Abstract
Introduction
Clinical trials investigating efficacy of immune checkpoint inhibitors (ICI) revealed sex-specific divergent outcomes in urothelial cancer (UC), suggesting that sex hormones might play an important role in gender-specific dimorphisms of response upon ICI. However, further clinical investigations are still needed to understand the influence of sex hormones in UC. The aim of this study was to get further insights on the prognostic and predictive value of sex hormone levels in patients with metastatic UC (mUC) who underwent ICI.
Material and methods
Sex hormone levels of patients with mUC including luteinizing hormone (LH), follicle-stimulating hormone (FSH), LH/FSH ratio, prolactin, testosterone and 17β-estradiol (E2) were evaluated at baseline and during ICI at 6/8 weeks and 12/14 weeks.
Results
Twenty-eight patients (10 women, 18 men) with a median age of 70 years were included. Metastatic disease was confirmed in 21 patients (75%) after radical cystectomy while seven patients showed mUC at first diagnosis. Twelve patients (42.8%) received first line and 16 patients second line pembrolizumab. The objective response rate (ORR) was 39% (CR in 7%). The median progression-free survival (PFS) and overall survival (OS) was 5.5 and 20 months. Focusing on changes of sex hormone levels during ICI, a significant increase in FSH levels and decrease of the LH/FSH ratio was noticed in responders (p = 0.035), yet without sex-specific significance. When adjusted for sex and treatment line, a significant increase of FSH levels was confirmed in men during second line pembrolizumab. Focusing on baseline levels, LH/FSH ratio was significantly higher in female responders (p = 0.043) compared to non-responders. In women, increased LH levels and LH/FSH ratio were associated with better PFS (p = 0.014 for LH, p = 0.016 for LH/FSH ratio) and OS (p = 0.026 and p = 0.018). In male patients, increased E2 levels were linked with improved PFS (p < 0.001) and OS (p = 0.039).
Conclusion
Increased LH and LH/FSH values in women as well as high E2 levels in men were significant predictors of better survival. Elevated LH/FSH ratio was predictive of better response to ICI in women. These results show first clinical evidence of the potential role of sex hormones as prognostic and predictive biomarker in mUC. Further prospective analyses are needed to corroborate our findings.
Plain language summary
Urothelial carcinoma (UC) presents as aggressive disease with a greater incidence in men, yet a more aggressive course of disease in women. Patients with metastatic UC receive a chemotherapy regimen as the gold standard, based on an included platin substance. In the case of having contraindications to chemotherapy, checkpoint immunotherapy, priming the immune system to the tumor, is the treatment of choice. Furthermore, immunotherapy is used as second line therapy in progressive disease after chemotherapy and as maintenance therapy in stable tumor conditions after completing the chemotherapy regimen.
Evidence shows that sex hormones of the hypothalamus–hypophysis axis influence development and course of UC. The sex hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH) stimulate estrogen (E2) production with a negative feedback function on the LH and FSH secretion. High levels of E2 present with a protective effect against UC. Sex has furthermore shown to predict potential response to immunotherapy. This study therefore focused on monitoring and correlating changes of sex hormone levels in 28 patients during therapy with checkpoint inhibitors.
This first study assessing changes in sex hormones and the influence of baseline sex hormone values on survival in UC shows that responders to immunotherapy had significantly increased FSH levels. FSH furthermore increased in male patients receiving second line immunotherapy. High values of LH and a high LH/FSH ratio at baseline correlated with better overall survival in female patients. High E2 levels were indicative of better survival in male patients. The study results represent first suggestive prognostic and predictive results to the response of immunotherapy in UC.
Highlights
-
Increased LH and LH/FSH values in women as well as high E2 levels in men were significant predictors of better survival.
-
Elevated LH/FSH ratio was predictive of better response to immunotherapy in women.
-
First clinical evidence of the potential role of sex hormones as prognostic and predictive biomarkers in metastatic urothelial cancer.
Similar content being viewed by others
Introduction
Urothelial cancer (UC) is one of the best-known cancer dignities exhibiting considerable sex-specific differences. Male sex is associated with a greater incidence of up to 5-times compared to females. However, women are more often diagnosed in a more advanced and aggressive disease stage [1,2,3,4]. Primary metastatic disease in UC accounts for approximately 5% of all newly diagnosed cases with a poor 5-year survival of only 5% [5]. According to international guidelines, aside from anatomical differences, female and male UC patients are clinically managed in the same way [6, 7]. Of note, a recent systematic review showed that female patients with muscle-invasive bladder cancer have a significantly increased risk of worse overall survival (OS) compared to male individuals [8].
Standard of care for first line treatment of metastatic UC (mUC) is a cisplatin-based chemotherapy combination [7]. Up to 50% remain ‘cisplatin-unfit’ by definition, which lead to implementation of a chemotherapy-regimen with carboplatin in these patients [9]. On the basis of two phase II clinical trials, two checkpoint inhibitors (ICI), namely the PD-1 receptor inhibitor pembrolizumab [10] and the PD-L1 inhibitor atezolizumab [11], have been approved by the FDA and EMA as the first line treatment in cisplatin-unfit mUC patients [10, 11].
Pembrolizumab demonstrated a significant OS improvement in patients with progressive disease after receiving first line platin-based chemotherapy [12]. On the basis of its high efficacy, pembrolizumab is currently recommended in international guidelines as second line therapy in mUC [7]. In patients with stable or remissive mUC after first line cisplatin-based chemotherapy, avelumab, a PD-L1 inhibitor is moreover approved as further maintenance therapy [13]. Resistance to standard therapies and high aggressiveness of mUC require a clinical shift towards individualized medicine, thus implying the need for further research in the field of sex-specific survival.
Several lines of evidence suggest a role of sex steroid hormones in UC, yet detailed mechanisms behind this remain unclarified. The sex hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are members of the gonadotropin family and secreted by the anterior pituitary gland. Both stimulate gonadal 17β-estradiol (E2) production with a negative feedback function on the pituitary LH and FSH secretion [14]. Literature indicates the potential role of E2 and androgens influencing the development and course of UC [15]. McGraph et al. described an increased risk of UC in postmenopausal women compared with premenopausal women, irrespectively of an experienced natural or surgical menopause [16]. This fact suggests E2 to significantly influence tumor physiology.
In addition, expression of the androgen receptor (AR) was repeatedly correlated with carcinogenesis [17, 18], accounting for the accumulation of male disease incidence. Loss of AR responsiveness has shown to lead to disease progression via androgen-independent pathways, maybe explaining the higher rates of progression in women [19, 20]. Given the common embryological origin of the bladder trigone and the upper portion of the vagina, there is presence of estrogen receptor expression in the female bladder. E2 seems to have a protective effect against bladder tumorigenesis via the estrogen receptor pathway [21]. Furthermore, female patients receiving E2 replacement therapy are suggested to have a reduced UC risk [22, 23].
Sex is also considered for being a responsible factor influencing the patients individual immune and immunotherapeutic response [21]. Moreover, sex has shown potential to predict response to PD-1/PD-L1 targeting ICI, presenting poorer outcomes in women [24]. PD-L1 and other immune checkpoints are likely to be implicated in the female bladder, which is exposed to higher estrogen levels than the male bladder [25].
We recently showed that sex hormones of the pituitary–gonadal axis have already shown to be accountable for differences in responses to checkpoint-based therapies in renal cell carcinoma [26], yet, to date, the influence of sex hormones on response of ICI in UC has not been described. Therefore, the aim of this study was for the first time to monitor and correlate changes of sex hormone levels during therapy with checkpoint inhibitors in mUC with focus on therapy response. Additionally, we evaluated if baseline levels of sex hormones could predict therapy response and survival.
Materials and methods
This is a retrospective observational study based on an Austrian uro-oncology cancer database. Consent of the local ethics commission of the Medical University Innsbruck was obtained with the study approval number 1006/2017. Research work was performed in accordance with the 1964 Helsinki Declaration, its later amendments and institutional ethical standards based on good clinical practice [27].
Patient population and data collection
Medical records of patients diagnosed with mUC and treated with the PD-1 inhibitor pembrolizumab in first and second line were reviewed retrospectively. All patients with a confirmed histopathology of mUC of the bladder, with no received hormone replacement therapy and eligible follow-up (FU) data at our outpatient department were included. Patients with no definitive histopathology, simultaneous other oncological disease, ongoing hormonal replacement therapy and those who were followed up elsewhere were excluded. Tumor classification was performed according to the 2016 tumor-node-metastasis (TNM) classification [28] and grading referring to the World Health Organization and International Society of Urological Pathology categorization [29]. Demographic patient data as well as tumor characteristics, sequence of systemic therapy, therapy response to PD-1 inhibition, dates of progression and time of death or last FU visit were collected. Pembrolizumab was administered as approved, intravenously at a fixed dosage of 200 mg once every three weeks [10, 12]. Since April 2020, the fixed dosage of 400 mg once every 6 weeks was additionally authorized.
Follow-up and blood sampling
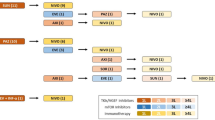
FU visits were scheduled every 3 months and consisted of blood analysis and radiographic imaging. Imaging consisted of a chest and abdominopelvic computer tomography scan with contrast medium and urographic phase. Analysis of sex hormones including LH, FSH, LH/FSH ratio, E2, testosterone and prolactin were performed from the patient’s serum with enzyme-linked immunosorbent assays at three different points in time defined as: baseline at the beginning of immunotherapy in week 0, interim analysis after week 6/8 and final analysis in week 12/14. An overview of the sampling process is shown in Fig. 1. Reference values for female patients were 0.8–7.6 U/L for LH, 1.6–20.4 U/L for FSH, 11–43 ng/L for E2, 1.70–4.90 µg/L for testosterone and 2.5–17.0 µg/L for prolactin. In male patients, reference levels were 0.8–7.6 U/L for LH, 1.6–20.4 U/L for FSH, 11–43 ng/L for E2, 1.70–4.90 µg/L for testosterone and 2.5–17.0 µg/L for prolactin. Complete remission (CR), partial remission (PR) or stable disease (SD) in CT imaging as well as good tolerance of therapy were necessary for continuation of treatment. Progressive disease (PD) lead to a further therapy regimen in line. ORR was defined as the number of patients with response divided by the total number of patients. OS was defined as time from first therapy administration to death from all causes. Progression-free survival was specified as time from therapy begin to tumor progression, treatment discontinuation or tumor-specific death. In patients who have not progressed and still alive, the last time of FU was used as endpoint in time.
Statistical analysis
Numeric features of the study cohort were presented as medians with interquartile ranges. Categorical variables were shown as percentages and total numbers within the set of complete observations. Normality of distribution of the hormone levels as well as normality of the individual differences in hormone levels between consecutive time points was investigated by the Shapiro–Wilk test and visual inspection of quantile–quantile plots. Changes in hormone levels were investigated by the Friedman test, for which patients were subdivided into two groups: stable disease/progressive disease (SD/PD) and complete remission/partial remission (CR/PR). Baseline hormone levels were compared using the Mann–Whitney test. Significance of differences in survival between hormone strata were assessed by Mantel–Haenszel test. The variable normality was checked separately for females and males. Statistical data analysis was performed with R (version 4.2.0), basic data transformation and re-coding tasks were done with the tidyverse package bundle and the rlang metaprogramming tool set. Numeric results were visualized with the ggplot and ExDA packages. Survival data were presented in Kaplan–Meier plots generated with the survminer and kmOptimizer packages. Report figures were built with the cowplot and figure packages. Report tables were created with the flextable package. To examine the sex hormone status and its associations with survival, patients were split into a high and low group using the gender-specific median as cutoff level. Overall response rate was defined as the number of patients with complete response or partial response divided by the total number of patients.
Results
Patient and tumor characteristics
Twenty-eight patients who were diagnosed and treated with mUC at our institution were included. Of these, 18 (64%) were male and 10 (36%) were female with a median age of 70 years (range 46–85). Age at diagnosis did not show any significance in baseline hormone values. 50% (n = 14) of all included patients had a smoking history and 75% (n = 21) underwent primary surgery after diagnosis according to the current recommended guidelines [7]. Histopathology revealed pure UC in all cases. In total, 3 (11%), 12 (43%), 4 (14%), 4 (14%), 3 (11%) and 2 (7.1%) patients presented with the tumors staged pT1, pT2a, pT2b, pT3a, pT3b and pT4a, respectively. Tumor grading resulted in 3.6% (n = 1) grade 2 and 96.4% (n = 27) grade 3. Thirteen (46%) patients had lymph node metastasis at diagnosis and seven (25%) patients presented in a metastatic disease. Twelve (42.8%) patients received pembrolizumab as first line therapy while 16 patients received it as second line therapy. No patient received ICI in any later therapy lines. Overall, the objective response rate (ORR) was 39% (n = 11) and 86% (n = 24) suffered disease progression in the study setting. Seventeen (61%) patients died before the date of completion of the study. Median duration of OS and progression-free survival (PFS) were 20 (range: 1–82) and 5.5 (range: 1–65) months.
Sex hormone level characteristics during immunotherapy
Change of levels of sex hormone levels including LH, FSH, LH/FSH ratio, prolactin, E2 and testosterone were collected during ICI for the whole patient cohort and analyzed separately for female and male patients. Alterations of the median blood hormone concentrations at baseline and during therapy were not significant in the entire cohort, as seen in Table 1. Additionally, there were no significant sex-specific deviations in hormone levels between time points, Additional file 1: Figure S1. An analogical analysis in therapy responders vs. non-responders, irrespective of gender, revealed a significant rise in circulating FSH levels in patients with complete or partial response (p = 0.035). This was paralleled by a significant decrease of the LH/FSH ratio in the responder subset (p = 0.035), Fig. 2. There were no differences in hormone levels between female responders and non-responders, yet a barely missed significant rise in FSH concentrations (p = 0.05) paralleled by a noticeable but non-significant drop of testosterone in male responders, Fig. 3. No significant changes of prolactin levels were observed in the female as well as in the male population. In females receiving first line ICI, only LH levels tended to decrease, yet just missing statistical significance (p = 0.074), Fig. 4. FSH levels significantly increased in men in second line ICI (p = 0.029), Fig. 5.
Higher baseline LH/FSH ratio in female immunotherapy responders
Comparison of baseline hormone concentrations between females with an overall therapy response (CR/PR) and without response (SD/PD) revealed a significantly higher LH/FSH ratio in responders (p = 0.043), as seen in Fig. 6. In male responders a direct trend towards lower testosterone levels could be noticed, this effect was however not significant, Fig. 7.
High LH in females and high E2 in males correlate with better survival
We analyzed potential sex-specific correlations between sex hormone levels assessed at baseline with response to ICI (CR/PR) or outcomes (PFS, OS). In women, high levels of LH at baseline were found to be significantly correlated with better PFS (p = 0.014) and OS (p = 0.026). An even more significant association was seen between an increased LH/FSH ratio an PFS (p = 0.018) as well as OS (p = 0.018), Figs. 8, 9. In male patients, higher baseline levels of estrogen correlated with better PFS (p ≤ 0.001) and OS (p = 0.039), Figs. 10, 11. No other endocrine parameters correlated with PFS or OS in either female or male patients.
Discussion
Sex differences with better anti-tumor responses in male patients have already been described in the past [24, 25, 30] which makes it necessary to evaluate potential influencing factors also in urooncological tumor entities. To the best of our knowledge, this is the first study assessing changes in sex hormones and the influence of baseline sex hormone values on survival in mUC receiving ICI.
Sex dimorphism of anti-tumor immune response and following efficacy of ICI relies on complex regulation and interactions of genes, microbiome composition and sex hormones [31]. The sole role of gonadotropins in UC has not yet been investigated. However, according to literature, E2 increases production of immunoglobulins [32] whereas androgens, including testosterone, have been reported to suppress immune cell activity [33]. Additionally, regulatory T cells increase with high E2 levels [34]. E2 enhances both cell mediated and humoral immune responses [34, 35], as well as secretion of IgG and IgM [33] and was found to directly up-regulate the expression of mediators of B cell survival [36].
In the study, overall responders to ICI had significantly increased FSH levels, combined with a physiologically associated lowered LH/FSH ratio, yet without sex-specific differences. In male therapy responders, FSH significantly increased when the patient received second line ICI. Testosterone in this cohort yet only showed a slight downward trend. Increased FSH values would physiologically lead to an increase in E2 values, which we, interestingly, we did not observe in our cohort. However, the small number of patients could also mask statistical significance. Based on the already described protective effect of E2 on bladder cancer, it can be hypothesized that the gonadotropic precursors also enhance the protective effect of E2.
Our data demonstrate that high LH values and a high LH/FSH ratio at baseline correlated with better PFS and OS in female patients. In male patients a high E2 level was also indicative for better survival with prolonged PFS and OS, also accounting for improved survival of patients when early sex hormone precursors are present. So far, literature has shown divergent results regarding the relationship between sex and response to ICI. Large meta-analysis showed no significant differences in therapy response to ICI including various cancer types [37, 38]. Further analysis revealed survival benefits for male but not female patients [25] undergoing ICI [24, 39]. However, we could not confirm such a trend in our study cohort and the detailed pathophysiological mechanisms remain unclear. Increased expression of PD-1 has been shown to be mediated by E2 [34]. This could result in a positive advantage in immunological and clinical response for women with higher LH and FSH baseline levels as seen in our cohort and could explain better survival in men with higher E2 levels.
Our study brings up certain limitations, primarily the small number of patients decreases the statistical power and these potential clinically relevant effects need to be validated in a larger collective. In addition, due to the low cohort size, effects of other factors impacting on survival such as tumor stage or metastasis status could not be included in the current analysis. It is of note that we only included postmenopausal women and our collective included patients treated with ICI in the first- and second line.
Perspectives and significance
First clinical data on modulation of sex hormones and gonadotropins during ICI in mUC are presented with this work. A significant increase in FSH in ICI responders was observed in the overall cohort, as well as in solely male individuals, but not in females. LH/FSH ratio at baseline was increased in female therapy responders. Increased baseline LH and LH/FSH ratio in females and increased baseline E2 in males correlated with better PFS and OS in our cohort. Further investigation of sex hormones and their association on efficacy of ICI is of utter importance to future research to provide more insights in these regulating mechanisms. To date, it is becoming apparent that sex hormones may well represent possible prognostic markers; therefore, more intensive observation of these interactions should become focus of larger cohort studies.
Conclusions
We provide first evidence that sex hormones can influence the response to immune checkpoint inhibitors as well as survival in patients with metastatic urothelial cancer. Thus, elucidating the interaction between sex hormones and immune responses in men and women could improve our understanding of resistance mechanisms to immune checkpoint inhibitors and better select patients who might benefit most from these specific therapies.
Availability of data and materials
Please contact author for data requests.
Abbreviations
- AR:
-
Androgen receptor
- UC:
-
Urothelial cancer
- CR:
-
Complete remission
- E2:
-
Estrogen
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- FSH:
-
Follicle-stimulating hormone
- FU:
-
Follow-up
- LH:
-
Luteinizing hormone
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- PD:
-
Progressive disease
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- PFS:
-
Progression-free survival
- PR:
-
Partial remission
- SD:
-
Stable disease
References
National Cancer Institute. SEER*Explorer: An interactive website for SEER cancer statistics [cited 2022 Dec 28]. Available from: URL: https://seer.cancer.gov/statistics-network/explorer/.
Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, et al. Gender and Bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69(2):300–10.
Liu S, Yang T, Na R, Hu M, Zhang L, Fu Y, et al. The impact of female gender on bladder cancer-specific death risk after radical cystectomy: a meta-analysis of 27,912 patients. Int Urol Nephrol. 2015;47(6):951–8.
Waldhoer T, Berger I, Haidinger G, Zielonke N, Madersbacher S. Sex differences of ≥ pT1 bladder cancer survival in Austria: a descriptive, long-term, nation-wide analysis based on 27,773 patients. Urol Int. 2015;94(4):383–9.
Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). 2020;8(1):15.
Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75–94.
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104.
Mori K, Yanagisawa T, Katayama S, Laukhtina E, Pradere B, Mostafaei H, et al. Impact of sex on outcomes after surgery for non-muscle-invasive and muscle-invasive bladder urothelial carcinoma: a systematic review and meta-analysis. World J Urol. 2022;41:909.
Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432–8.
Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92.
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–30.
Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, et al. Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic–pituitary–gonadal axis receptors. J Neurochem. 2009;110(3):1014–27.
Zhang Y. Understanding the gender disparity in bladder cancer risk: the impact of sex hormones and liver on bladder susceptibility to carcinogens. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2013;31(4):287–304.
McGrath M, Michaud DS, de Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol. 2006;163(3):236–44.
Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64(2):383–8.
Lombard AP, Mudryj M. The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer. 2015;22(5):R265–77.
Gakis G, Stenzl A. Gender-specific differences in muscle-invasive bladder cancer: the concept of sex steroid sensitivity. World J Urol. 2013;31(5):1059–64.
Jing Y, Di C, Guo W, Jiang J, Jiang B, Lu Y, et al. Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial-mesenchymal transition. Cancer Lett. 2014;348(1–2):135–45.
Özdemir bladderotto G-P. Sex Hormones and Anticancer Immunity. Clin Cancer Res. 2019;25(15):4603–10.
Daugherty SE, Lacey JV, Pfeiffer RM, Park Y, Hoover RN, Silverman DT. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2013;133(2):462–72.
Cantwell MM, Lacey JV, Schairer C, Schatzkin A, Michaud DS. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer. 2006;119(10):2398–401.
Botticelli A, Onesti CE, Zizzari I, Cerbelli B, Sciattella P, Occhipinti M, et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget. 2017;8(59):99336–46.
Conforti F, Pala L, Bagnardi V, de Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–46.
Tulchiner G, Pichler R, Ulmer H, Staudacher N, Lindner AK, Brunner A, et al. Sex-specific hormone changes during immunotherapy and its influence on survival in metastatic renal cell carcinoma. Cancer Immunol Immunother. 2021;70(10):2805–17.
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2000; 284(23):3043–5.
O’Sullivan B, Brierley J, Byrd D, Bosman F, Kehoe S, Kossary C, et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017;18(7):849–51.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part b: prostate and bladder tumours. Eur Urol. 2016;70(1):106–19.
Wang S, Cowley LA, Liu X-S. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules. 2019;24(18):3214.
Haupt S, Caramia F, Klein SL, Rubin JB, Haupt Y. Sex disparities matter in cancer development and therapy. Nat Rev Cancer. 2021;21(6):393–407.
Mirandola L, Wade R, Verma R, Pena C, Hosiriluck N, Figueroa JA, et al. Sex-driven differences in immunological responses: challenges and opportunities for the immunotherapies of the third millennium. Int Rev Immunol. 2015;34(2):134–42.
Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 1999;103(2 Pt 1):282–8.
Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84(2):370–8.
Lü FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128(1):10–20.
Sthoeger ZM, Chiorazzi N, Lahita RG. Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. J Immunol. 1988;141(1):91–8.
Wallis CJD, Butaney M, Satkunasivam R, Freedland SJ, Patel SP, Hamid O, et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5(4):529–36.
Yang F, Markovic SN, Molina JR, Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, et al. Association of sex, age, and eastern cooperative oncology group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(8): e2012534.
Nosrati A, Tsai KK, Goldinger SM, Tumeh P, Grimes B, Loo K, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–7.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RP and AKL conceived and designed research. FL performed the patient record reviews. PT contributed statistical analysis. RP, AKL, DB, AS and FK analyzed and interpreted the data. AKL and RP drafted the manuscript. MP, BT, MH and RP held supervision. All authors made substantial contributions to the manuscript draft, critical revised it and approved the submitted final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is a retrospective observational study based on an Austrian uro-oncology cancer database. Consent of the local ethics commission of the Medical University Innsbruck was obtained with the study approval number 1006/2017. Research work was performed in accordance with the 1964 Helsinki Declaration, its later amendments and institutional ethical standards based on good clinical practice.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Changes in blood levels of luteinizing hormone, follicle-stimulating hormone, LH to FSH ratio, prolactin, estrogen and testosterone prior to the therapy start, after 6/8 weeks and 12/14 weeks after the therapy begin in females and males. Statistical significance was assessed by Friedman test with Kendall’s W effect size statistic. Points represent single observations. Gray lines connect observations belonging to the same participant. Numbers of complete observations are displayed in the plot captions. Effect size and p values are presented in the plot facets. Female, male.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lindner, A.K., Lackner, F., Tymoszuk, P. et al. Sex hormones influence survival of patients with metastatic urothelial carcinoma undergoing immune checkpoint therapy. Biol Sex Differ 14, 38 (2023). https://doi.org/10.1186/s13293-023-00522-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-023-00522-x