to dysfunction of the autophagic-lysosomal system. α-Syn also affects mitochondrial, lysosomal, and autophagic functions [100,101,102]. Dopaminergic neurons are metabolically active and need high mitochondrial energy demand, and therefore are exposed to insufficient clearance of damaged mitochondria [103]. Accumulation of defective mitochondria will increase levels of reactive oxygen species (ROS), which damage surrounding healthy mitochondria and accelerate disease progression. Overall, elevated α-syn concentrations from either overproduction or reduced clearance, lead to α-syn aggregations and neurotoxicity. Therefore, lowering α-syn levels reduces oligomerization, aggregation, and deposition into LBs. This can result in a beneficial disease-modifying effect for synucleinopathies.
Recently, GWAS of a cohort of 2,591 patients diagnosed with DLB and 4,027 healthy controls from across 17 European and 27 North American sites resulted in identification of the highest independent five loci risks (SNCA-AS1, GBA, APOE, B1N1, and TMEM 175) [104]. GWAS and co-localization identified two loci risks, SNCA and SNCA antisense RNA 1 (SNCA-AS1), a non-coding RNA playing a role in regulating expression of α-syn. Transcriptomic studies showed overexpression of both genes. These works illustrated the impact on synaptogenesis and the role of SNCA-AS1 in cellular senescence and PD-related pathologies [104, 105]. Due to the overlap between α-syn, tau, and amyloid beta (Aβ) pathologies in DLB and PDD, an opportunity presents to compare genotypes with prognostic and therapeutic values between AD, PD, PDD, and DLB. Glucocerebrosidase (GBA) mutations are critical loci in DLB pathogenesis, and GBA gene variants were the first genetic risk factor identified for PD [106]. GBA encodes for the lysosomal hydrolase enzyme β-glucocerebrosidase (GCase) which catalyzes the hydrolysis of glucocerebroside to ceremide and glucose. This leads to increased GCase misfolding, endoplasmic reticulum (ER) trapping, and induction of both ER stress and ubiquitin proteasome (UPS) systems. This triggers ER associated degradation (ERAD) and unfolded protein response (UPR). Eventually, sustained activation of ERAD and UPR will increase apoptosis. The presence of misfolded GCase in lysosomes leads to accumulation and aggregation of α-syn. This results in decreasing α-syn degradation through chaperon mediated autophagy (CMA). GBA mutations also affect mitochondrial dysfunction by increasing ROS and decreasing ATP production and oxygen consumption [107].
Apolipoprotein E glycoprotein (APOE) maintains cholesterol hemostasis through facilitating transfer of phospholipids and cholesterol amongst cells. In the central nervous system (CNS), APOE is produced by astrocytes more than by microglia. This explains APOE upregulation in neurodegenerative diseases and its association with neuroinflammation. The process proceeds through activation of astrocytes and microglia. APOE lipidation mediates Aβ clearance through the astrocyte ATP-binding cassette A1 (ABCA1) cascade and inhibits Aβ plaque formation. One potential mechanism of Aβ plaque formation involves APOE protein overexpression, susceptibility to mutation, and accumulation of acids and triglycerides, which form complexes that bind Aβ aggregates to form Aβ plaques. Different APOE isoforms (APOE1, 2, 3, and 4) are present. APOE4 is associated with DLB and PDD pathologies [108]. In a cohort study of 100 PD patients, associations were made between APOE4 allele carriers and a higher risk of early dementia [109]. It is unknown whether APOE4 contributes directly to or is dependent on Aβ pathology that leads to α-syn accumulations. A prior study of APOE4 in an adeno-associated virus (AAV)-α-syn-overexpressing mouse model demonstrated increased neurodegenerative and behavior deficits, and neuroinflammatory responses which were independent from Aβ pathology compared to mice which expressed other APOE variants [108]. These results were supported by human postmortem brain examinations obtained from DLB with AD pathology. These examinations showed increased α-syn pathology in APOE4 carriers compared to non-carriers [108]. Certainly, additional studies are warranted to confirm the role of APOE and interacting proteins in neurodegenerative processes. Recent investigations demonstrate that the bridging integrator 1 protein (BIN1) is linked to endosomal trafficking leading to tau pathology. Meta-analysis shows positive association between BIN1 and the APOE4 carrier allele [110, 111].
Defective lysosomal acidification is associated with several neurodegenerative disorders [112]. The optimization of pH is determined by influx and efflux proton pump dynamics. Vacuolar-type H+-ATPase (V-ATPase) is a known proton pump with influx activity [113], while transmembrane protein 175 (TMEM175) is an endolysosomal potassium channel that is a selective permeable efflux H+ proton pump when the luminal domain faces an acidic pH environment, and functionally balances the effect of V-ATPase. The role of TMEM175 in PD pathology is somewhat controversial as to whether its depletion or overexpression is pathological in PD. Studies showed that TMEM175 deficiency in PD is associated with increased loss of dopaminergic neurons and deposition of α-syn aggregates [114, 115]. However, other studies showed that TMEM175 activity is correlated with Bcl-2 apoptosis regulator factor which plays a role in mitophagy. Upon binding to Bcl-2, TMEM175 is activated and induces ROS in a TMEM175-ROS positive feedback loop manner and exacerbates dopaminergic neurons loss and motor dysfunction in PD animal models [116]. Several studies investigated which alleles of TMEM175 are associated with synucleinopathies. As in the GWAS mentioned earlier, TMEM175 rs6599388-T was shown to be a risk allele for DLB [104]. A recent genomic analysis study in Italy included 400 PD patients and 300 healthy controls, showed a strong correlation between TMEM175 variant rs2290402 allele and PD pathology [117]. Taken together, more studies are required to illustrate how mutations and variants of TMEM175 and pH changes, affect synucleinopathy etiology and progression as well as explain the dichotomous discrepancies in TMEM175 expression and PD pathology.
Misfolded proteins
One mechanism common to several neurodegenerative diseases is the overproduction of aberrant proteins that has the potential to be harnessed for diagnostic benefit. The proteins linked to disease evolve, aggregate, and then accumulate as intra- and extra-cellular bodies. Collectively, they facilitate neuronal death in afflicted brain locations [118]. In each of the synucleinopathies, misfolded and accumulated α-syn proteins, presenting as fibrils and LBs, represent characteristic hallmarks of dopaminergic neurodegeneration as it occurs in the SNpc [119, 120]. Encoded by the SNCA gene, α-syn is a presynaptic protein in neurons. α-Syn is a small acidic protein expressed in the CNS, peripheral nervous system (PNS), blood, and other tissues [121]. α-Syn is natively unfolded monomer, however it is found naturally, in large part, as a folded tetramer of 58 KDa with little or no amyloid-like aggregation potential [122]. Monomer and tetramer forms exist together, but an unbalanced tetramer:monomer ratio leads to the predominance of pro-aggregating forms. α-Syn was found to have three main regions; each region has different molecular and biological properties [123]. The N-terminus, amino acid residues 1–60, is characterized by amphipathic repetitions which form an α-helix structure. This region of the protein controls the interaction of α-syn to the membranes [124]. The non-Aβ component (NAC region), amino acid residues 61–95, is the most aggregation-prone region. The C-terminus, amino acid residues 96–140, is involved in Ca2+ binding and chaperone-like activity [125]. It was found that binding of Ca2+ to the C terminus of α-syn also regulates binding to synaptic membranes [126]. The exact physiological functions of α-syn are not fully known, but most likely play a role in synaptic vesicle release. This is reflected by α-syn localization to the nerve terminal where neurotransmitter release is inhibited with α-syn blockage or deletion. In addition, α-syn aggregates localize more in brain stem and substantia nigra [127, 128]. In synucleinopathies, α-syn has a pathological β-sheet conformation that allows monomers to form oligomers and amyloid fibrils. α-Syn aggregates into LBs which localize in the neuron soma or into LNs in axons [123, 126]. While LBs are themselves not toxic, aggregates of α-syn are passed between neurons facilitating disease spread amongst adjacent brain regions [127]. During disease, α-syn filaments accumulate in amygdala and striatum forming LNs which inhibit axonal transport and reduce neuronal function and survival [129]. The localization of prion-like aggregates of α-syn is different among DLB and PD. In PD, α-syn aggregates localize in the brain stem and substantia nigra, while in DLB α-syn aggregates are diffuse throughout the brain [128]. In PD, LBs and LNs are in the mesencephalon, while in the cerebral cortex of DLB brain tissues [130].
DLB and PDD are heterogeneous disorders with overlapping clinical features amongst PD and AD, which complicate distinguishing between PDD and DLB as they share considerable clinical features [51]. Differences are seen solely in postmortem analyses and in part for imaging. Investigations in PD patients show that LB pathology is restricted to the brain stem and limbic subregions, while in PDD and DLB, LB pathology extends to the neocortex [131]. Striatal α-syn is detected in PD and PDD, but less in DLB. The dopaminergic loss in SN is higher in PD and PDD than in DLB. In contrast to DLB, more LB accumulates in neocortical and limbic regions in PDD where the temporal lobe and CA2 region of hippocampus are disease targets for PDD [132]. More postmortem studies to confirm differences in α-syn aggregation between the synucleinopathies remain in need. Postmortem studies also show that striatal Aβ loads are equivalent between AD and DLB. Aβ levels are higher in DLB than in PDD [18, 133]. Higher Aβ burdens are found in cortical and subcortical regions in postmortem samples of DLB patients than in PDD [18, 132, 133]. Hyperphosphorylated tau and Aβ, additional AD pathological hallmarks, are known to contribute to cognitive decline in PDD and DLB. DLB patients show advanced AD pathology compared to PDD patients, while patients with AD pathology are less likely to present with DLB clinical symptoms, even if diffuse LBs exist in the cortex [134]. Additionally, DLB- and PDD-associated cholinergic neuronal loss correlate better with cognitive decline. These findings affirm that cholinesterase inhibitors can provide improvement in cognitive function in DLB and PDD [135,136,137].
Immunity
Neuroinflammation in neurodegenerative disorders was reported in the early 1980’s, and has been confirmed by several subsequent studies showing the link between neuroinflammation and PD pathogenesis which includes increased pro-inflammatory cytokines in blood and CNS [138, 139]. Interleukin-1beta (IL-1β), an inflammatory cytokine, is part of the larger IL-1 family and plays an important role in controlling many innate immune responses [140]. IL-1β was found to increase dopaminergic neuron damage within substantia nigra in an adenoviral IL-1β expression vector model [141]. In a clinical trial, levels of serum IL-1β, IL-6, and IL-1 receptor antagonist (IL-1Ra) were significantly elevated in PD patients [142]. Additionally, in another study, IL-1β was found to be predictive of disease progression in PD [143]. Serum IL-6 has also been found to have a negative correlation with the Activities of Daily Living (ADL) scale, which contributes to the severity of disease [138]. Another pro-inflammatory cytokine of interest in PD pathogenesis is tumor necrosis factor-alpha (TNF-α) which is a crucial component of microglia-derived inflammatory responses. Serum TNF-α has been found to be increased in PD patients compared to controls and is positively correlated with UPDRS Part III scores [144]. In another study TNF-α levels in tears of PD patients were higher compared to healthy controls [145]. C-C motif chemokine ligand 5 (CCL5) has also been found to be associated with the severity and length of PD [146]. One area of interest is the association between gut inflammation and PD as many PD patients present intestinal maladies either prior to or during motor dysfunction. Congruent with those observations were increased expression of TNF-α, interferon-gamma (IFN-γ), IL-6, and IL-1β genes in ascending colons of PD patients compared to age matched healthy controls [147].
Several reports documented the activation of both innate and adaptive immune systems in synucleinopathies [148,149,150]. Evidence implicates misfolded α-syn itself as a main trigger of immune responses and a potent inducer of an inflammatory environment, which leads to neurodegeneration. In PD, α-syn aggregation occurs in the neurons of the SNpc in the CNS and those in the PNS [149]. Microglia are the resident immune cells in the brain and function as the primary contributor to innate immunity in the CNS [151]. Activated microglia surrounding degenerative dopaminergic neurons in the SNpc were found in PD brains [152]. The degree of microglial activation was shown by CD68 and major histocompatibility complex class II (MHC-II) staining [153]. Activation of innate immunity leads to production of pro-inflammatory cytokines. These include TNF-α, IL-1β, IL-6, and IFN-γ as well as production of chemokines and activation of the complement system. Although microglia are morphologically and functionally like circulating monocytes and tissue macrophages, they originate from a different lineage in the yolk sac and migrate into the brain during early development [154, 155]. A mixture of resident brain microglia and infiltrating peripheral monocytes exert different effects in PD pathogenesis. Cytokine and chemokine production are also upregulated by peripheral blood mononuclear cells in PD patients. Levels of chemokine CC-motif ligand 3 (CCL3), CCL5, IFN-γ, monocyte chemoattractant protein-1 (MCP-1 or CCL2), IL-1β, IL-8, and TNF-α in PBMCs at baseline or stimulated with lipopolysaccharide were found to correlate with motor function assessed by UPDRS Part III and Hoehn and Yahr (H&Y) stage [156]. Additionally, complement C1q was found to be associated with activated microglia surrounding degenerating neurons [157], with C3d, C4d, C7, and C9 components found co-localized with α-syn aggregations and degenerating neurons in PD autopsies [158]. Moreover, microglia can phagocytose extracellular aggregated α-syn from their environment and target it to light chain 3B (LC3B) immunoreactive autophagosomes for degradation, leading to induction of downstream nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-dependent signaling cascades including those facilitating chemokine production [159]. Oxidative stress and upregulation of ROS were also observed in response to phagocytosis of aggregated α-syn in rat primary microglia [160]. Although microglia treated with aggregated α-syn increased MHC-II expression and antigen processing, a robust MHC-II-dependent cytokine response to aggregated α-syn was only induced in primary microglia by co-culturing them with T cells [161]. Furthermore, pathway analysis in two independent GWAS reports uncovered significant associations between PD diagnosis and SNPs in pathways encoding cytokine signaling and regulation of leukocyte/lymphocyte activity [162], and indicated that modified α-syn led to microglial or monocytic activation with production of pro-inflammatory cytokines. This shows the interplay between innate and adaptive immunity in synucleinopathies. In parallel, distribution of toll-like receptor (TLR) is affected in response to α-syn. TLR-2 was found to be upregulated, while TLR-3 and TLR-7 were downregulated in microglia pretreated with wild-type oligomeric α-syn [163].
The involvement of adaptive immunity in PD pathogenesis is explicit with certain evidence of the ability of modified α-syn forms to modulate adaptive responses in PD animal models. Several studies showed increased numbers of T cells in both α-syn and toxin animal models of PD with their involvement in the neurodegeneration [164,165,166]. In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, adoptive transfer of T cells from mice immunized to the nitrated C-terminus of α-syn to mice administered MPTP leads to increased neurodegeneration [167]. In addition, with the polarization of T cells responsive to the nitrated C-terminus of α-syn to the Th1, Th2, and Th17 subtypes before adoptive transfer, both pro-inflammatory Th1 and Th17 cells were found to increase neurodegeneration in response to MPTP. Th17 cells showed a greater toxic effect than Th1 cells, while Th2 cells had no effect [168]. Similarly, in passive transfer studies into Rag1−/− mice, CD4+ T cells acted in a FasL-dependent, IFN-γ-independent manner to mediate MPTP neurotoxicity. In mice treated with MPTP, dopaminergic neurodegeneration was attenuated in CD4−/− animals, while neurodegeneration was unaffected in CD8a−/− animals [169]. In addition, overexpression of α-syn in AAV constructs in rat brains leads to dopaminergic neuronal loss with increased infiltration of CD4+ and CD8+ T cells within the substantia nigra 8 weeks post-injection [170].
Two subsets of functional T cells include effector T cells (Teffs) and regulatory T cells (Tregs). Functions of both subsets are maintained during homeostatic conditions to balance defense against infectious or neoplastic diseases (Teffs) and maintenance of immunological tolerance with control of overactive immune responses (Tregs). However, in neurodegenerative diseases, Teffs can recognize disease-specific modified self-proteins from oxidative stress and misfolding. These are presented by α-syn proteins in synucleinopathies, and Aβ and tau proteins in AD, which can break immune tolerance with expansion of self-reactive T cells [171, 172]. In synucleinopathies, Tregs have been found to have impaired immunosuppressive functions, whereby Teffs with neurotoxic effects are uncontrolled and expanded [173,174,175]. Studies from our own group showed that increased Teff phenotypes are associated with worsened UPDRS Part III scores and movement disorders in PD patients [176]. In addition, our research group, along with others, demonstrated that Tregs attenuate neuroinflammation and protect dopaminergic neurons from injury and loss in MPTP animal model [177,178,179]. Translationally, we have shown that strategies to increase Treg numbers or functions can modulate neuronal output and motor activity with improvement of UPDRS Part III scores in PD patients [180, 181]. Thus, modulating peripheral T cells represents viable therapeutic strategies for different neurodegenerative diseases that may express various underlying mechanisms for which these strategies are operative. Different underlying disease mechanisms contributing to synucleinopathies are summarized below (Fig. 2).
Pathogenesis of synucleinopathies. LB is mainly formed in neurons, and it is composed of misfolded, fibrillar α-syn (α-synuclein). Different genes are associated with synucleinopathies, mutations of these genes induce α-syn aggregation, mitochondrial dysfunction, autophagy dysregulation, oxidative stress, and inflammation (Gene dysregulation). Immune cells in the periphery contribute to the pathogenesis of synucleinopathies. Innate and adaptive immune cells (monocytes and lymphocytes, respectively) migrate into the brain. Activated macrophages release pro-inflammatory cytokines (Immunity). This results in generalized microglia and astrocyte activation which leads to neuronal damage (Immunity and neuroinflammation). Abbreviations: LB; Lewy body, BBB: blood–brain barrier, CNS: central nervous system. The figure was created with BioRender.com
Biomarkers
Biomarkers are measurable indicators that serve to describe normal biological processes, pathological processes, and pharmacological responses to therapeutic interventions. The main goal of using biomarkers for neurodegenerative disorders is the improvement of clinical diagnosis which increases the accuracy of differential diagnosis between different neurodegenerative diseases. For early diagnosis, the ideal biomarker should be sensitive and specific for early disease changes that discriminate between disease state and changes due to normal aging. In addition, biomarkers help in estimating disease stage and progression as well as reflecting therapeutic responses as either change in diagnostic biomarkers or responses from therapy [118]. The revelation of new biomarkers that signal positive therapeutic effects not normally assessed in diagnostic biomarkers, could import beneficial information for clinical studies. Indeed, therapeutic biomarkers assessed as products from DMTs in early phase 1 and 2 studies are generally more likely to show clinical effects in subsequent large-scale trials [182]. In addition, development of pharmacodynamic biomarkers that can identify relevant drug targets in vivo are crucial [25]. Currently, new techniques that incorporate transcriptomics, proteomics, and metabolomics, and can be comprehensively analyzed by bioinformatics are able to uncover unique candidates for biomarker analysis and validation (Fig. 3). Neurodegenerative diseases are characterized by the interaction of multiple molecular pathways that can best be evaluated from body fluids such as CSF and blood. CSF is a most useful biological fluid as it directly reflects biochemical processes and changes within the CNS and enables early pre-clinical diagnosis when CNS biomarkers are revealed [69]. However, monitoring biomarkers for neurodegenerative diseases would be more advantageous with a more accessible and less invasive option such as blood, which communicates with the brain via the hematoencephalic barrier, lymphatic vessels, and glymphatic system [118]. Nonetheless, analyzing blood has some limitations such as whether low levels of CNS biomarkers would be even lower in the blood and become undetectable in the periphery due to the substantial analyte dilution effect from the CSF: blood volume ratio. Another limitation is a biomarker that is not specific to the CNS and co-expressed in peripheral tissues, the contribution of the CNS could be potentially lost to the higher levels associated outside the CNS. A third limitation is possible analytical interference of blood proteins such as albumins, globulins, and transferrin [118]. Therefore, measuring biomarkers of neurodegenerative diseases in the blood would require sensitive and specific tests that are not confounded by blood or blood components. Some biomarkers have been established and are being used in clinical practice (such as α-syn, Aβ1-42, and tau) [30, 183, 184], while other biomarkers such as LRRK2, heme oxygenase-1 (HMOX1), TLR2, autophagy related 7 (ATG7), and GBA [185, 186] are undergoing methodological and analytical standardization, and yet confirmation of other biomarkers are expected in the future.
Biomarkers present or in development for neurodegenerative disorders. CSF and blood are the most common sources for samples collected for biomarker studies in neurodegenerative diseases. Samples collected from CSF and blood can be used for detecting misfolded protein by real-time quaking-induced conversion (RT-QuIC) (image [187]). Expression of genes dysregulated in neurodegenerative diseases can be assessed by different techniques such as microarrays, real-time polymerase chain reaction (qPCR), and single-cell RNA-sequencing (scRNA-seq) (Transcriptomics). Levels of proteins dysregulated in neurodegenerative diseases can be assessed by different techniques such as proteomic analysis, enzyme-linked immunosorbent assay (ELISA) (image [188]), and multiplex assay (image [189]) (Proteomics). Data from “omics” studies are processed by different bioinformatics tools generating heatmaps of altered signaling pathways, genes, and/or proteins as well as protein–protein interaction (PPI) networks (image [183]) (Bioinformatics). The figure was created with BioRender.com. Images taken from publications or web pages were referenced in the figure caption
LB disease-aggregated protein: α-syn
α-Syn oligomers have been found within exosomes of PD patients [190], which were upregulated in plasma exosomes from PD patients [191]. Exosomal transport and reuptake is hypothesized to be a mechanism of transferring toxic α-syn species between neurons in different synucleinopathies [192]. Thus, transmission of pathogenic oligomeric forms of α-syn from neurons to microglia is entirely plausible. Several neurodegenerative biomarkers have recently emerged. These biomarkers, which reflect types of accumulated pathological proteins, include α-syn in PD and other synuclein aggregation disorders. Several studies for biomarker identification and validation focused on the measurement of total α-syn species in CSF and blood [191, 193, 194]. In synucleinopathies, a decreased α-syn level in the CSF was observed [185, 194]. The attractiveness of α-syn as a biomarker is primarily due to its increased aggregation and accumulation in the CNS. The level of total α-syn in CSF can be used to differentiate synucleinopathies from other proteinopathies; however, it is not useful for differentiating between the different synucleinopathies, even though significantly lower concentrations have been observed in MSA than in PD and DLB [185]. Specific α-syn species, such as oligomeric α-syn, phosphorylated α-syn at residue Ser129, and pro-aggregated forms of α-syn, in CSF and blood have been considered as potential diagnostic biomarkers for synucleinopathies [184].
Real-time quaking-induced conversion (RT-QuIC) analysis can directly detect pathogenic proteins with prion-like properties and is very sensitive even in the early stages of diseases that express those type of proteins. This method has been used to detect α-syn misfolding in several different synucleinopathies [195]; however, technical complexity and cost/benefit concerns have precluded wider use of this ultrasensitive method. The concentration of oligomeric α-syn in CSF is generally higher in PD and DLB patients than in healthy controls. Moreover, higher levels of phosphorylated α-syn were observed in PD patients than in healthy controls, MSA patients, or PSP patients [30, 196]. While RT-QuIC of CSF samples are used to diagnose prion diseases, measurement of pro-aggregated α-syn has the potential to diagnose synucleinopathies in pre-symptomatic stages [197]. Using this method, α-syn aggregates in the CNS were detected in the CSF of RBD patients who later developed synucleinopathy. This technique was used to analyze isolated RBD (IRBD) individuals that also developed PD. The longitudinal study found that out of 52 IRBD patients, 47 had increased CSF α-syn compared to age-matched controls [198]. Additionally, it has been shown that RT-QuIC validated the presence of α-syn aggregates in CSF of PD and DLB patients with 92% and 95% sensitivity, respectively, and with 100% specificity compared to AD patients and healthy controls [199]. Interestingly, the properties of α-syn aggregates were different between PD/DLB and MSA patients suggesting that different conformational strains of α-syn exist as distinct species for each disease [200]. Different seeding aggregation assays show high concordance for the detection of misfolded CSF α-syn when evaluated in the same cohort, indicating high reproducibility among the assays [201]. Emerging evidence shows that α-syn seed amplification assay (SAA) of CSF has the potential to differentiate PD patients from healthy controls. In this study, 1,123 participants were enrolled from 33 participating academic neurology outpatient practices worldwide (Austria, Canada, France, Germany, Greece, Israel, Italy, Netherlands, Norway, Spain, UK, and USA) between July 7, 2010, and July 4, 2019. Sensitivity for PD was 87.7% (95% CI 84.9–90.5%), and specificity for healthy controls was 96.3% (93.4–99.2%). In addition, sensitivity of α-syn SAA in sporadic PD with typical olfactory deficit was 98.6% (96.4–99.4%) [202]. Another method that detects tandem α-syn and tau aggregates is surface-based fluorescence intensity distribution analysis (sFIDA). This technique features single-particle sensitivity through a microscopy-based readout [203] and uses linear epitopes to detect and count all subtypes of aggregated protein irrespective of higher-ordered structures, whereas other assays using structural epitopes can only determine subfractions of oligomers, fibrils, or other aggregates from a heterogeneous pool of structures [204]. A recent study showed that sFIDA could discriminate between α-syn from CSF of PD patients and DLB patients with a sensitivity of 73% and specificity of 65% [204]. Although α-syn can be reliably detected in CSF, with diminished levels in PD, DLB, and MSA, substantial overlap exists with healthy controls and other neurodegenerative diseases, thereby hindering its utility in clinical practice and trials [184].
Considering the invasive nature of CSF collection, detection of α-syn in less invasive and more easily accessible fluids and tissues would be a great step forward. Preliminary results indicate that skin biopsies, which include nerve terminals, are reliable samples for use in seeding aggregation assays to detect misfolded α-syn in PD, DLB, and MSA [205, 206]. Other potential peripheral tissues for detection of misfolded α-syn included olfactory mucosa and submandibular gland tissues. Salivary RT-QuIC has also been used to detect α-syn and showed 76% sensitivity and 94.4% specificity in differentiating patient with PD (75 subjects) from healthy controls (36 subjects) [207]. In another study, postmortem submandibular gland tissue was used to sample α-syn with RT-QuIC in 32 cases (13 PD patients, 3 incidental LBD, and 16 control cases) with 100% sensitivity and 95% specificity for PD with 100% concordance for elevated levels of pathological α-syn seeding activity in both PD and incidental LBD tissues compared to control tissues [208]. However, more data from different tissues are needed to verify the sensitivity and specificity of this ultrasensitive RT-QuIC method.
In the periphery, α-syn is largely expressed and measured in blood [209], however, the quantities in blood are strongly influenced by levels of red blood cell (RBC) contamination and hemolysis, even more than in CSF. RBCs are the major source (> 99%) of α-syn in blood and their abundance and fragility make it possible that even low RBC contamination could result in a false positive increase of α-syn in serum or plasma [210]. For this reason, levels of intracellular RBC α-syn are studied as an alternative measurement. Serum and plasma levels of total α-syn have been reported to be either higher [191], lower [194], or not significantly different [193] in PD patients compared to healthy controls. α-Syn levels in blood in light of RBC contamination, limit the utility of plasma or serum total α-syn measurement for diagnostic purposes in PD. For blood oligomeric α-syn, studies provided concordant results showing increased quantities in patients with PD, both in serum and RBCs [211,212,213] with remarkable diagnostic accuracy in serum (sensitivity 75% and specificity 100%) compared to controls [213], but requires confirmation in larger cohorts. Like oligomeric α-syn, plasma phosphorylated α-syn levels are higher in PD patients compared with healthy controls (AUC 0.71) [193]. In this context, RBC measurements of multiple post-translational modified forms of α-syn, such as nitrated, Tyr-125 phosphorylated, SUMOylated, and glycated species, were controversial in distinguishing PD patients from healthy controls (AUC 0.84) [214]. Although extensive evidence of α-syn measurements in CSF and blood exists, a definitive biomarker for PD has not yet been discovered due, in part to RBC contamination and overlap of α-syn forms found in CSF for different neurodegenerative disorders.
Other disease-linked proteins: Aβ, tau, and others
Other pathologic proteins have been assessed as biomarkers for different synucleinopathies, including those associated with AD pathology (Aβ1-42 and tau). In a small cohort (N = 28) of PD patients and age-matched controls, α-syn (total and oligomeric), Aβ1-42, and tau (total and phosphorylated) were determined in RBCs [212]. For the first time, those studies showed that PD patients exhibit α-syn heterocomplexes composed of Aβ1-42 and tau in RBCs. Moreover, concentrations of α-syn-Aβ1-42 were increased in PD subjects compared to healthy controls, and directly correlated with disease severity and motor deficits. In addition, total-α-syn levels were decreased in PD subjects and inversely related to their motor deficits. Furthermore, increased oligomeric α-syn and phosphorylated tau (ptau) from RBCs were detected in PD patients compared to controls. This study showed that combinations of total α-syn, ptau, and α-syn-Aβ1-42 concentrations provided the best fitting predictive index for discriminating PD patients from controls.
PDD pathologies include aggregated α-syn and LBs in the neocortex as well as Aβ plaques and tau neurofibrillary tangles. Therefore, α-syn, Aβ1-42, and tau in CSF have been used in several studies as biomarkers for PDD. Whereas Aβ was found to be reduced in PDD patients, those patients were still afflicted with attention deficits, executive function losses, and more rapid cognitive decline [185, 215]. Conversely, levels of total tau (t-tau) and ptau in CSF were found to be increased in PDD and more correlated to those cognitive deficits [216, 217]. In addition, the ratio of t-tau and Aβ in CSF has been used as a predictor of PDD with a low Aβ/t-tau ratio predictive of cognitive decline [218]. Conflicting results on the association of CSF α-syn levels with cognitive impairment in PD complicates its use as a biomarker for PDD [219, 220]. Data from these studies suggest that reduced Aβ and increased tau, but not α-syn in CSF can be predictors of cognitive decline in PD.
In DLB patients, Aβ1-42 levels were found to be significantly decreased compared to healthy controls, however decreased levels were also observed in AD patients [221, 222]. Additionally, lower Aβ1-42 levels were found in CSF of AD patients compared to DLB patients, with high specificity (94%), but low sensitivity (48%) [223]. Interestingly, the Aβ1-40 levels at different stages of the disease were found to be different between DLB and AD [224, 225]. In DLB, the decrease in Aβ1-40 levels is moderate compared to controls, but is not statistically significant [226]. On the other hand, in AD, Aβ1-40 levels rise sharply during the prodromal stage or before the onset of dementia [227]. Thus, although no significant differences in Aβ1-42 levels were found between DLB and AD, the decreases in Aβ1-40 levels in DLB results in higher Aβ1-42/Aβ1-40 ratio in DLB compared to AD. During the prodromal stage, lower Aβ1-42/Aβ1-40 ratio in AD cases helps to distinguish DLB patients from AD. This ratio can also distinguish AD from other forms of dementia, with specificity ranging from 73 to 94.7% and specificity ranging from 78 to 100% [228, 229]. Additionally, ptau protein at threonine-181 (pTau181) can also be used as a biomarker for DLB diagnosis [227]. The levels of pTau181 are higher in AD patients than in DLB patients and can be used to differentiate the diagnosis of DLB from AD with 75–94% sensitivity and 61–94% specificity. pTau181 levels are specific in discriminating AD from other forms of dementia as pTau181 levels remain unchanged in other dementias except for AD [230, 231]. The t-tau levels in CSF of DLB patients are shown to be normal or slightly lower than AD patients [232]. However, several studies refuted this finding by showing overlap of t-tau levels between DLB and AD patients and were verified by autopsy findings [233, 234]. Thus, differential diagnosis between AD and DLB is not supported based on t-tau levels in the CSF.
In recent years, other proteins have drawn attention in attempting to differentiate DLB from AD. These include chitinase-3-like protein 1 (CHI3L1, also known as YLK-40), neurogranin (NGRN), and visinin-like protein 1 (VILIP-1). YKL-40 is a glycoprotein that is expressed by a variety of cells, including macrophages, neutrophils, and chondrocytes [235, 236]. Elevated levels of YKL-40 have been found in several neurodegenerative diseases such as AD, PD, and DLB. Levels of YLK-40 in CSF were found to be significantly higher in AD compared to the DLB patients [237, 238]. NGRN is a postsynaptic protein involved in regulating synaptic plasticity and memory formation. This protein is highly expressed in the brain, particularly in the hippocampus and cortex, and has been implicated in AD and DLB pathologies. NGRN levels are higher in AD and DLB patients than in healthy controls. Despite noticeable synaptic dysfunction in DLB, levels of NGRN are significantly higher in AD compared to DLB [237]. VILIP-1 is a member of the neuronal calcium sensor protein family and has been identified as a biomarker for calcium-mediated neuronal injury [239]. VILIP-1 plays a critical role in linking calcium-mediated neurotoxicity and AD pathological changes [240]. One study has shown that CSF levels of VILIP-1 were significantly higher in AD patients compared to healthy controls and DLB patients [241]. It should be noted that overall the sensitivity and specificity of DLB diagnosis from AD has been found to range from 72–79% to 64–76%, respectively, depending on the detection limit of the analyte and the stage of each disease [227].
Disease-related biological/biochemical biomarkers
For the synucleinopathies, biomarkers of prime interest are those that play roles in mitochondrial dysfunction, oxidative stress, and lysosomal dysfunction. Protein deglycase (DJ-1) is a multifunctional protein involved in several cellular processes with diminished function leading to increased oxidative stress. Formerly, results of CSF concentrations of DJ-1 in neurodegenerative diseases were controversial. Using a new highly ultrasensitive Luminex ELISA, decreased CSF levels of DJ-1 were found among PD patients compared to control subjects and those afflicted with AD and MSA [242]. Another potential biomarker for PD is ubiquitin C-terminal hydrolase-L1 (UCH-L1) which participates in the degradation of abnormally modified proteins from neuronal cytoplasm. Significant decreases in UCH-L1 CSF levels were found in PD compared to PSP and MSA [30, 243]. Similarly, lysosomal hydrolase GBA, which is involved in α-syn degradation, is considered a major risk factor for PD when mutated leading to diminished ability to degrade misfolded α-syn. Decreased CSF levels of GBA in early stages of sporadic PD together with increased oligomeric α-syn/total α-syn ratios have been suggested as a combined candidate diagnostic biomarker of early PD [185]. Other potential biomarkers include low serum levels of uric acid [244], epidermal growth factor [245], and insulin-like growth factor [246] that were predictive of cognitive decline in PD, and highly predictive of cognitive decline in PDD. Thus, combining several serum analytes with neuroimaging biomarkers may provide higher accuracy in diagnosing and assessing progression of PDD.
Focus on several unique micro-ribonucleic acids (miRNAs) may also provide promising biomarkers for PD. miRNAs are single stranded chains of non-coding RNA involved in regulating the expression of different genes. The dysfunction of these miRNAs in synucleinopathies can result in problems including, but are not limited to, overexpression of α-syn [247], upregulation of LRRK2 protein [248], downregulation of DJ-1 protein [249], and dysregulation of pro-inflammatory mediators [250]. miRNAs are assumed to be tissue-specific, abundant, highly stable, and quantifiable. Their upregulation or downregulation can occur several years before the onset of PD; thus, miRNAs may serve as putative biomarkers for early stages of PD [29, 30]. For example, miR-137 and miR-124 are widely expressed in the CNS and are involved in neurogenesis, neurotransmission, morphology of synapses, inflammation, autophagy, and mitochondrial function [251]. Clinical evidence showed that serum miR-137 levels are significantly increased for PD patients compared to healthy controls, while miR-124 levels were significantly down-regulated [252]. Downregulation of miR-124 was observed in early stages of neurodegeneration, implying that its reduction may not only reflect dopamine-induced cell death, but may even contribute to the initial biological process of neurodegeneration in PD [253].
The CSF-to-plasma ratio of albumin as a reflection of the integrity of the BBB was found to be elevated in most dementia disorders independent of AD pathology represents another potential biomarker for neurodegenerative diseases [254]. Several other non-specific biomarkers candidates include markers of axonal damage and degeneration, such as the well-studied neurofilament light-chain (NFL) [118]. Measured in both blood and CSF, this biomarker reflects axonal degeneration and injury, irrespective of cause, and its levels are increased in amyotrophic lateral sclerosis (ALS), FTD, and atypical Parkinsonian disorders (PSP, MSA, and corticobasal syndrome) [255]. NFL has also been found to be suggestive of PDD development and as such have certain predictive associations with PDD. High NFL protein and heart-type fatty acid-binding protein (H-FABP), in combination with low Aβ, in CSF was found to be highly predictive of future PDD [256]. NFL levels are also increased in AD, and studies on autosomal dominant AD show that the rate of change in blood NFL increases 15 years prior to onset of symptoms [257]. Importantly, higher levels of NFL are associated with faster disease progression and higher brain atrophy rates in most neurodegenerative diseases [258, 259]. Therefore, NFL can be considered as a measure of the intensity of ongoing neurodegeneration, regardless of specific disorder. Effective DMTs can normalize NFL levels, such as in treatment of multiple sclerosis and spinal muscular atrophy, by reducing NFL levels, and as such, serve as a therapeutic response biomarker that correlates with clinical efficacy of treatment [260, 261]. Other non-specific biomarkers include, but are not limited to, proteins that delineate synaptic, lysosomal, and mitochondrial functions involved in the formation of intracellular proteins, and participating in the degradation and clearance of abnormally modified proteins or molecules associated with glial activation [25].
Synucleinopathies are associated with impairment of the autophagy-lysosomal pathway which represents a main route for the intracellular degradation of α-syn [262], thus lysosomal activities of the CSF have been a prime area of study for possible diagnosis of synucleinopathies [185, 263, 264]. One such lysosomal enzyme, GCase has been shown to exhibit decreased activity in PD patients compared to healthy controls [185, 265]. Moreover from the BioFIND cohort of 79 PD patients and 61 healthy controls, significant decreases in CSF GCase and cathepsin D activities (-28% and -21%, respectively) were found in PD compared to healthy controls, and a similar trend was also observed for β-hexosaminidase activity (-9% in PD patients) [265]. Moreover, 13% of PD patients and 5% of healthy controls were found to be carriers of mutations of the GCase coding gene (GBA). Although GCase activity was lower in carriers compared to non-carriers (-27%), the overall decrease was independent of GBA mutation carrier status (-25% in non-carrier PD patients versus non-carrier healthy controls). Receiver Operating Characteristic (ROC) curve analyses showed suboptimal diagnostic accuracies for GCase (sensitivity 67%, specificity 77%) and cathepsin D (sensitivity 61%, specificity 77%). The diagnostic performance improved by combining the panel of all measured lysosomal enzyme activities (sensitivity 71%, specificity 85%), and was further augmented with the inclusion of amyloid, tau, and α-syn pathology biomarkers added to the model [265].
Exosomes can contribute to the pathogenesis of different synucleinopathies as principal mediators of α-syn cell-to-cell transmission. In addition, they can transport RNA, primarily miRNA involved in regulating the expression of different genes associated with PD, DLB, and MSA [266,267,268]. In several synucleinopathies, α-syn is secreted directly into the extracellular space or transmitted via exosome pathways, and secretion is regulated via intracellular calcium concentrations. Additionally, exosomes containing α-syn are released by damaged neurons to further transmit aberrant α-syn beyond neuronal confines. Thus, exosomes can alter α-syn spread from neuron-to-neuron to neuron-to-glial cell; the latter activates microglia and induces inflammatory foci in areas of the brain [269]. While exosomes carry low levels of α-syn, they were also found to provide an ideal environment for α-syn aggregation, transmission, and synucleinopathy. This is supported by the finding that α-syn oligomers in exosomes are more easily transmitted and accepted by cells than free-form of α-syn species [270]. Exosomes were also found to contribute to non-cell autonomous mediation of neurotoxicity, which further facilitates wide-range transportation of α-syn throughout the CNS and to the peripheral immune system [267, 271]. A potential method of measuring neuron-derived biomarkers of neurodegeneration in the periphery is to measure levels of CNS-specific genes and/or proteins in the exosomes isolated from blood [272]. This offers a less invasive method compared to CSF measurements, however, the sensitivities of some immunoassays, such as ELISA, are not sufficient for quantifying the concentration of CNS biomarkers in blood exosomes [118]. To overcome this problem, advanced and highly sensitive techniques and analytical methods, such as Meso Scale Discovery (MSD) platform, single molecule array (SIMOA), and scRNA-seq, were developed to improve the detection of peripheral biomarkers contained within CNS-derived exosomes [118, 273].
Therapeutic biomarkers for neurodegenerative diseases
As seen in this review, extensive evidence identified promising diagnostic and prognostic biomarkers for different synucleinopathies that can be measured in CSF or blood. However, the knowledge and identification of therapeutic biomarkers, which track responses to disease treatment, remain enigmatic. For different neurodegenerative disorders, the current approved therapies are palliative for symptomatic relief, and have little or no curative effect on motor and cognitive dysfunctions seen commonly in neurodegenerative disorders. Therefore, an urgent need is warranted for development of DMTs that better control disease progression in neurodegenerative disorders. Identifying therapeutic biomarkers that measure therapy responses and efficacy in blood is an unmet need for clinical studies wherein potential DMTs are evaluated. Our research group, along with others, demonstrated that Tregs attenuate neuroinflammation and protect dopaminergic neurons from injury and loss [178,179,180]. Our works demonstrated that granulocyte–macrophage colony stimulating factor (GM-CSF, sargramostim, Leukine®) increases Treg numbers and function, protects dopaminergic neurons in PD animal models [178, 274], and improves motor function as determined by UPDRS and magnetoencephalography (MEG)-recorded cortical output in PD patients [180, 181, 275]. Our research group was the first to assess the transcriptomic and proteomic profiles of peripheral blood lymphocytes [180] and monocytes [186] in PD patients treated with an immune modulator drug (sargramostim). Significant increases in IL-10 gene expression by 2 and 6 months after treatment initiation were found, thus supporting the immunosuppressive biomarker expression observed in Treg function. In addition, proteomic analysis indicated that sargramostim treatment downregulates calcineurin and NF-κB expression significantly by 2 months of treatment and their levels remained reduced after 6 months of treatment, suggesting the reduction of inflammation-mediated neurodegeneration and a consequent protective effect in PD patients [180].
In neurodegenerative diseases, the actions of microglia, the resident myeloid cells in the CNS, may diverge from or intersect with those of recruited monocytes to drive immune-mediated pathology [276]. Therefore, we studied the association between monocyte profiles and clinical motor function and disease progression during immune modulatory therapy with sargramostim in PD patients [186]. We showed that monocyte transcriptomic and proteomic signature profiles demonstrate a neuroprotective signature that includes antioxidant, anti-inflammatory, and autophagy genes and proteins (LRRK2, HMOX1, TLR2, TLR8, transcription factor p65; RELA, ATG7, and GABA type A receptor associated protein like 2; GABARAPL2). Our findings showed the predictive potential of LRRK2 gene and protein expression for UPDRS Part III scores and changes in scores. In addition, HMOX1, TLR2, and ATG7 gene expression, and RELA, TLR2, and ATG7 protein expression, also showed predictive potential for UPDRS Part III scores and changes in scores, suggesting the utility of these genes/proteins as putative biomarkers for sargramostim therapy. Therefore, these genes/proteins may serve as potential biomarkers to predict therapeutic response in synucleinopathies treated with sargramostim or similar immunomodulatory therapies. Due to the small sample size in that study [186], the therapeutic biomarkers identified are currently being validated in ten PD patients through a twelve-month study in our laboratories (ClinicalTrials.gov Identifier: NCT05677633) [277].
Conclusions
The need for translational biomarkers for different neurodegenerative diseases is extremely warranted. To uncover potential translational biomarkers, different aspects need be taken into consideration including (1) availability of highly sensitive assays, which are specific to the target biomarker; (2) less invasive sample collection method, such as blood-based samples; (3) mechanisms by which peripheral biomarkers interact with CNS compartments and contribute to disease pathogenesis should be known and well-established [e.g., the intersection between roles of peripheral monocytes and CNS microglia in neurodegenerative disorders]; (4) availability of high-throughput “omics” technologies to investigate the entirety of the genome, proteome, and metabolome for biomarker discovery; (5) availability of up-to-date and well-curated bioinformatics tools for “omics” data analysis; and 6) revelation of drug targets for identification of pharmacodynamic and therapeutic biomarkers, such as Tregs [180] and/or monocytes [186] for sargramostim therapy. Biomarkers for neurodegenerative diseases are needed in the clinic to improve the differential diagnosis, and in drug discovery to facilitate the development and monitoring of effective DMTs.
Availability of data and materials
Not applicable.
Abbreviations
- 11C-DTBZ:
-
11C-dihydrotetrabenazine
- 123I-MIBG:
-
123Iodine-meta-iodobenzylguanidine
- 18F-FDG:
-
18F-fluorodeoxyglucose
- AAV:
-
Adeno-associated virus
- ABCA1:
-
ATP-binding cassette A1
- AD:
-
Alzheimer’s disease
- APOE:
-
Apolipoprotein E glycoprotein
- ATG7:
-
Autophagy related 7
- Aβ:
-
Amyloid beta
- BIN1:
-
Bridging integrator 1 protein
- BBB:
-
Blood–brain barrier
- CCL5:
-
C-C motif chemokine ligand 5
- CHI3L1:
-
Chitinase-3-like protein 1
- CMA:
-
Chaperon mediated autophagy
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DAT:
-
Dopamine transporter
- DJ-1:
-
Deglycase
- DLB:
-
Dementia with LBs
- DMTs:
-
Disease-modifying therapies
- DTI:
-
Diffusion tensor imaging
- ELISA:
-
Enzyme-linked immunosorbent assays
- ER:
-
Endoplasmic reticulum
- ERAD:
-
ER associated degradation
- FTD:
-
Frontotemporal dementia
- GBA:
-
Glucocerebrosidase; GCase coding gene
- GCase:
-
β-Glucocerebrosidase
- GWAS:
-
Genome-wide association studies
- HMOX1:
-
Heme oxygenase 1
- IFN-γ:
-
Interferon-gamma
- IL-1β:
-
Interleukin-1beta
- IRBD:
-
Isolated RBD
- LBD:
-
Lewy body dementia
- LBs:
-
Lewy bodies
- LNs:
-
Lewy neurites
- LRRK2:
-
Leucine-rich repeat kinase 2
- MHC-II:
-
Major histocompatibility complex class II
- miRNAs:
-
Micro-ribonucleic acids
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRI:
-
Magnetic resonance imaging
- MSA:
-
Multiple system atrophy
- NBM:
-
Nucleus basalis of Meynert
- NFL:
-
Neurofilament light-chain
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NGRN:
-
Neurogranin
- PD:
-
Parkinson’s disease
- PDD:
-
PD dementia
- PET:
-
Positron emission tomography
- PNS:
-
Peripheral nervous system
- PPI:
-
Protein–protein interaction
- p-tau:
-
Phosphorylated tau
- pTau181 :
-
P-tau protein at threonine-181
- RBC:
-
Red blood cell
- RBD:
-
Rapid-eye-movement (REM) sleep behavior disorder
- RELA:
-
Transcription factor p65
- ROS:
-
Reactive oxygen species
- RT-QuIC:
-
Real-time quaking-induced conversion
- SAA:
-
Seed amplification assay
- scRNA-seq:
-
Single-cell RNA-sequencing
- sFIDA:
-
Surface-based fluorescence intensity distribution analysis
- SNCA :
-
α-Syn gene
- SNCA-AS1 :
-
SNCA Antisense RNA 1
- SNpc:
-
Substantia nigra pars compacta
- SPECT:
-
Single-photon emission computed tomography
- Teffs:
-
Effector T cells
- TLR:
-
Toll-like receptor
- TMEM175:
-
Transmembrane protein 175
- TNF-α:
-
Tumor necrosis factor-alpha
- Tregs:
-
Regulatory T cells
- t-tau:
-
Total tau
- UCH-L1:
-
Ubiquitin C-terminal hydrolase-L1
- UPDRS:
-
United Parkinson’s Disease Rating scale
- UPR:
-
Unfolded protein response
- V-ATPase:
-
Vacuolar-type H+-ATPase
- VILIP-1:
-
Visinin-like protein 1
- VMAT2:
-
Vesicular monoamine type 2 transporter
- α-syn:
-
α-Synuclein
References
Koga S, Sekiya H, Kondru N, Ross OA, Dickson DW. Neuropathology and molecular diagnosis of synucleinopathies. Mol Neurodegener. 2021;16(1):83. https://doi.org/10.1186/s13024-021-00501-z.
Goedert M, Jakes R, Spillantini MG. The synucleinopathies: twenty years on. J Parkinsons Dis. 2017;7(s1):S51–69. https://doi.org/10.3233/JPD-179005.
McCann H, Stevens CH, Cartwright H, Halliday GM. alpha-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014;20(Suppl 1):S62–7. https://doi.org/10.1016/S1353-8020(13)70017-8.
Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. https://doi.org/10.1111/j.1749-6632.2000.tb06900.x.
Parkinson’s Foundation. https://www.parkinson.org/understanding-parkinsons/statistics. Accessed 5 Sept 2023.
Hess CW, Okun MS. Diagnosing Parkinson disease. Continuum Minneap Minn. 2016;22(4):1047–63. https://doi.org/10.1212/CON.0000000000000345.
World Health Organization. Parkinson disease. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease. Accessed 5 Sept 2023.
Astrom DO, et al. High risk of developing dementia in Parkinson’s disease: a Swedish registry-based study. Sci Rep. 2022;12(1):16759. https://doi.org/10.1038/s41598-022-21093-8.
Aarsland D, Tandberg E, Larsen JP, Cummings JL. Frequency of dementia in Parkinson disease. Arch Neurol. 1996;53(6):538–42. https://doi.org/10.1001/archneur.1996.00550060082020.
Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289(1–2):18–22. https://doi.org/10.1016/j.jns.2009.08.034.
Hanagasi HA, Tufekcioglu Z, Emre M. Dementia in Parkinson’s disease. J Neurol Sci. 2017;374:26–31. https://doi.org/10.1016/j.jns.2017.01.012.
Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol. 2010;20(3):633–9. https://doi.org/10.1111/j.1750-3639.2009.00369.x.
Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–92. https://doi.org/10.1001/archneur.60.3.387.
Coughlin DG, Hurtig HI, Irwin DJ. Pathological Influences on Clinical Heterogeneity in Lewy Body Diseases. Mov Disord. 2020;35(1):5–19. https://doi.org/10.1002/mds.27867.
Galvin JE, Balasubramaniam M. Lewy body dementia: the under-recognized but common FOE. Cerebrum. 2013;2013:13.
McKeith IG, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88–100. https://doi.org/10.1212/WNL.0000000000004058.
Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561–6.
Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet. 2015;386(10004):1683–97. https://doi.org/10.1016/S0140-6736(15)00462-6.
Kane JPM, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther. 2018;10(1):19. https://doi.org/10.1186/s13195-018-0350-6.
Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70(11):1396–402. https://doi.org/10.1001/jamaneurol.2013.3579.
Chen Y, et al. The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimers Dement. 2019;15(7):899–906. https://doi.org/10.1016/j.jalz.2019.03.015.
Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 2017;13(1):28–37. https://doi.org/10.1016/j.jalz.2016.04.002.
Desai U, et al. Epidemiology and economic burden of Lewy body dementia in the United States. Curr Med Res Opin. 2022;38(7):1177–88. https://doi.org/10.1080/03007995.2022.2059978.
Hunter CA, Kirson NY, Desai U, Cummings AK, Faries DE, Birnbaum HG. Medical costs of Alzheimer’s disease misdiagnosis among US Medicare beneficiaries. Alzheimers Dement. 2015;11(8):887–95. https://doi.org/10.1016/j.jalz.2015.06.1889.
Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27(6):954–63. https://doi.org/10.1038/s41591-021-01382-x.
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. https://doi.org/10.1038/nrdp.2015.56.
Smedinga M, Darweesh SKL, Bloem BR, Post B, Richard E. Towards early disease modification of Parkinson’s disease: a review of lessons learned in the Alzheimer field. J Neurol. 2021;268(2):724–33. https://doi.org/10.1007/s00415-020-10162-5.
Sheeler C, Rosa JG, Ferro A, McAdams B, Borgenheimer E, Cvetanovic M. Glia in neurodegeneration: the housekeeper, the defender and the perpetrator. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21239188.
Cova I, Priori A. Diagnostic biomarkers for Parkinson’s disease at a glance: where are we? J Neural Transm (Vienna). 2018;125(10):1417–32. https://doi.org/10.1007/s00702-018-1910-4.
He R, Yan X, Guo J, Xu Q, Tang B, Sun Q. Recent advances in biomarkers for Parkinson’s disease. Front Aging Neurosci. 2018;10:305. https://doi.org/10.3389/fnagi.2018.00305.
Chin KS, Teodorczuk A, Watson R. Dementia with Lewy bodies: challenges in the diagnosis and management. Aust N Z J Psychiatry. 2019;53(4):291–303. https://doi.org/10.1177/0004867419835029.
Pagano G, Niccolini F, Politis M. Imaging in Parkinson’s disease. Clin Med Lond. 2016;16(4):371–5. https://doi.org/10.7861/clinmedicine.16-4-371.
MedlinePlus Trusted Health Information for You. https://medlineplus.gov/ency/imagepages/19515.htm. Accessed 5 Sept 2023.
Kaneko Y, et al. Association between clinical symptoms and post-mortem neuropathology in dementia with Lewy bodies. Geriatr Gerontol Int. 2020;20(3):261–2. https://doi.org/10.1111/ggi.13853.
Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(Pt 11):2131–46. https://doi.org/10.1093/brain/124.11.2131.
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. https://doi.org/10.1136/jnnp.2007.131045.
Palakurthi B, Burugupally SP. Postural instability in Parkinson’s disease: a review. Brain Sci. 2019. https://doi.org/10.3390/brainsci9090239.
Greenland JC, Barker RA. The differential diagnosis of Parkinson’s disease. In: Stoker TB, Greenland JC, editors. Parkinson’s disease: pathogenesis and clinical aspects. Brisbane: Exon Publications; 2018. p. 2018.
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. https://doi.org/10.1016/S0140-6736(14)61393-3.
Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):14–20. https://doi.org/10.1111/j.1468-1331.2008.02056.x.
Fullard ME, Morley JF, Duda JE. Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull. 2017;33(5):515–25. https://doi.org/10.1007/s12264-017-0170-x.
He R, et al. Olfactory dysfunction predicts disease progression in Parkinson’s disease: a longitudinal study. Front Neurosci. 2020;14: 569777. https://doi.org/10.3389/fnins.2020.569777.
Iranzo A, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443–53. https://doi.org/10.1016/S1474-4422(13)70056-5.
Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811–26. https://doi.org/10.1016/bs.irn.2017.06.003.
Biundo R, Weis L, Antonini A. Cognitive decline in Parkinson’s disease: the complex picture. NPJ Parkinsons Dis. 2016;2:16018. https://doi.org/10.1038/npjparkd.2016.18.
Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther. 2014;6(4):46. https://doi.org/10.1186/alzrt274.
Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559–68. https://doi.org/10.1016/S1474-4422(18)30127-3.
Zhu K, van Hilten JJ, Marinus J. Predictors of dementia in Parkinson’s disease; findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism Relat Disord. 2014;20(9):980–5. https://doi.org/10.1016/j.parkreldis.2014.06.006.
Anang JB, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253–60. https://doi.org/10.1212/WNL.0000000000000842.
Pagano G, De Micco R, Yousaf T, Wilson H, Chandra A, Politis M. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology. 2018;91(10):e894–905. https://doi.org/10.1212/WNL.0000000000006134.
Outeiro TF, et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener. 2019;14(1):5. https://doi.org/10.1186/s13024-019-0306-8.
McKeith IG. Author response: diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2018;90(6):300–1. https://doi.org/10.1212/WNL.0000000000004919.
Yamada M, et al. Diagnostic criteria for dementia with Lewy bodies: updates and future directions. J Mov Disord. 2020;13(1):1–10. https://doi.org/10.14802/jmd.19052.
Gomperts SN. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum Minneap Minn. 2016;22(2):435–63. https://doi.org/10.1212/CON.0000000000000309.
Hoops S, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–45. https://doi.org/10.1212/WNL.0b013e3181c34b47.
Emre M, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–707. https://doi.org/10.1002/mds.21507.
Rodnitzky RL. Cognitive impairment and dementia in Parkinson disease. Waltham Publishers. https://www.uptodate.com/contents/cognitive-impairment-and-dementia-in-parkinson-disease. Accessed 5 Sept 2023.
McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. J Alzheimers Dis. 2006;9(3 Suppl):417–23. https://doi.org/10.3233/jad-2006-9s347.
Prell T. Structural and functional brain patterns of non-motor syndromes in Parkinson’s disease. Front Neurol. 2018;9:138. https://doi.org/10.3389/fneur.2018.00138.
Prakash KG, Bannur BM, Chavan MD, Saniya K, Sailesh KS, Rajagopalan A. Neuroanatomical changes in Parkinson’s disease in relation to cognition: an update. J Adv Pharm Technol Res. 2016;7(4):123–6. https://doi.org/10.4103/2231-4040.191416.
Cerasa A, et al. Increased prefrontal volume in PD with levodopa-induced dyskinesias: a voxel-based morphometry study. Mov Disord. 2011;26(5):807–12. https://doi.org/10.1002/mds.23660.
Cullen NC, et al. Comparing progression biomarkers in clinical trials of early Alzheimer’s disease. Ann Clin Transl Neurol. 2020;7(9):1661–73. https://doi.org/10.1002/acn3.51158.
Righini A, et al. Thin section MR study of the basal ganglia in the differential diagnosis between striatonigral degeneration and Parkinson disease. J Comput Assist Tomogr. 2002;26(2):266–71. https://doi.org/10.1097/00004728-200203000-00018.
Ghassaban K, et al. Regional high iron in the substantia nigra differentiates Parkinson’s disease patients from healthy controls. Front Aging Neurosci. 2019;11:106. https://doi.org/10.3389/fnagi.2019.00106.
Cho SJ, et al. Iron-sensitive magnetic resonance imaging in Parkinson’s disease: a systematic review and meta-analysis. J Neurol. 2021;268(12):4721–36. https://doi.org/10.1007/s00415-021-10582-x.
Elschot EP, et al. A comprehensive view on MRI techniques for imaging blood-brain barrier integrity. Invest Radiol. 2021;56(1):10–9. https://doi.org/10.1097/RLI.0000000000000723.
Stoessl AJ, Martin WW, McKeown MJ, Sossi V. Advances in imaging in Parkinson’s disease. Lancet Neurol. 2011;10(11):987–1001. https://doi.org/10.1016/s1474-4422(11)70214-9.
Brooks DJ. Imaging approaches to Parkinson disease. J Nucl Med. 2010;51(4):596–609. https://doi.org/10.2967/jnumed.108.059998.
Matuskey D, et al. Synaptic changes in Parkinson disease assessed with in vivo imaging. Ann Neurol. 2020;87(3):329–38. https://doi.org/10.1002/ana.25682.
Mecca AP, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 2020;16(7):974–82. https://doi.org/10.1002/alz.12097.
Chetelat G, et al. Amyloid-PET and (18)F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19(11):951–62. https://doi.org/10.1016/S1474-4422(20)30314-8.
Sanchez-Catasus CA, Bohnen NI, Yeh FC, D’Cruz N, Kanel P, Muller M. Dopaminergic nigrostriatal connectivity in early Parkinson disease: in vivo neuroimaging study of (11)C-DTBZ PET combined with correlational tractography. J Nucl Med. 2021;62(4):545–52. https://doi.org/10.2967/jnumed.120.248500.
Delva A, et al. Quantification and discriminative power of (18)F-FE-PE2I PET in patients with Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2020;47(8):1913–26. https://doi.org/10.1007/s00259-019-04587-y.
Jakobson Mo S, et al. Dopamine transporter imaging with clinical comparison. EJNMMI Res. 2018;8(1):100. https://doi.org/10.1186/s13550-018-0450-0.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29. https://doi.org/10.1016/j.nurt.2007.05.011.
Zhang Y, Burock MA. Diffusion tensor imaging in Parkinson’s disease and Parkinsonian syndrome: a systematic review. Front Neurol. 2020;11: 531993. https://doi.org/10.3389/fneur.2020.531993.
Atkinson-Clement C, Pinto S, Eusebio A, Coulon O. Diffusion tensor imaging in Parkinson’s disease: review and meta-analysis. NeuroImage Clin. 2017;16:98–110. https://doi.org/10.1016/j.nicl.2017.07.011.
Schulz J, Pagano G, Fernandez Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain. 2018;141(5):1501–16. https://doi.org/10.1093/brain/awy072.
Chen B, Fan GG, Liu H, Wang S. Changes in anatomical and functional connectivity of Parkinson’s disease patients according to cognitive status. Eur J Radiol. 2015;84(7):1318–24. https://doi.org/10.1016/j.ejrad.2015.04.014.
Lee JE, et al. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014;85(1):7–16. https://doi.org/10.1136/jnnp-2013-305062.
Kandiah N, et al. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(11):1203–8. https://doi.org/10.1016/j.parkreldis.2014.08.024.
Sakakibara R, Tateno F, Kishi M, Tsuyusaki Y, Terada H, Inaoka T. MIBG myocardial scintigraphy in pre-motor Parkinson’s disease: a review. Parkinsonism Relat Disord. 2014;20(3):267–73. https://doi.org/10.1016/j.parkreldis.2013.11.001.
Skowronek C, Zange L, Lipp A. Cardiac 123I-MIBG scintigraphy in neurodegenerative Parkinson syndromes: performance and pitfalls in clinical practice. Front Neurol. 2019;10:152. https://doi.org/10.3389/fneur.2019.00152.
Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson’s disease: progress and therapeutic implications. Mov Disord. 2013;28(1):14–23. https://doi.org/10.1002/mds.25249.
George G, Singh S, Lokappa SB, Varkey J. Gene co-expression network analysis for identifying genetic markers in Parkinson’s disease - a three-way comparative approach. Genomics. 2019;111(4):819–30. https://doi.org/10.1016/j.ygeno.2018.05.005.
Edwards TL, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74(2):97–109. https://doi.org/10.1111/j.1469-1809.2009.00560.x.
Gandhi S, Wood NW. Genome-wide association studies: the key to unlocking neurodegeneration? Nat Neurosci. 2010;13(7):789–94. https://doi.org/10.1038/nn.2584.
Satake W, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41(12):1303–7. https://doi.org/10.1038/ng.485.
Simon-Sanchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–12. https://doi.org/10.1038/ng.487.
Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–73. https://doi.org/10.1002/ana.10795.
Khalaf O, et al. The H50Q mutation enhances alpha-synuclein aggregation, secretion, and toxicity. J Biol Chem. 2014;289(32):21856–76. https://doi.org/10.1074/jbc.M114.553297.
Hoffman-Zacharska D, et al. Novel A18T and pA29S substitutions in alpha-synuclein may be associated with sporadic Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):1057–60. https://doi.org/10.1016/j.parkreldis.2013.07.011.
Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. https://doi.org/10.1017/S1462399409001148.
Aasly JO, et al. Novel pathogenic LRRK2 p.Asn1437His substitution in familial Parkinson’s disease. Mov Disord. 2010;25(13):2156–63. https://doi.org/10.1002/mds.23265.
Deng H, et al. Genetic analysis of LRRK2 mutations in patients with Parkinson disease. J Neurol Sci. 2006;251(1–2):102–6. https://doi.org/10.1016/j.jns.2006.09.017.
Bardien S, Lesage S, Brice A, Carr J. Genetic characteristics of leucine-rich repeat kinase 2 (LRRK2) associated Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(7):501–8. https://doi.org/10.1016/j.parkreldis.2010.11.008.
Kouli A, Torsney KM, Kuan WL. Parkinson’s disease: etiology, neuropathology, and pathogenesis. In: Stoker TB, Greenland JC, editors. Parkinson’s disease: pathogenesis and clinical aspects. Brisbane: Exon publications; 2018.
Tanji K, Mori F, Kakita A, Takahashi H, Wakabayashi K. Alteration of autophagosomal proteins (LC3, GABARAP and GATE-16) in Lewy body disease. Neurobiol Dis. 2011;43(3):690–7. https://doi.org/10.1016/j.nbd.2011.05.022.
Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13(4):473–81. https://doi.org/10.1111/j.1750-3639.2003.tb00478.x.
Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200–10. https://doi.org/10.1016/j.tibs.2015.02.003.
Xilouri M, Brekk OR, Stefanis L. Autophagy and alpha-synuclein: relevance to Parkinson’s disease and related synucleopathies. Mov Disord. 2016;31(2):178–92. https://doi.org/10.1002/mds.26477.
Caballero B, et al. Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell. 2018. https://doi.org/10.1111/acel.12692.
Haddad D, Nakamura K. Understanding the susceptibility of dopamine neurons to mitochondrial stressors in Parkinson’s disease. FEBS Lett. 2015;589(24 Pt A):3702–13. https://doi.org/10.1016/j.febslet.2015.10.021.
Chia R, et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet. 2021;53(3):294–303. https://doi.org/10.1038/s41588-021-00785-3.
Rey F, et al. Alpha-synuclein antisense transcript SNCA-AS1 regulates synapses- and aging-related genes suggesting its implication in Parkinson’s disease. Aging Cell. 2021;20(12): e13504. https://doi.org/10.1111/acel.13504.
Pradas E, Martinez-Vicente M. The consequences of GBA deficiency in the autophagy-lysosome system in Parkinson’s disease associated with GBA. Cells. 2023. https://doi.org/10.3390/cells12010191.
Migdalska-Richards A, Schapira AH. The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem. 2016;139(Suppl 1):77–90. https://doi.org/10.1111/jnc.13385.
Zhao N, et al. APOE4 exacerbates alpha-synuclein pathology and related toxicity independent of amyloid,". Sci Transl Med. 2020. https://doi.org/10.1126/scitranslmed.aay1809.
Tropea TF, et al. APOE, thought disorder, and SPARE-AD predict cognitive decline in established Parkinson’s disease. Mov Disord. 2018;33(2):289–97. https://doi.org/10.1002/mds.27204.
Seshadri S, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–40. https://doi.org/10.1001/jama.2010.574.
Zhu J, Liu X, Yin H, Gao Y, Yu H. Convergent lines of evidence support BIN1 as a risk gene of Alzheimer’s disease. Hum Genomics. 2021;15(1):9. https://doi.org/10.1186/s40246-021-00307-6.
Lo CH, Zeng J. Defective lysosomal acidification: a new prognostic marker and therapeutic target for neurodegenerative diseases. Transl Neurodegener. 2023;12(1):29. https://doi.org/10.1186/s40035-023-00362-0.
Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. https://doi.org/10.1146/annurev-physiol-012110-142317.
Jinn S, et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases alpha-synuclein aggregation. Proc Natl Acad Sci U S A. 2017;114(9):2389–94. https://doi.org/10.1073/pnas.1616332114.
Wie J, et al. A growth-factor-activated lysosomal K(+) channel regulates Parkinson’s pathology. Nature. 2021;591(7850):431–7. https://doi.org/10.1038/s41586-021-03185-z.
Qu L, et al. Lysosomal K(+) channel TMEM175 promotes apoptosis and aggravates symptoms of Parkinson’s disease. EMBO Rep. 2022;23(9):53234. https://doi.org/10.15252/embr.202153234.
Palomba NP, et al. Common and rare variants in TMEM175 gene concur to the pathogenesis of Parkinson’s disease in Italian patients. Mol Neurobiol. 2023;60(4):2150–73. https://doi.org/10.1007/s12035-022-03203-9.
Konickova D, et al. Biomarkers of neurodegenerative diseases: biology, taxonomy, clinical relevance, and current research status. Biomedicines. 2022. https://doi.org/10.3390/biomedicines10071760.
Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. https://doi.org/10.1016/s0896-6273(03)00568-3.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. https://doi.org/10.1038/42166.
Burre J, Sharma M, Sudhof TC. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb Perspect Med. 2018. https://doi.org/10.1101/cshperspect.a024091.
Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–10. https://doi.org/10.1038/nature10324.
Fusco G, et al. Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour. Nat Commun. 2014;5:3827. https://doi.org/10.1038/ncomms4827.
Vamvaca K, Volles MJ, Lansbury PT Jr. The first N-terminal amino acids of alpha-synuclein are essential for alpha-helical structure formation in vitro and membrane binding in yeast. J Mol Biol. 2009;389(2):413–24. https://doi.org/10.1016/j.jmb.2009.03.021.
Nielsen MS, Vorum H, Lindersson E, Jensen PH. Ca2+ binding to alpha-synuclein regulates ligand binding and oligomerization. J Biol Chem. 2001;276(25):22680–4. https://doi.org/10.1074/jbc.M101181200.
Lautenschlager J, et al. C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat Commun. 2018;9(1):712. https://doi.org/10.1038/s41467-018-03111-4.
Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–5. https://doi.org/10.1073/pnas.0903691106.
Bousiges O, Blanc F. Biomarkers of dementia with Lewy bodies: differential diagnostic with Alzheimer’s disease. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23126371.
Nury T, Lizard G, Vejux A. Lipids nutrients in Parkinson and Alzheimer’s diseases: cell death and cytoprotection. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21072501.
Kosaka K. Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol. 1978;42(2):127–34. https://doi.org/10.1007/BF00690978.
Walker L, Stefanis L, Attems J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—current issues and future directions. J Neurochem. 2019;150(5):467–74. https://doi.org/10.1111/jnc.14698.
Jellinger KA. Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm Vienna. 2018;125(4):615–50. https://doi.org/10.1007/s00702-017-1821-9.
Halliday GM, Song YJ, Harding AJ. Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm Vienna. 2011;118(5):713–9. https://doi.org/10.1007/s00702-011-0641-6.
Tsuboi Y, Uchikado H, Dickson DW. Neuropathology of Parkinson’s disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 2007;13(Suppl 3):S221–4. https://doi.org/10.1016/S1353-8020(08)70005-1.
Bohnen NI, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol. 2006;253(2):242–7. https://doi.org/10.1007/s00415-005-0971-0.
Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD006504.pub2.
Shimada H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273–8. https://doi.org/10.1212/WNL.0b013e3181ab2b58.
Hofmann KW, et al. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem Res. 2009;34(8):1401–4. https://doi.org/10.1007/s11064-009-9921-z.
Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000;60:277–90. https://doi.org/10.1007/978-3-7091-6301-6_19.
Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflam Regen. 2019;39(1):12. https://doi.org/10.1186/s41232-019-0101-5.
Ferrari CC, et al. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165(5):1827–37. https://doi.org/10.1016/s0002-9440(10)63438-4.
Karpenko MN, Vasilishina AA, Gromova EA, Muruzheva ZM, Miliukhina IV, Bernadotte A. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-alpha levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell Immunol. 2018;327:77–82. https://doi.org/10.1016/j.cellimm.2018.02.011.
Williams-Gray CH, et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord. 2016;31(7):995–1003. https://doi.org/10.1002/mds.26563.
Xiromerisiou G, et al. Peripheral inflammatory markers TNF-alpha and CCL2 revisited: association with Parkinson’s disease severity. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms24010264.
Çomoğlu SS, Güven H, Acar M, Öztürk G, Koçer B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson’s disease. Neurosci Lett. 2013;553:63–7. https://doi.org/10.1016/j.neulet.2013.08.019.
Tang P, et al. Correlation between serum RANTES levels and the severity of Parkinson’s disease. Oxid Med Cell Longev. 2014;2014: 208408. https://doi.org/10.1155/2014/208408.
Devos D, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–8. https://doi.org/10.1016/j.nbd.2012.09.007.
Iba M, et al. Neuroinflammation is associated with infiltration of T cells in Lewy body disease and alpha-synuclein transgenic models. J Neuroinflammation. 2020;17(1):214. https://doi.org/10.1186/s12974-020-01888-0.
Allen Reish HE, Standaert DG. Role of alpha-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis. 2015;5(1):1–19. https://doi.org/10.3233/JPD-140491.
Schonhoff AM, Williams GP, Wallen ZD, Standaert DG, Harms AS. Innate and adaptive immune responses in Parkinson’s disease. Prog Brain Res. 2020;252:169–216. https://doi.org/10.1016/bs.pbr.2019.10.006.
Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193(6):2615–21. https://doi.org/10.4049/jimmunol.1400716.
McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–91. https://doi.org/10.1212/wnl.38.8.1285.
Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. https://doi.org/10.1186/1742-2094-2-14.
Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. https://doi.org/10.1126/science.1194637.
Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. https://doi.org/10.1126/science.1219179.
Reale M, et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23(1):55–63. https://doi.org/10.1016/j.bbi.2008.07.003.
Depboylu C, Schafer MK, Arias-Carrion O, Oertel WH, Weihe E, Hoglinger GU. Possible involvement of complement factor C1q in the clearance of extracellular neuromelanin from the substantia nigra in Parkinson disease. J Neuropathol Exp Neurol. 2011;70(2):125–32. https://doi.org/10.1097/NEN.0b013e31820805b9.
Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84(1):100–4. https://doi.org/10.1007/BF00427222.
Cao S, Standaert DG, Harms AS. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J Neuroinflammation. 2012;9:259. https://doi.org/10.1186/1742-2094-9-259.
Zhang W, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19(6):533–42. https://doi.org/10.1096/fj.04-2751com.
Harms AS, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592–600. https://doi.org/10.1523/JNEUROSCI.5610-12.2013.
Holmans P, et al. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum Mol Genet. 2013;22(5):1039–49. https://doi.org/10.1093/hmg/dds492.
Roodveldt C, et al. Preconditioning of microglia by alpha-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation. PLoS ONE. 2013;8(11): e79160. https://doi.org/10.1371/journal.pone.0079160.
Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67(12):1149–58. https://doi.org/10.1097/NEN.0b013e31818e5e99.
Watson MB, et al. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237(2):318–34. https://doi.org/10.1016/j.expneurol.2012.06.025.
Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson’s disease. Acta Neurobiol Exp. 1999;59(1):1–8.
Benner EJ, et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3(1):e1376. https://doi.org/10.1371/journal.pone.0001376.
Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184(5):2261–71. https://doi.org/10.4049/jimmunol.0901852.
Brochard V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182–92. https://doi.org/10.1172/JCI36470.
Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5(1):e8784. https://doi.org/10.1371/journal.pone.0008784.
Anderson KM, Olson KE, Estes KA, Flanagan K, Gendelman HE, Mosley RL. Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders. Transl Neurodegener. 2014;3(1):25. https://doi.org/10.1186/2047-9158-3-25.
Mosley RL, Gendelman HE. T cells and Parkinson’s disease. Lancet Neurol. 2017;16(10):769–71. https://doi.org/10.1016/S1474-4422(17)30276-4.
Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int. 2014;2014: 308654. https://doi.org/10.1155/2014/308654.
Kustrimovic N, et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation. 2018;15(1):205. https://doi.org/10.1186/s12974-018-1248-8.
Lindestam Arlehamn CS, et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun. 2020;11(1):1875. https://doi.org/10.1038/s41467-020-15626-w.
Saunders JA, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7(4):927–38. https://doi.org/10.1007/s11481-012-9402-z.
Huang Y, Liu Z, Cao BB, Qiu YH, Peng YP. Treg cells attenuate neuroinflammation and protect neurons in a mouse model of Parkinson’s disease. J Neuroimmune Pharmacol. 2020;15(2):224–37. https://doi.org/10.1007/s11481-019-09888-5.
Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J Neuroimmunol. 2013;265(1–2):1–10. https://doi.org/10.1016/j.jneuroim.2013.10.009.
Olson KE, et al. Selective VIP receptor agonists facilitate immune transformation for dopaminergic neuroprotection in MPTP-intoxicated mice. J Neurosci. 2015;35(50):16463–78. https://doi.org/10.1523/JNEUROSCI.2131-15.2015.
Olson KE, et al. Safety, tolerability, and immune-biomarker profiling for year-long sargramostim treatment of Parkinson’s disease. EBioMedicine. 2021;67: 103380. https://doi.org/10.1016/j.ebiom.2021.103380.
Olson KE, et al. An open-label multiyear study of sargramostim-treated Parkinson’s disease patients examining drug safety, tolerability, and immune biomarkers from limited case numbers. Transl Neurodegener. 2023;12(1):26. https://doi.org/10.1186/s40035-023-00361-1.
Bateman RJ, Klunk WE. Measuring target effect of proposed disease-modifying therapies in Alzheimer’s disease. Neurotherapeutics. 2008;5(3):381–90. https://doi.org/10.1016/j.nurt.2008.05.009.
Muller-Linow M, Hilgetag CC, Hutt MT. Organization of excitable dynamics in hierarchical biological networks. PLoS Comput Biol. 2008;4(9):e1000190. https://doi.org/10.1371/journal.pcbi.1000190.
Parnetti L, et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019;18(6):573–86. https://doi.org/10.1016/S1474-4422(19)30024-9.
Parnetti L, et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov Disord. 2014;29(8):1019–27. https://doi.org/10.1002/mds.25772.
Abdelmoaty MM, et al. Monocyte biomarkers define sargramostim treatment outcomes for Parkinson’s disease. Clin Transl Med. 2022;12(7): e958. https://doi.org/10.1002/ctm2.958.
Shi S, Mitteregger-Kretzschmar G, Giese A, Kretzschmar HA. Establishing quantitative real-time quaking-induced conversion (qRT-QuIC) for highly sensitive detection and quantification of PrPSc in prion-infected tissues. Acta Neuropathol Commun. 2013;1:44. https://doi.org/10.1186/2051-5960-1-44.
Boster antibody and ELISA experts. https://www.bosterbio.com/blog/post/5-pitfalls-to-avoid-for-elisa. Accessed 21 June 2023.
Reaction Biology Biomarker Discovery. https://www.reactionbiology.com/services/biomarker-discovery/multiplex-immunoassay-service. Accessed 21 June 2023.
Danzer KM, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. https://doi.org/10.1186/1750-1326-7-42.
Shi M, et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128(5):639–50. https://doi.org/10.1007/s00401-014-1314-y.
Russo I, Bubacco L, Greggio E. Exosomes-associated neurodegeneration and progression of Parkinson’s disease. Am J Neurodegener Dis. 2012;1(3):217–25.
Foulds PG, et al. A longitudinal study on alpha-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci Rep. 2013;3:2540. https://doi.org/10.1038/srep02540.
Besong-Agbo D, et al. Naturally occurring alpha-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology. 2013;80(2):169–75. https://doi.org/10.1212/WNL.0b013e31827b90d1.
Zerr I, et al. Optimization of the real-time quaking-induced conversion assay for prion disease diagnosis. Front Bioeng Biotechnol. 2020;8: 586890. https://doi.org/10.3389/fbioe.2020.586890.
Wang Y, et al. Phosphorylated alpha-synuclein in Parkinson’s disease. Sci Transl Med. 2012;4(121):121ra20. https://doi.org/10.1126/scitranslmed.3002566.
Hermann P, et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20(3):235–46. https://doi.org/10.1016/S1474-4422(20)30477-4.
Iranzo A, et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021;20(3):203–12. https://doi.org/10.1016/s1474-4422(20)30449-x.
Fairfoul G, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3(10):812–8. https://doi.org/10.1002/acn3.338.
Shahnawaz M, et al. Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578(7794):273–7. https://doi.org/10.1038/s41586-020-1984-7.
Kang UJ, et al. Comparative study of cerebrospinal fluid alpha-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord. 2019;34(4):536–44. https://doi.org/10.1002/mds.27646.
Siderowf A, et al. Assessment of heterogeneity among participants in the Parkinson’s progression markers initiative cohort using alpha-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023;22(5):407–17. https://doi.org/10.1016/S1474-4422(23)00109-6.
Herrmann Y, et al. Nanoparticle standards for immuno-based quantitation of alpha-synuclein oligomers in diagnostics of Parkinson’s disease and other synucleinopathies. Clin Chim Acta. 2017;466:152–9. https://doi.org/10.1016/j.cca.2017.01.010.
Blomeke L, et al. Quantitative detection of alpha-synuclein and tau oligomers and other aggregates by digital single particle counting. NPJ Parkinsons Dis. 2022;8(1):68. https://doi.org/10.1038/s41531-022-00330-x.
Manne S, et al. Blinded RT-QuIC analysis of alpha-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord. 2020;35(12):2230–9. https://doi.org/10.1002/mds.28242.
Wang Z, et al. Skin alpha-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol. 2020;78(1):1–11. https://doi.org/10.1001/jamaneurol.2020.3311.
Luan M, et al. Diagnostic Value of Salivary Real-Time Quaking-Induced Conversion in Parkinson’s Disease and Multiple System Atrophy. Mov Disord. 2022;37(5):1059–63. https://doi.org/10.1002/mds.28976.
Manne S, et al. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov Disord. 2020;35(2):268–78. https://doi.org/10.1002/mds.27907.
El-Agnaf OM, et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17(13):1945–7. https://doi.org/10.1096/fj.03-0098fje.
Barbour R, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5(2):55–9. https://doi.org/10.1159/000112832.
Zhao HQ, Li FF, Wang Z, Wang XM, Feng T. A comparative study of the amount of alpha-synuclein in ischemic stroke and Parkinson’s disease. Neurol Sci. 2016;37(5):749–54. https://doi.org/10.1007/s10072-016-2485-1.
Daniele S, et al. Alpha-synuclein heterocomplexes with beta-amyloid are increased in red blood cells of Parkinson’s disease patients and correlate with disease severity. Front Mol Neurosci. 2018;11:53. https://doi.org/10.3389/fnmol.2018.00053.
Williams SM, Schulz P, Sierks MR. Oligomeric alpha-synuclein and beta-amyloid variants as potential biomarkers for Parkinson’s and Alzheimer’s diseases. Eur J Neurosci. 2016;43(1):3–16. https://doi.org/10.1111/ejn.13056.
Vicente Miranda H, et al. Posttranslational modifications of blood-derived alpha-synuclein as biochemical markers for Parkinson’s disease. Sci Rep. 2017;7(1):13713. https://doi.org/10.1038/s41598-017-14175-5.
Liu C, et al. CSF tau and tau/Abeta42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(3):271–6. https://doi.org/10.1016/j.parkreldis.2014.12.027.
Compta Y, et al. High cerebrospinal tau levels are associated with the rs242557 tau gene variant and low cerebrospinal beta-amyloid in Parkinson disease. Neurosci Lett. 2011;487(2):169–73. https://doi.org/10.1016/j.neulet.2010.10.015.
Gmitterova K, Gawinecka J, Llorens F, Varges D, Valkovic P, Zerr I. Cerebrospinal fluid markers analysis in the differential diagnosis of dementia with Lewy bodies and Parkinson’s disease dementia. Eur Arch Psychiatry Clin Neurosci. 2020;270(4):461–70. https://doi.org/10.1007/s00406-018-0928-9.
Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol. 2017;16(1):66–75. https://doi.org/10.1016/S1474-4422(16)30328-3.
Stewart T, et al. Cerebrospinal fluid alpha-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol. 2014;184(4):966–75. https://doi.org/10.1016/j.ajpath.2013.12.007.
Skogseth RE, et al. Associations between cerebrospinal fluid biomarkers and cognition in early untreated Parkinson’s disease. J Parkinsons Dis. 2015;5(4):783–92. https://doi.org/10.3233/JPD-150682.
Bousiges O, et al. Cerebrospinal fluid Alzheimer biomarkers can be useful for discriminating dementia with Lewy bodies from Alzheimer’s disease at the prodromal stage. J Neurol Neurosurg Psychiatry. 2018;89(5):467–75. https://doi.org/10.1136/jnnp-2017-316385.
H. J. Tschampa et al., "Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies," Neurology, vol. 56, no. 4, p. 576, Feb 27 2001, doi: https://doi.org/10.1212/wnl.56.4.576.
Bibl M, et al. CSF diagnosis of Alzheimer’s disease and dementia with Lewy bodies. J Neural Transm. 2006;113:1771–8.
Mollenhauer B, et al. Follow-up investigations in cerebrospinal fluid of patients with dementia with Lewy bodies and Alzheimer’s disease. J Neural Transm. 2005;112:933–48.
Zhong X-M, et al. Alterations of CSF cystatin C levels and their correlations with CSF Αβ40 and Αβ42 levels in patients with Alzheimer’s disease, dementia with Lewy bodies and the atrophic form of general paresis. PLoS ONE. 2013;8(1): e55328.
Bousiges O, et al. Cerebrospinal fluid Alzheimer biomarkers can be useful for discriminating dementia with Lewy bodies from Alzheimer’s disease at the prodromal stage. J Neurol Neurosurg Psychiatry. 2018;89(5):467–75.
Bousiges O, Blanc F. Diagnostic value of cerebro-spinal fluid biomarkers in dementia with lewy bodies. Clin Chim Acta. 2019;490:222–8. https://doi.org/10.1016/j.cca.2018.11.027.
Dumurgier J, et al. Cerebrospinal fluid amyloid-beta 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimers Res Ther. 2015;7(1):30. https://doi.org/10.1186/s13195-015-0114-5.
Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheimers Dis. 2015;43(1):183–91. https://doi.org/10.3233/JAD-140771.
Koopman K, Le Bastard N, Martin JJ, Nagels G, De Deyn PP, Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau(181P). Neurochem Int. 2009;55(4):214–8. https://doi.org/10.1016/j.neuint.2009.02.017.
Vanderstichele H, et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin Chem Lab Med. 2006;44(12):1472–80. https://doi.org/10.1515/CCLM.2006.258.
Vanderstichele H, et al. Analytical performance and clinical utility of the INNOTEST® PHOSPHO-TAU (181P) assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clinical Chemistry and Laboratory Medicine (CCLM). 2006;44(12):1472–80.
Tschampa H, et al. Decreased CSF amyloid β42 and normal tau levels in dementia with Lewy bodies. Neurology. 2001;56(4):576–576.
Kaerst L, Kuhlmann A, Wedekind D, Stoeck K, Lange P, Zerr I. Using cerebrospinal fluid marker profiles in clinical diagnosis of dementia with Lewy bodies, Parkinson’s disease, and Alzheimer’s disease. J Alzheimers Dis. 2014;38(1):63–73.
Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation. 2010;7:1–8.
Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:1–12.
Janelidze S, et al. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3(1):12–20.
Wennström M, et al. The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with Lewy bodies. PLoS ONE. 2015;10(8): e0135458.
Stejskal D, Sporova L, Svestak M, Karpisek M. Determination of serum visinin like protein-1 and its potential for the diagnosis of brain injury due to the stroke: a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(3):263–8. https://doi.org/10.5507/bp.2011.049.
Schnurra I, Bernstein HG, Riederer P, Braunewell KH. The neuronal calcium sensor protein VILIP-1 is associated with amyloid plaques and extracellular tangles in Alzheimer’s disease and promotes cell death and tau phosphorylation in vitro: a link between calcium sensors and Alzheimer’s disease? Neurobiol Dis. 2001;8(5):900–9. https://doi.org/10.1006/nbdi.2001.0432.
Luo X, et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J Neurochem. 2013;127(5):681–90.
Herbert MK, et al. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat Disord. 2014;20(1):112–5. https://doi.org/10.1016/j.parkreldis.2013.09.003.
Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JA. Cerebrospinal fluid biochemical studies in patients with Parkinson’s disease: toward a potential search for biomarkers for this disease. Front Cell Neurosci. 2014;8:369. https://doi.org/10.3389/fncel.2014.00369.
Moccia M, et al. Is serum uric acid related to non-motor symptoms in de-novo Parkinson’s disease patients? Parkinsonism Relat Disord. 2014;20(7):772–5. https://doi.org/10.1016/j.parkreldis.2014.03.016.
Pellecchia MT, et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J Neurol. 2013;260(2):438–44. https://doi.org/10.1007/s00415-012-6648-6.
Pellecchia MT, et al. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naive Parkinson’s disease. Eur J Neurol. 2014;21(5):802–7. https://doi.org/10.1111/ene.12137.
Zhang Z, Cheng Y. miR-16-1 promotes the aberrant alpha-synuclein accumulation in parkinson disease via targeting heat shock protein 70. ScientificWorldJournal. 2014;2014: 938348. https://doi.org/10.1155/2014/938348.
Rassu M, et al. Role of LRRK2 in the regulation of dopamine receptor trafficking. PLoS ONE. 2017;12(6): e0179082. https://doi.org/10.1371/journal.pone.0179082.
Xiong R, et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol Aging. 2014;35(3):705–14. https://doi.org/10.1016/j.neurobiolaging.2013.09.027.
Zhou Y, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016;11:28. https://doi.org/10.1186/s13024-016-0094-3.
Santos MC, et al. miR-124, -128, and -137 orchestrate neural differentiation by acting on overlapping gene sets containing a highly connected transcription factor network. Stem Cells. 2016;34(1):220–32. https://doi.org/10.1002/stem.2204.
Li N, et al. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol Sci. 2017;38(5):761–7. https://doi.org/10.1007/s10072-017-2841-9.
Angelopoulou E, Paudel YN, Piperi C. miR-124 and Parkinson’s disease: a biomarker with therapeutic potential. Pharmacol Res. 2019;150: 104515. https://doi.org/10.1016/j.phrs.2019.104515.
Musaeus CS, Gleerup HS, Hogh P, Waldemar G, Hasselbalch SG, Simonsen AH. Cerebrospinal fluid/plasma albumin ratio as a biomarker for blood-brain barrier impairment across neurodegenerative dementias. J Alzheimers Dis. 2020;75(2):429–36. https://doi.org/10.3233/JAD-200168.
Magdalinou NK, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86(11):1240–7. https://doi.org/10.1136/jnnp-2014-309562.
Backstrom DC, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72(10):1175–82. https://doi.org/10.1001/jamaneurol.2015.1449.
Quiroz YT, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. 2020;19(6):513–21. https://doi.org/10.1016/S1474-4422(20)30137-X.
Kouchaki E, et al. Neurofilament light chain as a biomarker for diagnosis of multiple sclerosis. EXCLI J. 2021;20:1308–25. https://doi.org/10.17179/excli2021-3973.
Johnson EB, et al. Neurofilament light protein in blood predicts regional atrophy in Huntington disease. Neurology. 2018;90(8):e717–23. https://doi.org/10.1212/WNL.0000000000005005.
Kuhle J, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–15. https://doi.org/10.1212/WNL.0000000000007032.
Zanella I, Blasco H, Filosto M, Biasiotto G. Editorial: the impact of neurofilament light chain (NFL) quantification in serum and cerebrospinal fluid in neurodegenerative diseases. Front Neurosci. 2022;16: 915115. https://doi.org/10.3389/fnins.2022.915115.
Moors T, et al. Lysosomal dysfunction and alpha-synuclein aggregation in Parkinson’s disease: diagnostic links. Mov Disord. 2016;31(6):791–801. https://doi.org/10.1002/mds.26562.
Balducci C, et al. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov Disord. 2007;22(10):1481–4. https://doi.org/10.1002/mds.21399.
Parnetti L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in Dementia with Lewy Bodies. Neurobiol Dis. 2009;34(3):484–6. https://doi.org/10.1016/j.nbd.2009.03.002.
Parnetti L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in parkinson’s disease patients. Mov Disord. 2017;32(10):1423–31. https://doi.org/10.1002/mds.27136.
Dutta S, et al. alpha-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021;142(3):495–511. https://doi.org/10.1007/s00401-021-02324-0.
Pinnell JR, Cui M, Tieu K. Exosomes in Parkinson disease. J Neurochem. 2021;157(3):413–28. https://doi.org/10.1111/jnc.15288.
Ngolab J, et al. Brain-derived exosomes from dementia with Lewy bodies propagate alpha-synuclein pathology. Acta Neuropathol Commun. 2017;5(1):46. https://doi.org/10.1186/s40478-017-0445-5.
Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–71. https://doi.org/10.1016/j.cca.2018.11.009.
Wang X, et al. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid Med Cell Longev. 2020;2020:3232869. https://doi.org/10.1155/2020/3232869.
Kitamura Y, et al. Proteomic profiling of exosomal proteins for blood-based biomarkers in Parkinson’s disease. Neuroscience. 2018;392:121–8. https://doi.org/10.1016/j.neuroscience.2018.09.017.
Swift IJ, et al. Fluid biomarkers in frontotemporal dementia: past, present and future. J Neurol Neurosurg Psychiatry. 2021;92(2):204–15. https://doi.org/10.1136/jnnp-2020-323520.
Obrocki P, et al. Perspectives in fluid biomarkers in neurodegeneration from the 2019 biomarkers in neurodegenerative diseases course-a joint PhD student course at University College London and University of Gothenburg. Alzheimers Res Ther. 2020;12(1):20. https://doi.org/10.1186/s13195-020-00586-6.
Olson KE, et al. Neuroprotective activities of long-acting granulocyte-macrophage colony-stimulating factor (mPDM608) in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-intoxicated mice. Neurotherapeutics. 2020;17(4):1861–77. https://doi.org/10.1007/s13311-020-00877-8.
Gendelman HE, et al. Evaluation of the safety and immunomodulatory effects of sargramostim in a randomized, double-blind phase 1 clinical Parkinson’s disease trial. NPJ Parkinsons Dis. 2017;3:10. https://doi.org/10.1038/s41531-017-0013-5.
Spiteri AG, Wishart CL, Pamphlett R, Locatelli G, King NJC. Microglia and monocytes in inflammatory CNS disease: integrating phenotype and function. Acta Neuropathol. 2022;143(2):179–224. https://doi.org/10.1007/s00401-021-02384-2.
Biomarker validation following sargramostim treatment in Parkinson’s disease. ClinicalTrials.gov Identifier: NCT05677633. NIH U.S. National Library of Medicine. ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05677633. Accessed 30 Oct 2023.
Acknowledgements
We would like to thank BioRender.com for using their program to create the review figure and the INBRE grant from NIH (2P20GM103427) for supporting a site license to EndNote software. A special thank you is made to Ms. Joan H. Squires for her continued support and encouragement.
Funding
This work is supported by the University of Nebraska Foundation, which includes community donations from the Carol Swarts, M.D. Emerging Neuroscience Research Laboratory, the Margaret R. Larson Professorship, the Eisenberg Parkinson’s Research Fund, and the Frances and Louie Blumkin and Harriet Singer Research Foundations and National Institutes of Health grants P01 DA028555, R01 NS36126, P01 NS31492, P01 MH64570, P01 NS43985, P30 MH062261, and R01 AG043540 (HEG), and 2R01 NS034239 (HEG and RLM).
Author information
Authors and Affiliations
Contributions
MMA and HEG. developed the proposed outline and design of the article. The first draft was divided into sections, researched, and written by MMA, EL, RK, EGF, and SB Sections were compiled by MMA and refined and proofed by RLM and HEG. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
All authors have given their approval for submitting the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdelmoaty, M.M., Lu, E., Kadry, R. et al. Clinical biomarkers for Lewy body diseases. Cell Biosci 13, 209 (2023). https://doi.org/10.1186/s13578-023-01152-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-023-01152-x