Abstract
Purpose
To assess the prognostic value of sex for non-muscle-invasive/muscle-invasive bladder urothelial carcinoma (NMIBC/MIBC) treated with radical surgery.
Methods
The PubMed, Web of Science, and Scopus databases were searched in November 2021 according to the Preferred Reporting Items for Systematic Review and Meta-analysis statement. Studies were deemed eligible if they involved the comparison of the overall, cancer-specific, progression, and recurrence-free survival of patients with NMIBC/MIBC. Formal sex-stratified meta-analyses of these outcomes were performed.
Results
Thirty-one studies, which included 32,525 patients with NMIBC, and 63 studies, which included 85,132 patients with MIBC, were eligible for review and meta-analysis. Female sex was associated with worse cancer-specific survival (pooled hazard ratio [HR], 1.21; 95% confidence interval [CI], 1.11–1.31) and overall survival (pooled HR, 1.02; 95% CI, 1.00–1.05) in patients with MIBC. In contrast, however, sex was not associated with cancer-specific survival (pooled HR, 1.01; 95% CI, 0.70–1.46), progression-free survival (pooled HR, 1.04; 95% CI, 0.88–1.24), and recurrence-free survival (pooled HR, 1.06; 95% CI, 0.98–1.16) in patients with NMIBC.
Conclusions
Sex is associated with an increased risk of worse survival outcomes in patients with MIBC but not in those with NMIBC. Given the genetic and social differences between sexes, sex may represent a key factor in the clinical decision-making process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urothelial carcinoma of the bladder (UCB) is the ninth most diagnosed cancer in the world. Approximately 75–85% of patients with UCB in developed countries present with disease confined to the mucosa or submucosa, i.e., non-muscle-invasive bladder cancer (NMIBC) [1]. Transurethral resection (TUR) is an initial clinical step in the diagnosis and management of NMIBC [2, 3]. Patients who undergo TUR generally show favorable cancer-specific survival (CSS) and overall survival (OS) [4]. However, the disease recurs in > 50% of these patients, with progression to muscle-invasive bladder cancer (MIBC) in approximately 20% of patients [5, 6]. Risk stratification of patients is key to formulating an appropriate management strategy in light of their probability of recurrence and progression [7]. Radical cystectomy (RC) with lymph node dissection remains the standard treatment for very high-risk NMIBC and MIBC [1, 8]. Despite definitive therapy with curative intent, the 5-year OS of patients remains poor (< 60%) [9, 10]. Thus, various clinical and pathological factors have been explored to improve the risk stratification of patients with UCB to facilitate clinical decision-making and patient counseling [11,12,13,14,15,16].
Sex-specific differences in the outcomes of UCB have been extensively investigated [14]. Men have a threefold greater risk of developing UCB than women. Additionally, although the incidence of UCB increased 25% faster in men than in women over the past decade, the female sex was also found to be an independent adverse prognostic factor for UCB [17, 18]. Smoking habits, tumor biology, occupational risk factors, response to BCG therapy/immunotherapy, and anatomical and hormonal factors may affect these reported sex-specific disparities in UCB statistics [14]. However, no meta-analysis on the sex-stratified comparison of the outcomes of NMIBC and MIBC has been conducted to date. Moreover, sex-specific differences in the outcomes of UCB are often overlooked in the clinical practice. Understanding the factors associated with disparities in the presentation, evaluation, and management of UCB in men and women could result in improvements in the timeliness and intensity of healthcare delivery [19]. For instance, it should be deemed crucial to ensure that women with suspected hematuria likely due to infection they are prone to, e.g., cystitis, are screened for UCB as well, just as men are, to avoid overlooking it, given that reliable detection of early-stage UCB in women is likely to lead to improvements in their life prognosis. In addition, such efforts may lessen the magnitude of the sex-specific disparities in UCB outcomes [19]. Therefore, the aim of this systematic review and meta-analysis was to summarize the existing data and determine whether sex-specific differences may predict oncological outcomes in patients treated with surgery for NMIBC and MIBC. This study also explored the effects of sex on the prognosis of UCB.
Methods
Search strategy
The systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [20]. The PubMed, Web of Science, and Scopus databases were searched in November 2021 to identify reports on the prognostic value of sex in UCB. The keywords used in our search strategy were: (transurethral resection OR cystectomy) AND (gender OR sex) AND (survival OR mortality). The primary outcomes of interest in NMIBC were recurrence-free survival (RFS) and progression-free survival (PFS) with the secondary outcomes being CSS. The primary outcome of interest in MIBC was CSS with the secondary outcomes being OS. Initial screening was performed independently by two investigators based on titles and abstracts to exclude ineligible reports, and the reasons for exclusions were noted. Potentially relevant reports were subjected to a full-text review and their relevance confirmed after the data extraction process. Disagreements were resolved via consensus with a third investigator.
Inclusion and exclusion criteria
Studies were included if they had involved female patients treated for UCB (Patients) who had received surgery (Intervention) compared to male patients (Comparison) to assess the independent predictive value of sex on CSS, OS, RFS, and PFS (Outcome) utilizing multivariate Cox regression analysis (Study design) in nonrandomized observational, randomized, or cohort studies. Excluded from analysis were reviews, letters, editorials, meeting abstracts, replies from authors, case reports and non-English articles. Where the database studies were concerned, our analysis was limited to one national database study to avoid redundancies. In case of duplicate publications on the same study population, the study of a higher quality or of the most recent date was selected. The references listed in the manuscripts included were also explored for further studies of interest.
Data extraction
Two investigators independently extracted the following information from the included articles: first author’s name, publication year, country of recruitment, period of patient recruitment, number of patients, age, sex, oncological outcome, and follow-up duration. Subsequently, hazard ratios (HR) and 95% confidence intervals (CI) were extracted from multivariate analyses for any sex difference found to be associated with each outcome of interest and all discrepancies in data extraction were resolved by consensus with a third investigator.
Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the nonrandomized studies included according to the Cochrane Handbook for Systematic Reviews of Interventions. The scale rates selection (1–4 points), comparability (1–2 points), and exposure (1–3 points), with total scores ranging from 0 (lowest) to 9 (highest). The main confounders were identified as the important prognostic factors of cancer-specific, overall, progression-free, and recurrence-free survival. The presence of confounders was determined by consensus and review of the literature. We identified studies with scores above 6 as high-quality choices.
Risk of bias (RoB) assessment
Each of the studies included in this analysis was assessed for RoB according to the Cochrane Handbook for Systematic Reviews of Interventions (Supplementary Fig. 1, Supplementary Fig. 2). Due to the nature of these nonrandomized studies, the RoB was determined for each study by examining the risk of pre-assigned confounders. The confounding factors were identified as the most important of prognostic factors at the time of treatment. Each study was assessed for RoB independently by two authors, with the overall RoB level defined as “low”, “intermediate” or “high”.
Statistical analyses
Forest plots were used to assess and summarize the multivariate HRs to describe the relationships between sex differences and CSS, OS, RFS, and PFS. Studies were not considered eligible for meta-analysis if they had used Kaplan–Meier log-rank, univariate Cox proportional hazard regression, or general logistic regression analyses. For all studies reporting only HRs and P values, the corresponding 95% CIs were calculated [21, 22]. The studies included in the meta-analysis were evaluated for heterogeneity in outcome using the Cochrane’s Q test and the I2 statistic. Significant heterogeneity was indicated by a P < 0.05 in Cochrane’s Q tests and a ratio of > 50% in I2 statistics. Fixed-effects models were used to calculate pooled HRs for non-heterogeneous outcomes [23,24,25]. Sensitivity analyses were conducted to assess the robustness of the results based on the quality of the studies included. All statistical analyses were performed using Review Manager 5.3 and Stata/MP 14.2 (Stata Corp., College Station, TX) with the level of statistical significance set at P < 0.05.
Results
Study selection and characteristics
Our initial search identified a total of 1512 publications; of these, a total of 1243 were available after exclusion of duplicates (Supplementary Fig. 3). A total of 1,071 articles were excluded after screening of their titles and abstracts, and 172 articles were available for full-text review. A total of 31 studies in NMIBC which accounted for 32,525 patients, as well as 63 studies in MIBC which accounted for 85,132 patients, were identified as meeting the selection criteria for the current meta-analysis. Data extracted from the 94 studies are summarized in Supplementary Tables 1 and 2. All studies included in this study were published between 2003 and 2021 with 31, 21, 23, and 18 conducted in Europe, North America, Asia and internationally, and with patient accrual for these studies occurring between 1971 and 2020. Males and females accounted for 25,317 (77.8%) and 7208 (22.2%), respectively, in the NMIBC cohort, as well as for 65,986 (77.5%) and 19,146 (22.5%), respectively, in the MIBC cohort (median age, 61.4–75 years), with a median follow-up of 10.2–223.2 months.
Meta-analysis
Association of sex difference with CSS in MIBC
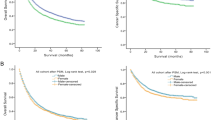
Forty-two studies involving 42,794 patients provided data on the association of sex difference with CSS in MIBC. The forest plot (Fig. 1A) revealed that sex difference was significantly associated with CSS in MIBC (pooled HR, 1.21; 95% CI, 1.11–1.31). The Cochrane’s Q test (P < 0.001) and I2 test (I2 = 65%) revealed significant heterogeneity.
Association of sex difference with OS in MIBC
Forty-five studies involving 63,170 patients provided data on the association of sex difference with OS in MIBC. The forest plot (Fig. 1B) revealed that sex difference was significantly associated with OS in MIBC (pooled HR, 1.02; 95% CI, 1.00–1.05). The Cochrane’s Q test (P = 0.002) and I2 test (I2 = 42%) revealed no significant heterogeneity.
Association of sex difference with PFS in NMIBC
Twelve studies involving 22,934 patients provided data on the association of sex difference with PFS in NMIBC. The forest plot (Fig. 2A) revealed that sex difference was not significantly associated with PFS in NMIBC (pooled HR, 1.04; 95% CI, 0.88–1.24). The Cochrane’s Q test (P = 0.016) and I2 test (I2 = 52.7%) revealed significant heterogeneity.
Association of sex difference with RFS in NMIBC
Thirty studies involving 31,408 patients provided data on the association of sex difference with RFS in NMIBC. The forest plot (Fig. 2B) revealed that sex difference was not significantly associated with RFS in NMIBC (pooled HR, 1.06; 95% CI, 0.98–1.16). The Cochrane’s Q test (P ≤ 0.001) and I2 test (I2 = 57.2%) revealed significant heterogeneity.
Association of sex difference with CSS in NMIBC
Six studies involving 11,026 patients provided data on the association of sex difference with CSS in NMIBC. The forest plot (Fig. 2C) revealed that sex difference was not significantly associated with RFS in NMIBC (pooled HR, 1.01; 95% CI, 0.70–1.46). The Cochrane’s Q test (P = 0.006) and I2 test (I2 = 69%) revealed significant heterogeneity.
Sensitivity analyses
Sensitivity analyses were performed based on the quality of the studies included, which demonstrated that the overall HRs were not significantly influenced in high-quality group, suggesting the robustness and reliability of the results in our meta-analysis.
Discussion
In this systematic review and meta-analysis, we investigated the prognostic value of sex for UCB. The results showed that female patients with MIBC have a significantly increased risk for worse CSS and OS compared to their male counterparts. However, there were no sex-associated differences in the prognosis of patients with NMIBC.
Our findings on the outcomes of MIBC corroborate the frequently reported fact that female patients with UCB are at a prognostically and clinically significant risk of succumbing to their disease. There are multiple reasons for this disparity. First, women with UCB are more likely to present with advanced cancer and are often older, which is a risk factor for poorer outcomes after RC [26, 27]. Second, hematuria and irritative voiding symptoms in female patients are first interpreted as symptoms of gynecologic diseases or urinary tract infections, leading to delays in the detection of UCB in women [28, 29]. Delayed UCB diagnosis increases cancer mortality, independent of the cancer stage and grade [30]. Thus, continued efforts are warranted to educate physicians on the need to follow a standardized diagnostic approach for patients with hematuria, regardless of sex [31, 32]. Third, it is hypothesized that embryologic and anatomical differences between men and women account for the sex-specific differences in UCB prognoses. The embryonic development of the trigone and the posterior bladder neck, which form a common origin with the upper part of the vagina, may contribute to the more invasive extension of UCB in women [33]. In contrast to men whose prostate and Denonvilliers’ fascia prevent direct tumor spread and lymphovascular invasion from the bladder neck to adjacent organs, women have no barriers in place between the bladder neck and the anterior vaginal wall [34]. Thus, the prognosis of UCB varies widely between sexes, as observed in pT4 disease involving vaginal or prostate invasion. Another relevant anatomic difference between men and women is bladder wall thickness. With advancing age, men develop thicker detrusor muscles secondary to changes caused by the resistance associated with benign prostatic hyperplasia [35]. While these anatomical differences between the sexes may affect UCB prognosis, it has been shown that the impact of these differences on prognosis is particularly significant in MIBC, but limited in NMIBC. In other words, if the disease remains in the NMIBC stage in women, it is likely to be diagnosed relatively earlier, thus causing minimal anatomical damage and reducing the magnitude of the differences between the prognosis of UCB in men and women. However, this is only a theoretical hypothesis that is yet to be validated. Indeed, the results of the present meta-analysis did not show any significant differences in RFS, PFS, and CSS between men and women with NMIBC.
Another hypothesis regarding the prognostic differences between male and female patients with UCB is the influence of sex steroids and their receptors. Numerous studies have indicated a potential role of androgens and estrogens and their receptors in influencing the development and course of UCB [36, 37]. For example, the expression of the androgen receptor (AR) is inversely correlated with UCB stage, suggesting that its expression may promote carcinogenesis [38, 39]. Loss of AR responsiveness may account for an increased risk of disease progression via an androgen-independent mechanism, a hypothesis that could explain the earlier progression of UCB in women [40, 41]. In contrast, female sex hormones, predominantly estrogens, protect against bladder tumorigenesis through the estrogen receptor (ER) pathway [42]. ER expression in the female bladder is not surprising, given the common embryological origin of the bladder trigone and the upper portion of the vagina. Furthermore, several lines of epidemiological evidence suggest a reduced risk for UCB in women who had menarche at an older age, those with multiparity, and those receiving combined estrogen and progestin hormone replacement therapy [43,44,45], suggesting a role of sex steroids in the risk for UCB. However, the exact effect of sex steroids and their receptors on UCB is not fully established yet.
Sex-specific differences have been noted in the responses to various therapeutic agents. Sex-specific differences in immunity remain a critical factor that dictates host immune responses [46, 47]. Notably, women are more likely to be affected by urinary tract infection or painful bladder syndrome than men, suggesting sex-specific differences in immunity in the bladder. In the context of infection, which may be analogous to BCG immunotherapy, there are stark differences in the innate immune responses of men and women, given the presence of non-commensal bacteria in the bladder lumen [48]. Increased susceptibility to urinary tract infections among postmenopausal women is also attributed to their lower estrogen levels. These sex-specific disparities may be attributed to the influence of estrogen signaling on BCG response [49]. In BCG response, the antitumor properties of BCG are mediated by the binding of BCG to bladder cells via the integrin-a5b1 receptor complex, which is upregulated by the cytokine interleukin (IL)-6 [50], whose expression is, in turn, inhibited by estrogen [51]. Furthermore, blocking the binding of BCG to the bladder mucosa has been shown to inhibit BCG-related antitumor activity [52], which was recently reported to occur due to pathway downregulation [49]. In the context of PD-1/PD-L1 targeting immunotherapy, sex has recently been reported as an important variable in determining response to treatment, which explains the poor response in women [53, 54]. Notably, the expression of PD-L1 is also regulated by estrogen and several X-linked micro-RNAs [55]. Along with these regulatory mechanisms, the increased expression of PD-L1 and/or other immune checkpoints is likely implicated in the female bladder, which is exposed to more pathogenic microbial challenges, as well as higher estrogen levels, than the male bladder [54]. However, further studies of different cancer states that are conducted under normal physiological conditions are warranted to obtain definitive supporting evidence of the clinical utility of this hypothesis.
Another available line of evidence is that smoking, the most established risk factor for UCB, has a larger impact in women than in men [56, 57]. Although smoking rates are generally declining, tobacco use has recently increased among women and is expected to double between 2005 and 2025. In addition, although cigarette consumption is still estimated to be higher in men than in women, female smokers are not only at greater risk of developing UCB [58], but also have worse prognoses than male smokers [59].
Despite the common assertion that the urinary tract is sterile, evidence of the presence of a commensal bacterial community in the urinary tract emerged as early as 1997 [48]. Notably, greater diversity was observed between healthy women and those with bacterial vaginosis than between male and female microbiota samples. Although reports of differences between the microbiome of patients with UCB and those of healthy individuals are beginning to emerge, no study has been conducted to directly assess how the urinary microbiome may change during tumorigenesis, intravesical instillation of therapeutic agents, or systemic administration of anticancer treatments, or to determine whether there may be changes or differences between the sexes in any of these aspects [60]. In addition to appropriate powering of any future study, careful planning of sample acquisition, storage, and analysis are important for an accurate depiction of the urinary microbiome.
This study had several limitations. First, all the studies included in the analysis were retrospective studies; thus, the risk of selection bias in the analysis is increased. Second, reporting bias may have led to negative results not being published. Third, heterogeneity was detected in the analyses, suggesting the limited value of these results. As a result, these papers were not of equal rank (The weight in analyses is far difference among each studies). Moreover, the study did not adequately address the heterogeneity in methods for determining survival (local databases or social security/national registries) or progression/recurrence (radiographic or biopsy-proven). Although a random-effects model was used to minimize heterogeneity among the studies, the conclusions should be interpreted with caution. Fourth, the chemotherapy protocols used in the included studies were heterogeneous; hence, the analysis of individual treatment strategies was limited. Moreover, our analysis was not adjusted for known sex-specific differences in response to immunotherapy, including BCG. Furthermore, as no studies were excluded from analysis in this study on account of the operative methods (e.g., lymph node dissection and urinary diversion) involved, which varied between the studies. Therefore, the possibility cannot be ruled out that this may have affected our study results and contributed to heterogeneity among the studies evaluated, and our results need to be interpreted with these factors in mind. Fifth, of the patients evaluated, some were found to have progressed from NMIBC to MIBC, likely suggesting an overlap of the two populations. Again, those with prior NMIBC and those with MIBC should have been evaluated separately in the first place; however, the data available from the studies included in this analysis did not allow for this analysis, which constituted a limitation of our study. Sixth, high-risk NMIBC patients were not excluded from MIBC patients. Thus, it must be borne in mind that those undergoing RC included not only MIBC but high-risk NMIBC patients. Seventh, while sex difference was significantly associated with OS in MIBC, the HR included 1.0 and was subject to rounding. Finally, automation was not used to screen the initial 1,512 articles made available prior to independent investigator reviews. Therefore, well-designed, prospective studies with long follow-up periods are needed to validate the prognostic value of sex-specific differences for UCB in the clinical setting, as well as to determine whether consideration of these differences may improve the current risk stratification tools and clinical decision-making for patients with UCB.
Conclusion
Current evidence suggests that the female sex is a negative prognostic factor for survival after radical surgery for MIBC. In contrast, sex was not associated with adverse survival outcomes in patients with NMIBC. Further study is warranted to verify whether sex is a potential prognostic factor worth including in validated prognostic tables and nomograms to facilitate more accurate diagnosis and risk stratification of patients with UCB.
References
Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ (2022) European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in Situ). Eur Urol 81(1):75–94. https://doi.org/10.1016/j.eururo.2021.08.010
Mori K, D’Andrea D, Enikeev DV, Egawa S, Shariat SF (2020) En bloc resection for nonmuscle invasive bladder cancer: review of the recent literature. Curr Opin Urol 30(1):41–47. https://doi.org/10.1097/mou.0000000000000697
Mori K, Miura N, Babjuk M, Karakiewicz PI, Mostafaei H, Laukhtina E, Quhal F, Motlagh RS, Pradere B, Kimura S, Egawa S, Shariat SF (2020) Low compliance to guidelines in nonmuscle-invasive bladder carcinoma: a systematic review. Urol Oncol 38(10):774–782. https://doi.org/10.1016/j.urolonc.2020.06.013
Wallerand H, Bernhard JC, Culine S, Ballanger P, Robert G, Reiter RE, Ferrière JM, Ravaud A (2011) Targeted therapies in non-muscle-invasive bladder cancer according to the signaling pathways. Urol Oncol 29(1):4–11. https://doi.org/10.1016/j.urolonc.2009.07.025
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63(2):234–241. https://doi.org/10.1016/j.eururo.2012.07.033
Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ, Sagalowsky AI, Schoenberg MP, Lerner SP (2006) Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the bladder cancer research consortium. J Urol 176(6 Pt 1):2414–2422. https://doi.org/10.1016/j.juro.2006.08.004
Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek RS, Lotan Y, Trinh QD, Karakiewicz PI, Holmang S, Scherr DS, Zerbib M, Vickers AJ, Shariat SF (2013) Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer 109(6):1460–1466. https://doi.org/10.1038/bjc.2013.372
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A, Neuzillet Y, Rouanne M, Thalmann GN, Veskimäe E, Ribal MJ, van der Heijden AG (2021) European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 79(1):82–104. https://doi.org/10.1016/j.eururo.2020.03.055
Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF, Perrotte P, Montorsi F, Karakiewicz PI, Sun M (2013) Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 37(3):219–225. https://doi.org/10.1016/j.canep.2013.02.002
Zargar H, Espiritu PN, Fairey AS, Mertens LS, Dinney CP, Mir MC, Krabbe LM, Cookson MS, Jacobsen NE, Gandhi NM, Griffin J, Montgomery JS, Vasdev N, Yu EY, Youssef D, Xylinas E, Campain NJ, Kassouf W, Dall’Era MA, Seah JA, Ercole CE, Horenblas S, Sridhar SS, McGrath JS, Aning J, Shariat SF, Wright JL, Thorpe AC, Morgan TM, Holzbeierlein JM, Bivalacqua TJ, North S, Barocas DA, Lotan Y, Garcia JA, Stephenson AJ, Shah JB, van Rhijn BW, Daneshmand S, Spiess PE, Black PC (2015) Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 67(2):241–249. https://doi.org/10.1016/j.eururo.2014.09.007
Kluth LA, Black PC, Bochner BH, Catto J, Lerner SP, Stenzl A, Sylvester R, Vickers AJ, Xylinas E, Shariat SF (2015) Prognostic and prediction tools in bladder cancer: a comprehensive review of the literature. Eur Urol 68(2):238–253. https://doi.org/10.1016/j.eururo.2015.01.032
Putluri N, Shojaie A, Vasu VT, Vareed SK, Nalluri S, Putluri V, Thangjam GS, Panzitt K, Tallman CT, Butler C, Sana TR, Fischer SM, Sica G, Brat DJ, Shi H, Palapattu GS, Lotan Y, Weizer AZ, Terris MK, Shariat SF, Michailidis G, Sreekumar A (2011) Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Can Res 71(24):7376–7386. https://doi.org/10.1158/0008-5472.can-11-1154
Mori K, Abufaraj M, Mostafaei H, Quhal F, Karakiewicz PI, Briganti A, Kimura S, Egawa S, Shariat SF (2020) A systematic review and meta-analysis of variant histology in urothelial carcinoma of the bladder treated with radical cystectomy. J Urol 204(6):1129–1140. https://doi.org/10.1097/ju.0000000000001305
Mori K, Mostafaei H, Enikeev DV, Lysenko I, Quhal F, Kimura S, Karakiewicz PI, Egawa S, Shariat SF (2020) Differential effect of sex on outcomes after radical surgery for upper tract and bladder urothelial carcinoma: a systematic review and meta-analysis. J Urol 204(1):58–62. https://doi.org/10.1097/ju.0000000000000788
Mori K, Miura N, Mostafaei H, Quhal F, Motlagh RS, Lysenko I, Kimura S, Egawa S, Karakiewicz PI, Shariat SF (2020) Prognostic value of preoperative hematologic biomarkers in urothelial carcinoma of the bladder treated with radical cystectomy: a systematic review and meta-analysis. Int J Clin Oncol 25(8):1459–1474. https://doi.org/10.1007/s10147-020-01690-1
Mori K, Schuettfort VM, Katayama S, Laukhtina E, Pradere B, Quhal F, Sari Motlagh R, Mostafaei H, Grossmann NC, Rajwa P, König F, Aydh A, Soria F, Moschini M, Karakiewicz PI, Lotan Y, Scherr D, Haydter M, Nyirady P, Teoh JYC, Egawa S, Compérat E, Shariat SF (2021) The value of preoperative plasma VEGF levels in urothelial carcinoma of the bladder treated with radical cystectomy. Eur Urol Focus. https://doi.org/10.1016/j.euf.2021.08.006
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA: Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Lucca I, Klatte T, Fajkovic H, de Martino M, Shariat SF (2015) Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat Rev Urol 12(10):585–592. https://doi.org/10.1038/nrurol.2015.232
Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA (2016) Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol 69(2):300–310. https://doi.org/10.1016/j.eururo.2015.08.037
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res Ed) 372:n71. https://doi.org/10.1136/bmj.n71
Altman DG, Bland JM (2011) How to obtain the P value from a confidence interval. BMJ 343:d2304. https://doi.org/10.1136/bmj.d2304
Altman DG, Bland JM (2011) How to obtain the confidence interval from a P value. BMJ 343:d2090. https://doi.org/10.1136/bmj.d2090
DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28(2):105–114. https://doi.org/10.1016/j.cct.2006.04.004
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Scosyrev E, Noyes K, Feng C, Messing E (2009) Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 115(1):68–74. https://doi.org/10.1002/cncr.23986
Nielsen ME, Shariat SF, Karakiewicz PI, Lotan Y, Rogers CG, Amiel GE, Bastian PJ, Vazina A, Gupta A, Lerner SP, Sagalowsky AI, Schoenberg MP, Palapattu GS (2007) Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol 51(3):699–706. https://doi.org/10.1016/j.eururo.2006.11.004
Otto W, May M, Fritsche HM, Dragun D, Aziz A, Gierth M, Trojan L, Herrmann E, Moritz R, Ellinger J, Tilki D, Buchner A, Höfner T, Brookman-May S, Nuhn P, Gilfrich C, Roigas J, Zacharias M, Denzinger S, Hohenfellner M, Haferkamp A, Müller SC, Kocot A, Riedmiller H, Wieland WF, Stief CG, Bastian PJ, Burger M (2012) Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend Med 9(6):481–489. https://doi.org/10.1016/j.genm.2012.11.001
Mansson A, Anderson H, Colleen S (1993) Time lag to diagnosis of bladder cancer–influence of psychosocial parameters and level of health-care provision. Scand J Urol Nephrol 27(3):363–369. https://doi.org/10.3109/00365599309180448
Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Skolarus TA, Kim SP, Montie JE, Lee CT, Wood DP Jr, Miller DC (2010) Delays in diagnosis and bladder cancer mortality. Cancer 116(22):5235–5242. https://doi.org/10.1002/cncr.25310
Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, Messing EM, Miller SD, Peterson AC, Turk TM, Weitzel W (2012) Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 188(6 Suppl):2473–2481. https://doi.org/10.1016/j.juro.2012.09.078
Bergman J, Neuhausen K, Chamie K, Scales CD, Carter S, Kwan L, Lerman SE, Aronson W, Litwin MS (2013) Building a medical neighborhood in the safety net: an innovative technology improves hematuria workups. Urology 82(6):1277–1282. https://doi.org/10.1016/j.urology.2013.08.015
Saez S, Martin PM (1981) Evidence of estrogen receptors in the trigone area of human urinary bladder. J Steroid Biochem 15:317–320. https://doi.org/10.1016/0022-4731(81)90291-0
Schilling D, Horstmann M, Nagele U, Sievert KD, Stenzl A (2008) Cystectomy in women. BJU Int 102(9 Pt B):1289–1295. https://doi.org/10.1111/j.1464-410X.2008.07972.x
Madeb R, Messing EM (2004) Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol 22(2):86–92. https://doi.org/10.1016/s1078-1439(03)00139-x
Zhang Y (2013) Understanding the gender disparity in bladder cancer risk: the impact of sex hormones and liver on bladder susceptibility to carcinogens. J Environ Sci Health, Part C Environ Carcinog Ecotoxicol Rev 31(4):287–304. https://doi.org/10.1080/10590501.2013.844755
Bolenz C, Lotan Y, Ashfaq R, Shariat SF (2009) Estrogen and progesterone hormonal receptor expression in urothelial carcinoma of the bladder. Eur Urol 56(6):1093–1095. https://doi.org/10.1016/j.eururo.2009.06.032
Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, Scherr DS (2004) Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology 64(2):383–388. https://doi.org/10.1016/j.urology.2004.03.025
Lombard AP, Mudryj M (2015) The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer 22(5):R265-277. https://doi.org/10.1530/erc-15-0209
Gakis G, Stenzl A (2013) Gender-specific differences in muscle-invasive bladder cancer: the concept of sex steroid sensitivity. World J Urol 31(5):1059–1064. https://doi.org/10.1007/s00345-013-1037-z
Jing Y, Cui D, Guo W, Jiang J, Jiang B, Lu Y, Zhao W, Wang X, Jiang Q, Han B, Xia S (2014) Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial-mesenchymal transition. Cancer Lett 348(1–2):135–145. https://doi.org/10.1016/j.canlet.2014.03.018
Özdemir BC, Dotto GP (2019) Sex hormones and anticancer immunity. Clin Cancer Res: Off J Am Assoc Cancer Res 25(15):4603–4610. https://doi.org/10.1158/1078-0432.ccr-19-0137
McGrath M, Michaud DS, De Vivo I (2006) Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol 163(3):236–244. https://doi.org/10.1093/aje/kwj028
Daugherty SE, Lacey JV Jr, Pfeiffer RM, Park Y, Hoover RN, Silverman DT (2013) Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP diet and health study. Int J Cancer 133(2):462–472. https://doi.org/10.1002/ijc.28022
Cantwell MM, Lacey JV Jr, Schairer C, Schatzkin A, Michaud DS (2006) Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer 119(10):2398–2401. https://doi.org/10.1002/ijc.22175
Abelson B, Sun D, Que L, Nebel RA, Baker D, Popiel P, Amundsen CL, Chai T, Close C, DiSanto M, Fraser MO, Kielb SJ, Kuchel G, Mueller ER, Palmer MH, Parker-Autry C, Wolfe AJ, Damaser MS (2018) Sex differences in lower urinary tract biology and physiology. Biol Sex Differ 9(1):45. https://doi.org/10.1186/s13293-018-0204-8
Webb K, Peckham H, Radziszewska A, Menon M, Oliveri P, Simpson F, Deakin CT, Lee S, Ciurtin C, Butler G, Wedderburn LR, Ioannou Y (2018) Sex and Pubertal Differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol 9:3167. https://doi.org/10.3389/fimmu.2018.03167
Koti M, Ingersoll MA, Gupta S, Lam CM, Li X, Kamat AM, Black PC, Siemens DR (2020) Sex differences in bladder cancer immunobiology and outcomes: a collaborative review with implications for treatment. Eur Urol Oncol 3(5):622–630. https://doi.org/10.1016/j.euo.2020.08.013
Shang Z, Li Y, Hsu I, Zhang M, Tian J, Wen S, Han R, Messing EM, Chang C, Niu Y, Yeh S (2016) Targeting estrogen/estrogen receptor alpha enhances Bacillus Calmette-Guérin efficacy in bladder cancer. Oncotarget 7(19):27325–27335. https://doi.org/10.18632/oncotarget.8756
Zhang GJ, Crist SA, McKerrow AK, Xu Y, Ladehoff DC, See WA (2000) Autocrine IL-6 production by human transitional carcinoma cells upregulates expression of the alpha5beta1 firbonectin receptor. J Urol 163(5):1553–1559
Guise AI, Chen F, Zhang G, See W (2011) The effects of physiological estrogen concentration on the immune response of urothelial carcinoma cells to bacillus Calmette-Guérin. J Urol 185(1):298–304. https://doi.org/10.1016/j.juro.2010.09.004
Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL (1990) Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa requirement for the expression of an antitumor response. J Clin Investig 85(1):62–67. https://doi.org/10.1172/jci114434
Wang PF, Song HF, Zhang Q, Yan CX (2020) Pan-cancer immunogenomic analyses reveal sex disparity in the efficacy of cancer immunotherapy. Eur J Cancer (Oxf, Engl) 126:136–138. https://doi.org/10.1016/j.ejca.2019.12.008
Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 19(6):737–746. https://doi.org/10.1016/s1470-2045(18)30261-4
Carè A, Bellenghi M, Matarrese P, Gabriele L, Salvioli S, Malorni W (2018) Sex disparity in cancer: roles of microRNAs and related functional players. Cell Death Differ 25(3):477–485. https://doi.org/10.1038/s41418-017-0051-x
Mori K, Mostafaei H, Abufaraj M, Yang L, Egawa S, Shariat SF (2020) Smoking and bladder cancer: review of the recent literature. Curr Opin Urol 30(5):720–725. https://doi.org/10.1097/mou.0000000000000804
Rink M, Zabor EC, Furberg H, Xylinas E, Ehdaie B, Novara G, Babjuk M, Pycha A, Lotan Y, Trinh QD, Chun FK, Lee RK, Karakiewicz PI, Fisch M, Robinson BD, Scherr DS, Shariat SF (2013) Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol 64(3):456–464. https://doi.org/10.1016/j.eururo.2012.11.039
Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC (2011) Association between smoking and risk of bladder cancer among men and women. JAMA 306(7):737–745. https://doi.org/10.1001/jama.2011.1142
Rink M, Xylinas E, Trinh QD, Lotan Y, Margulis V, Raman JD, Fisch M, Lee RK, Chun FK, Abdennabi J, Seitz C, Pycha A, Zlotta AR, Karakiewicz PI, Babjuk M, Scherr DS, Shariat SF (2013) Gender-specific effect of smoking on upper tract urothelial carcinoma outcomes. BJU Int 112(5):623–637. https://doi.org/10.1111/bju.12014
Bi H, Tian Y, Song C, Li J, Liu T, Chen Z, Chen C, Huang Y, Zhang Y (2019) Urinary microbiota - a potential biomarker and therapeutic target for bladder cancer. J Med Microbiol 68(10):1471–1478. https://doi.org/10.1099/jmm.0.001058
Funding
Open access funding provided by Medical University of Vienna. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
KM: Protocol/project development, Data management, Data analysis, Manuscript writing/editing; TY: Data collection, Manuscript writing/editing; SK: Data collection, Manuscript writing/editing; EL: Manuscript writing/editing; BP: Manuscript writing/editing; HM: Data analysis, Manuscript writing/editing; FQ: Manuscript writing/editing; PR: Manuscript writing/editing; MM: Manuscript writing/editing; FS: Manuscript writing/editing; DD: Manuscript writing/editing; MA: Manuscript writing/editing; SA: Manuscript writing/editing; WK: Manuscript writing/editing; WF: Manuscript writing/editing; JM: Manuscript writing/editing; TK: Manuscript writing/editing; SE: Manuscript writing/editing; JYCT: Protocol/project development, Manuscript writing/editing; SFS: Protocol/project development, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest to disclose.
Research involving human participants and/or animals
This is a systematic review. No ethical approval is required.
Informed consent
This is a systematic review. No informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mori, K., Yanagisawa, T., Katayama, S. et al. Impact of sex on outcomes after surgery for non-muscle-invasive and muscle-invasive bladder urothelial carcinoma: a systematic review and meta-analysis. World J Urol 41, 909–919 (2023). https://doi.org/10.1007/s00345-022-04116-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04116-x