Abstract
Liver damage caused by toxicity can lead to various severe conditions, such as acute liver failure (ALF), fibrogenesis, and cirrhosis. Among these, liver cirrhosis (LC) is recognized as the leading cause of liver-related deaths globally. Unfortunately, patients with progressive cirrhosis are often on a waiting list, with limited donor organs, postoperative complications, immune system side effects, and high financial costs being some of the factors restricting transplantation. Although the liver has some capacity for self-renewal due to the presence of stem cells, it is usually insufficient to prevent the progression of LC and ALF. One potential therapeutic approach to improving liver function is the transplantation of gene-engineered stem cells. Several types of mesenchymal stem cells from various sources have been suggested for stem cell therapy for liver disease. Genetic engineering is an effective strategy that enhances the regenerative potential of stem cells by releasing growth factors and cytokines. In this review, we primarily focus on the genetic engineering of stem cells to improve their ability to treat damaged liver function. We also recommend further research into accurate treatment methods that involve safe gene modification and long-term follow-up of patients to increase the effectiveness and reliability of these therapeutic strategies.
Similar content being viewed by others
Introduction
Toxins in the liver can lead to acute liver failure (ALF) or progress to chronic liver disease and cirrhosis. Liver cirrhosis (LC) is the main cause of worldwide liver-related mortality [1]. It causes irreversible liver damage with loss of hepatocytes and has limited therapeutic authority [2]. Infection by viruses, drugs, autoimmune disorders, sinusoidal obstruction syndrome [3], genetic diseases, chronic alcohol abuse, and obesity are significant causes of LC [4]. Most patients in the first stage of liver disease do not show any symptoms. In these cases, advanced LC, the final pathological pathway of liver disease, occurs if essential care is not taken. Eventually, liver transplantation is the last treatment for LC disease.
This treatment has limitations because of a shortage of donor's livers, postoperative problems, immune-suppression side effects, and expensive health system governance services [5, 6]. Therefore, finding new unrestricted techniques with spectacular results is urgently needed.
Several cellular and molecular factors contribute to ALF and cirrhosis progression. Hepatocytes are frequently involved in liver regeneration; hepatic stellate cells (HSCs) generate collagen and another extracellular matrix (ECM); sinusoidal endothelial cells are present as the agents of defenestration and development of capillaries, Kupffer cells stimulate the activation of HSCs, and they destroy the hepatocytes, which contribute to the progression of LC. In the case of cirrhosis, molecular factors such as cytokines mediate complex signaling pathways that have anti-oxidative, anti-inflammatory, and anti-apoptotic effects. MiRNAs also play a significant role in cirrhosis by regulating the transcription and translation of several genes [7, 8]. The liver has an intrinsic renewal capacity [9]. But in patients with the end stages of liver disease, local stem cells could not regenerate the whole damaged tissue. So, administration of engineered stem cells can be a therapeutic option with enhanced function.
According to recent studies, stem cells have been promised as a new strategy for improving liver function [10, 12]. Commonly, two mechanisms are proposed for stem cells: the paracrine effect, which enhances the de novo generation of hepatocytes (8).
The curative properties of many types of stem cells have been investigated in LC conditions [13, 14].
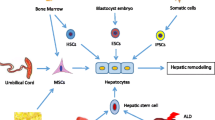
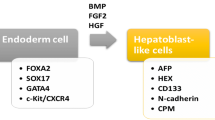
As hepatocyte-like cells (HLCs) are known to contribute to the remodeling of the cirrhosis liver, some kinds of cells, such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and endothelial progenitor cells (EPCs) that have differentiation potential into HLCs, can promote liver disease [15,16,17]. Therefore, various sources of mesenchymal stem cells, because of their beneficial attributes, have been suggested for stem cell therapy of liver disease [18].
Other kinds of stem cells, like induced pluripotent stem cells [19] and embryonic stem cells (ESCs), can differentiate into HLCs [20].
Stem cells' regenerative abilities, due to the release of growth factors, chemokines, cytokines, microRNAs, and exosomes, make them one of the best choices for cell-based therapy [21]. So, gene engineering of stem cells is a strategy to improve their natural function and therapeutic potential. In pre-clinical experiments, different types of genetically modified stem cells, pretreated stem cells, and cell-free therapy were investigated last year. In this review, we discussed the current literature according to the different approaches (Table 1) and the future outlooks of gene-engineered stem cell-based therapy in liver disease, as shown in Fig. 1.
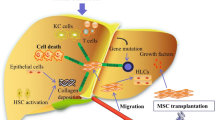
Therapeutic potential of genetically modified stem cells in liver injuries. MSCs can originate from several tissues such as umbilical cord, bone marrow, adipose, peripheral blood, etc. Genetic manipulation increases regenerative capacities of MSCs in liver diseases. Injection of engineered MSCs can attenuate activation of HSCs, collagen deposition, inflammation, apoptosis and fibrotic processes. MSCs, mesenchymal stem cells; HSCs, hepatic stellate cells
Common gene editing methods
Gene therapy involves introducing genetic material into target cells using non-viral or viral vehicles. This genetic material is used to treat or prevent diseases by replacing or repairing damaged genes. Viruses are the most common method for exogenous transgene overexpression and external genetic manipulations. Gene therapy practices ex vivo and/or in vivo techniques to produce benefits. During ex vivo gene editing, target cells such as stem cells are taken, genetically modified, and injected into the patient [12]. Several gene delivery techniques have been used to treat various disorders like liver diseases. It has been investigated whether defective genes can be replaced using gene-editing technologies such as clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9 (CRISPR-Cas9), zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and mega-nucleases has been investigated [13]. In recent years, viral vector-mediated gene therapy has been used in pre-clinical tests and clinical trials to treat various diseases. Adenovirus, adeno-associated virus (AAV), retroviral, and lentiviral vectors are the most efficient viral vectors, as reported in several studies [14, 15]. The first gene therapy product, based on an AAV gene delivery strategy, was approved in Europe for lipoprotein lipase deficiencies in 2012 [16]. After that, numerous viral vector drugs were approved by the US Food and Drug Administration (FDA) [17,18,19]. Furthermore, the FDA predicted that by 2025, 10–20 new cell and gene therapy products would be authorized annually. But an efficient drug based on genetically modified stem cells has not yet been tested in clinical studies.
Modification of genes rolled in cell survival and migration
C-X-C chemokine receptor type 4 (CXCR-4)
Stromal-derived factor 1 alpha (SDF1α), also known as CXCL12, is the ligand of the CXC family, bound to C-X-C chemokine receptor type 4 (CXCR-4) that is expressed throughout bone marrow stromal cells. Several studies have shown essential roles for the CXCL12–CXCR4 axis in the survival, homing, and improved colonization of stem cells. Downstream signaling pathways of CXCR4 can control cell proliferation and movement via PI3K-Akt and mTORC signaling [22, 23]. In addition, CXCL12 can activate the migration of stem cells from bone marrow to destroyed organs such as the liver, lung, heart, and brain through the chemoattraction of CXCR4 on stem cell membranes. It has been suggested that CXCL12–CXCR4 can enhance stem cell transplantation, which is necessary to regenerate damaged tissue [24,25,26]. Indeed, MSCs express CXCR4 at low levels, so overexpression of CXCR4 seems advantageous to improve organ functions.
Ma et al. [22] injected modified CXCR4 gene MSCs with lentiviral transduction and null-MSCs intravenously into nude mice a day after chemical carbon tetrachloride (CCL4) administration. They indicated that after ALF, the concentration of CXCL12 was increased. Furthermore, in vivo imaging techniques illustrated that CXCR4-MSCs mobilized more than null-MSCs to the damaged liver. Results demonstrated that genetically modified cells enhanced the homing of MSCs. So, in the following analysis, Ki-67 immunohistochemical assays showed raised cell proliferation and levels of hepatocyte-generating factors (HGF) and vascular endothelial growth factor (VEGF) and better liver function.
Tyrosine-protein kinase Met (c-Met) or hepatocyte growth factor receptor
Several studies [27,28,29] found that hepatocyte growth factor (HGF), a motility and trophic factor secreted by MSCs, protects against liver damage. HGF is the ligand of c-Met, a tyrosine kinase receptor family member. The HGF/c-Met axis is crucial in the proliferation, regeneration, development, protection, scattering process, and differentiation of BMSCs into hepatocytes [27, 30,31,32,33]. However, the insufficient capacity of stem cells to reside in the damaged liver has been a concern for their therapeutic properties.
Wang and his colleagues [34] overexpressed c-Met protein in BMSCs using lenti-C-Met-GFP vectors (C-Met-BMSCs). In vitro assays demonstrated the increased migration activity of c-Met-BMSCs against the control BMSC group associated with HGF. This study showed that improving the homing of BMSCs through increased cell surveillance and decreasing the hepatic activity index (HAI) scores could all facilitate repairing the ALF rat model.
In a similar study, Liu et al. [35] investigated whether HGF/c-Met signaling effectively promoted MSC migration and regeneration of the injured liver induced by intestinal ischemia–reperfusion in rats.
Akt
A serine/threonine kinase, Akt, is well-known as protein kinase B, which has crucial roles in cellular processes like proliferation, migration, angiogenesis [36], and anti-apoptosis. As a member of the Bcl2 family, BAD is phosphorylated by Akt and reduces its pro-apoptotic property [37]. The Akt family has three different isoforms: Akt1, Akt2, and Akt3, which are encoded by distinct genes.
Despite the beneficial effect of MSCs in diverse regeneration aspects, a low survival rate in apoptosis sites has been a restrictive factor. To improve the viability of transplanted cells, Zhou et al. [38] suggested genetic modification of BMSCs with the AKT1 gene in concanavalin A (ConA) in the injured liver of a C57BL/6 mouse. In vitro and in vivo analysis of AKT1-BMSCs have illustrated more viability and better homing capacity than the control group. Notably, AKT1-BMSCs treated mice produced lower levels of ALT, AST, TNF-α, IFN-γ, and higher concentrations of VEGF, HGF, and immunosuppressive factors like IL-10 in serum and injured liver.
Ma et al. [39] evaluated the therapeutic effect of exosomes derived from AKT gene-modified human umbilical cord mesenchymal stem cells (Akt-Exo). They reported that Akt-Exo significantly upregulated the expression of PDGF, which caused an improvement in the proliferation, migration, and angiogenesis of endothelial cells.
Gene modification of trophic factors
Hepatocyte growth factor (HGF)
HGF is a liver-regenerative factor that is elevated during liver injuries and hepatectomy [48,49,50]. HGF has been identified as a potent multifunctional cytokine that can stimulate mitogenesis, morphogenesis, cell growth, differentiation, and motility [51, 52]. Subsequent HGF binding to its specific receptor, c-Met, initiates a signaling cascade of critical biological actions like development, homeostasis, and regeneration [50, 53, 54]. So, the HGF/c-Met signaling pathway is considered to regulate liver damage.
Zhang et al. [55] investigated the protective effect of BM-MSCs genetically engineered with HGF with an adenoviral vector containing a green fluorescent protein (EGFP) label (HGF-BM-MSC group). Different groups were transplanted intravenously in the rat cirrhosis model induced by CCL4. After 4 weeks of treatment, the data analysis showed that the HGF-BM-MSC group enhanced the mRNA and protein expression levels of HNF-4α, CK18, and ALB in the liver. On the other hand, some liver injury markers, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (TBIL), showed significant elevation in the serological test. Due to the exogenous HGF’s short half-life, HGF-BM-MSC seems affordable for treating liver cirrhosis and disease.
In another experiment, Tang et al. [56] discovered the therapeutic potential of human umbilical cord-derived mesenchymal stem cells (UCMSCs) that overexpress HGF in the ALF mouse model. Their results indicated that HGF-UCMSCs could gain the activity of γ-glutamyl cysteine synthetase (γ-GCS), superoxide dismutase (SOD), and catalase (CAT), which are involved in cellular redox homeostasis. Additionally, this group had anti-apoptotic features by downregulating Bax and TNFα and upregulating BCL-2 genes.
Fibroblast growth factor (FGF)
Fibroblast growth factors (FGFs) comprise a large family of cell signaling proteins. This growth factor is highly conserved in gene and amino acid sequencing between vertebrates [57]. The FGF family is composed of 22 ligands that have their special tyrosine kinase receptors (FGFRs). The FGF/FGFR signaling pathway can regulate biological processes like cell survival, proliferation, differentiation, migration, embryonic development, organogenesis, metabolism, and regeneration [58].
A previous cirrhosis liver study reported that FGF4 can induce BMSCs into HLCs, which indicated that this differentiation might be the effect of secreted cytokines from damaged liver cells [59]. Wang et al. [60] modified BMSCs with a recombinant FGF4 lentiviral vector and found that FGF4-BMSC improved BMSC engraftment in cirrhotic liver rats. Furthermore, compared to another group, they observed more proliferating cell nuclear antigen (PCNA), Jagged-1, and epithelial cell adhesion molecule (EpCAM)-positive hepatocytes. These results suggest that FGF-4-modified BMSCs might be involved in liver regeneration by ameliorating engraftment and proliferation of BMSCs and modulating cirrhotic liver cell microenvironments.
Gene modification enrolled in the anti-apoptosis process
B-cell lymphoma 2 (Bcl2)
Apoptosis is a crucial biological process for tissue development and homeostasis. This programmed cell death procedure mediates hepatic cirrhosis and influences the liver’s regenerative ability [40, 41]. The mechanism of apoptosis occurs via two main pathways; one is the extrinsic way, which is found by the interaction between a ligand and tumor necrosis factor (TNF) death receptors. Death-inducing signaling complexes then bind to their adaptors and lead to activation of caspase-8, caspase-3 cascades, and finally, cell death [42, 43]. The other is an intrinsic way that is caused by endogenous cellular stresses like metabolic disturbances, growth factor deprivation, DNA damage, and oxidative stress. Then, due to the intrinsic pathway, mitochondrial depolarization and cytochrome c release occur. Cytochrome c can bind to apoptosis protease-activating factor 1 (APAF1) and procaspase-9, assembling an apoptosome that triggers the activation of caspase-9. Therefore, downstream caspase-3, caspase-7, and caspase-6 are activated [43]. The Bcl-2 family of proteins controls the critical activity of the intrinsic apoptotic pathway by regulating mitochondrial outer membrane permeabilization (MOMP) [44]. In several research studies [45, 46], the BCL-2 gene has been known as an apoptosis repressor gene.
Jin and co-workers [47] transplanted BMSCs with BCL-2 overexpression into cirrhotic rats induced by CCL4. Expression of albumin (ALB), cytokeratin 18 (CK18), and hepatocyte nuclear factor 4a (HNF4a) was examined in HLCs, which were integrated with adeno-associated virus (AAV) as a vector for overexpressing the BCL-2 gene (AAV-BCL-2). The BMSCs-AAV-BCL-2 cirrhosis group indicated the highest mRNA level and hepatocyte markers, such as ALB, CK18, and HNF4a, on day 28. Finally, they reported that genetic modification of BMSCs with the BCL-2 gene enhanced cell survival, differentiation to HLCs, and recovered liver function in cirrhotic rat models.
Phosphatase of regenerating liver-1 (PRL-1)
Phosphatase of regenerating liver-1 (PRL-1), as a member of the PRL family, is a tyrosine phosphatase and primary response gene in liver cell repair [65]. Although the PRL‐1 mRNA expression value varies in different tissues, higher levels of PRL-1 have been found in growing hepatic cells. The early growth response protein 1 (Egr‐1) transcription factor induces PRL‐1 expression in liver regeneration [66, 67]. PRL-1 can regulate cell proliferation and differentiation and affect the migration and invasion processes by promoting matrix metalloproteinase (MMP)-2 and MMP-9 expression via the proto-oncogene c-Src and ERK1/2 pathways [68,69,70,71,72,73]. Jiao et al. [74] reported that PRL-1 is necessary for the normal timing of cell cycle progression within liver regeneration and has an anti-apoptotic effect.
Kim et al. [75] generated placenta-derived mesenchymal stem cells (PD-MSCs) overexpressing PRL-1 (PD-MSCsPRL-1) to analyze their performance in rat liver cirrhosis induced by administration of bile duct ligation (BDL) for 10 days. Outcomes showed that enhanced PRL-1 expression in PD-MSCs could improve ATP production, mitochondrial biogenesis, and metabolism, better engraft into damaged areas, and eventually accelerate liver function through mitochondrial dynamics.
Interleukin-1 receptor antagonist (IL-1RA)
Another member of the IL-1 family of cytokines is IL-1Ra, which inhibits the pro-inflammatory activity of IL-1α and IL-1β and modulates their immune and inflammatory reactions [76, 77]. IL-1Ra can bind to the IL-1 receptor and prevent signal transduction into the cell. As a result, the natural equivalence of IL-1 and IL-1Ra is critical and mediates inflammatory events [78, 79]. Previous findings report that IL-1Ra can prevent apoptosis [80] and have hepatoprotective effects [81].
Zheng et al. [82] investigated IL-1Ra overexpression in amniotic fluid-derived mesenchymal stem cells (IL-1Ra-AF-MSCs) in a rat model of fulminant hepatic failure (FHF). The implantation of IL-1Ra-AF-MSCs into the damaged liver via the portal vein resulted in the downregulation of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α; improved MSC migration rates; higher potential in hepatic differentiations; the prevention of hepatocyte apoptosis; and significant liver function.
Gene modification plays a role in the anti-inflammatory properties
Hepatocyte nuclear factor-4 alpha (HNF4α)
HNF-4 is a nuclear transcription factor that controls the morphogenesis and maturation of liver cells and is known as a consequential regulator of hepatocyte differentiation [83, 84]. Furthermore, regular HNF-4 expression can restore hepatocyte function [85, 86]. It was reported that HNF-4α has less expression in liver disease conditions; therefore, researchers suggested overexpression of HNF-4α can increase the curative effect of this factor on liver damage [86].
Ye et al. [87] used CCL4 to induce a liver cirrhosis model. Three weeks after the induction of cirrhosis, MSCs modified by HNF-4α overexpression adenoviruses (HNF-4α-MSCs) were injected into the mice's tail vein. The result showed that HNF-4α could improve the anti-inflammatory properties of MSCs by enhancing nitric oxide synthase (iNOS) expression by activating the NF-κB signaling pathway.
Yu et al. [88] administered human umbilical cord mesenchymal stem cells with overexpressed HNF4α (HuMSC-HNF4α) to mice with ALF induced by D-galactosamine/lipopolysaccharide (D-galN/LPS). They discovered that HuMSC-HNF4 promoted Kupffer cell polarization to the M2 phenotype, inhibited macrophage inflammatory responses by secreting higher levels of IL-10 and macrophage colony-stimulating factor (M-CSF), and reduced the expression of inflammatory factors such as TNF- and IL-1, which inhibited inflammation and regenerated injuries.
Interleukin 1 beta (IL-1β)
IL-1β is a primary cytokine that activates immune and inflammatory responses encoded by the IL1B gene. Following tissue injury, activated macrophages produce excessive IL1 and recruit inflammatory cells [89,90,91]. Small interfering RNAs (siRNA) benefit from high target selectivity and low toxicity and can help regulate inflammatory responses. Ma et al. [92] prepared MSC combined with IL-1β siRNA adenovirus for implantation into the tail vein of the ALF mouse model. The results showed notably reduced levels of CXCL1, IL-1β, and IL-6 as the primary inflammation cytokines by MSC + IL-1β siRNA treatment, and ALT and AST levels changed significantly compared to the control group. In addition, models treated with MSC and IL-1 siRNA had better liver regeneration, higher survival rates, and lower inflammatory cytokines, indicating an effective ALF strategy.
Gene modification role in the anti-fibrotic process
Insulin growth factor-I (IGF-I)
Insulin growth factor I (IGF-I) is a hormone produced by the liver and is involved in anabolic reactions. Experiments demonstrated this hormone level had decreased in the cirrhotic liver [61]. IGF-I can stimulate cell growth and metabolic pathways [62].
Fiore et al. [63] aimed to study the efficacy of IGF-I overexpressing BMSCs on fibrotic liver mice. A day after genetically modified MSCs to express IGF-I (AdIGF-I-MSCs) transplantation, PCNA mRNA values were upregulated, especially in hepatocytes. Also, collagen deposition reduction and suppression immunogenicity against adenoviral antigens were elucidated in this group.
In another study, Fiore and his colleagues [64] found that hepatic macrophage (hMø) numbers increased in AdIGF-I-MSCs-treated fibrosis mice and demonstrated a reduced pro-inflammatory and pro-fibrogenic gene expression profile and decreased oxidative stress levels. Furthermore, expression profile analyses showed downregulation of pro-inflammatory markers and significant gene regulation in the DNA repair and synthesis cell cycle. In addition, they reported that hMø participated in AdIGF-I-MSCs anti-fibrotic activities. After being treated with AdIGF-I-MSCs, fibrotic livers had expression profile analyses for cell cycle markers performed on them. The profiles showed significant gene regulation related to DNA synthesis and repair quality control, cell cycle progression, and DNA damage/cellular stress compatible with the early induction of pro-regenerative and hepatoprotective mechanisms.
Mothers against decapentaplegic homolog 7 (Smad7)
Smad7 is a member of the Smad family that regulates transforming growth factor (TGF-β) signaling [93, 94]. TGF-β ligands stimulate the Smad-2/3 pathway and the expression of several profibrotic genes, including various types of collagens [95, 96], plasminogen activator inhibitor-1 (PAI-1) [97, 98], integrins [99], some proteoglycans [100, 101], MMPs [102], and connective tissue growth factor [103]. Smad7 is a negative modulator of TGF-β [104]. Wu et al. discovered that increased SMAD7 gene expression in rat MSCs could prevent fibrogenesis in HSCs [105].
Su et al. [106] investigated the curative potential of genetically modified MSCs overexpressing the SMAD7 gene in an 8-week CCL4-induced cirrhosis liver rat model. 7 and 21 days after injection of Smad7-MSCs into the main lobes of the cirrhotic liver, both protein and mRNA values of Smad7 were increased. Treatment with Smad7-MSCs diminished the serum levels of collagen I, III, and collagenase I, III. This approach caused a reduction in the mRNA levels of TGF-β1, α-SMA, TIMP-1, TGFBR1, laminin, and hyaluronic acid. In this in vivo study, cell-based gene therapy was applied to improve cirrhosis liver function by inhibiting TGF-β1 signaling.
microRNAs manipulation
MicroRNAs (miRNAs) are short non-coding RNAs that can control gene expression at the posttranscriptional or translational levels [107]. miRNAs involve various biological processes, like proliferation, differentiation, immune responses, apoptosis, tumorigenesis, and tissue remodeling [108, 109]. Recent research suggests that miRNAs play a role in liver regeneration and could be a therapeutic strategy in liver disease [110, 111].
Qu et al. [112] showed the anti-fibrotic effects of exosomes derived from miRNA‐181‐5p overexpressed adipose‐derived mesenchymal stem cells (ADMSCs) in the CCL4‐induced liver fibrosis mouse model. In addition, exosomes containing miR181‐5p downregulated STAT3 and BCL-2 expression and activated autophagy, which revealed the reduction in extracellular matrix components.
In another study on ALF, Liu's team [113] used exosomes isolated from miR-17-knockdown adipose tissue-derived MSCs (AMSC-ExomiR-17-KD) to find the role of miR-17 in AMSC-Exo-based therapy. They determined miR-17 can target thioredoxin-interacting protein (TXNIP) and inhibit nucleotide-binding and oligomerization domain-like receptor 3 (NLRP3) inflammasome activation in macrophages. NLRP3 is significantly expressed in the liver and plays a role in fibrosis. Lou et al. [114] identified a new potential approach for improving liver fibrosis by administering MiR‐122‐modified AMSCs. Data analysis showed serum markers such as hyaluronic acid (HA), procollagen III‐N‐peptide (P‐III‐P), ALT, and decreased AST levels. In addition, the expression levels of TGF‐β1 and α‐SMA notably downregulated, and, importantly, MiR122‐ AMSCs suppressed HSC proliferation and collagen maturation.
Conclusion
Until now, stem cells, especially MSCs, have been considered remarkable applicants for regenerative medicine in liver disease due to their beneficial properties, including differentiation into hepatocyte-like cells, producing chemokine factors, immunomodulatory effects, anti-fibrotic, anti-apoptotic, and anti-oxidant activities [115]. However, there are still some challenges in clinical administration, like the genetic modification of stem cells through the manipulation of target genes, which can enhance the rate of stem cell survival and engraftment in damaged liver tissue and improve their therapeutic potential. In addition to the advantages of genetic engineering, there are still several limitations to having a functional next-generation MSC-based cell therapy. For instance, the sufficient dosage of transplanted cells, optimal timing, and injection frequency is still being determined. Furthermore, the transplantation route is unclear, and the risk of unanticipated differentiation and tumorigenicity causes safety concerns. Due to different types of liver damage, we recommend further studies on accurate treatment methods with optimal conditions, safe gene modification, and long-term follow-up of cases to increase the reliability of these therapeutic strategies.
Availability of data and materials
All data are available in this article.
Abbreviations
- ALF:
-
Acute liver failure
- LC:
-
Liver cirrhosis
- HSCs:
-
Hepatic stellate cells
- ECM:
-
Extracellular matrix
- HLCs:
-
Hepatocyte-like cells
- MSCs:
-
Mesenchymal stem cells
- EPCs:
-
Endothelial progenitor cells
- ESCs:
-
Embryonic stem cells
- CXCR-4:
-
C-X-C chemokine receptor type 4
- SDF1α:
-
Stromal-derived factor 1 alpha
- CCL4:
-
Chemical carbon tetrachloride
- HGF:
-
Hepatocyte-generating factors
- VEGF:
-
Vascular endothelial growth factor
- C-Met:
-
Tyrosine-protein kinase Met
- Bcl2:
-
B-cell lymphoma 2
- TNF:
-
Tumor necrosis factor
- ALB:
-
Expression of albumin
- CK18:
-
Cytokeratin 18
- HNF4a:
-
Hepatocyte nuclear factor 4a
- FGFs:
-
Fibroblast growth factors
- IGF-I:
-
Insulin growth factor like-I
- PCNA:
-
Proliferating cell nuclear antigen
- PRL-1:
-
Phosphatase of regenerating liver-1
- IL-1RA:
-
Interleukin-1 receptor antagonist
- HNF4α:
-
Hepatocyte nuclear factor-4 alpha
- IL-1β:
-
Interleukin 1 beta
- Smad7:
-
Mothers against decapentaplegic homolog 7
References
Roth GA, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88.
Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12(1):1–3.
Volarevic V, et al. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem cells. 2014;32(11):2818–23.
Karageorgos SA, et al. Long-term change in incidence and risk factors of cirrhosis and hepatocellular carcinoma in Crete, Greece: a 25-year study. Ann Gastroenterol. 2017;30(3):357.
Ge PS, Runyon BA. Treatment of patients with cirrhosis. N Engl J Med. 2016;375(8):767–77.
Zhu W, et al. Effects of xenogeneic adipose-derived stem cell transplantation on acute-on-chronic liver failure. Hepatobiliary Pancreat Dis Int. 2013;12(1):60–7.
Libo C, Zhen Y, Fazu Q. Studies on hepatocyte apoptosis, proliferation and oncogene c-fos expression in carbon tetrachloride-induced cirrhotic rat liver. J Tongji Med Univ. 1999;19(1):53–5.
Sánchez-Valle V, et al. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19(28):4850–60.
Kung JW, Forbes SJ. Stem cells and liver repair. Curr Opin Biotechnol. 2009;20(5):568–74.
Zhang Z, Wang F-S. Stem cell therapies for liver failure and cirrhosis. J Hepatol. 2013;59(1):183–5.
Gramignoli R, et al. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res. 2015;54(3–4):162–77.
Cho KA, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Liver Int. 2011;31(7):932–9.
AdiwinataPawitan J. Exploring the most promising stem cell therapy in liver failure: a systematic review. Stem Cells Int. 2019;2019:1–15.
Kwak K-A, et al. Current perspectives regarding stem cell-based therapy for liver cirrhosis. Can J Gastroenterol Hepatol. 2018;2018:1–19.
Terai S, et al. Timeline for development of autologous bone marrow infusion (ABMi) therapy and perspective for future stem cell therapy. J Gastroenterol. 2012;47(5):491–7.
Amer M-EM, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23(10):936–41.
Terai S, et al. Status and prospects of liver cirrhosis treatment by using bone marrow-derived cells and mesenchymal cells. Tissue Eng Part B Rev. 2014;20(3):206–10.
Yang X, et al. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. 2020;10(1):1–18.
Si-Tayeb K, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305.
Hay DC, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci. 2008;105(34):12301–6.
Lin L, Du L. The role of secreted factors in stem cells-mediated immune regulation. Cell Immunol. 2018;326:24–32.
Ma H-C, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol WJG. 2014;20(40):14884.
Delgado-Martín C, et al. Chemokine CXCL12 uses CXCR4 and a signaling core formed by bifunctional Akt, extracellular signal-regulated kinase (ERK) 1/2, and mammalian target of rapamycin complex 1 (mTORC1) proteins to control chemotaxis and survival simultaneously in mature dendritic cells. J Biol Chem. 2011;286(43):37222–36.
Pawig L, et al. Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol. 2015;6:429.
Imitola J, et al. Directed migrationof neural stem cellsto sites of CNS injury by thestromal cell-derived factor1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci. 2004;101(52):18117–22.
Döring Y, et al. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212.
Huh C-G, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci. 2004;101(13):4477–82.
Valdés-Arzate A, et al. Hepatocyte growth factor protects hepatocytes against oxidative injury induced by ethanol metabolism. Free Radic Biol Med. 2009;47(4):424–30.
Phaneuf D, Chen S-J, Wilson JM. Intravenous injection of an adenovirus encoding hepatocyte growth factor results in liver growth and has a protective effect against apoptosis. Mol Med. 2000;6(2):96–103.
Giordano S, Columbano A. Met as a therapeutic target in HCC: facts and hopes. J Hepatol. 2014;60(2):442–52.
Wang H, et al. The function of the HGF/c-Met axis in hepatocellular carcinoma. Front Cell Dev Biol. 2020;8:55.
Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–10.
Zhu C, et al. Gene transfer of c-met confers protection against D-galactosamine/lipopolysaccharide-induced acute liver failure. Dig Dis Sci. 2012;57(4):925–34.
Wang K, et al. Overexpression of c-Met in bone marrow mesenchymal stem cells improves their effectiveness in homing and repair of acute liver failure. Stem Cell Res Ther. 2017;8(1):1–10.
Liu J, et al. HGF/c-Met signaling mediated mesenchymal stem cell-induced liver recovery in intestinal ischemia reperfusion model. Int J Med Sci. 2014;11(6):626.
Chen J, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–96.
Ruvolo P, Deng X, May W. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15(4):515–22.
Chang W, Song B-W, Hwang K-C. Mesenchymal stem cell survival in infarcted myocardium: adhesion and anti-death signals. In: Haya MA, editor. Stem cells and cancer stem cells, vol. 10. Springer; 2013. p. 35–43.
Ma J, et al. Exosomes derived from AKt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2017;6(1):51–9.
Takehara T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127(4):1189–97.
Pritchard DJ, Butler WH. Apoptosis—the mechanism of cell death in dimethylnitrosamine-induced hepatotoxicity. J Pathol. 1989;158(3):253–60.
Yan G, Elbadawi M, Efferth T. Multiple cell death modalities and their key features. World Acad Sci J. 2020;2(2):39–48.
Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):147–71.
Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–9.
Lawrence MS, et al. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci. 1996;16(2):486–96.
Shimazaki K, et al. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7(14):1244–9.
Jin S, et al. Mesenchymal stem cells with enhanced Bcl-2 expression promote liver recovery in a rat model of hepatic cirrhosis. Cell Physiol Biochem. 2016;40(5):1117–28.
Garcia-Ocaña A, et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275(2):1226–32.
Nakamura T, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342(6248):440–3.
Nakamura T, et al. Hepatocyte growth factor twenty years on: much more than a growth factor. J Gastroenterol Hepatol. 2011;26:188–202.
Oliveira AG, et al. The role of hepatocyte growth factor (HGF) in insulin resistance and diabetes. Front Endocrinol. 2018;9:503.
Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119(4):591–600.
Bottaro DP, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251(4995):802–4.
Matsumoto K, et al. HGF–Met pathway in regeneration and drug discovery. Biomedicines. 2014;2(4):275–300.
Martins I, et al. Anticancer chemotherapy and radiotherapy trigger both non-cell-autonomous and cell-autonomous death. Cell Death Dis. 2018;9(7):1–18.
Tang Y, et al. Therapeutic potential of HGF-expressing human umbilical cord mesenchymal stem cells in mice with acute liver failure. Int J Hepatol. 2016;2016:1–13.
Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3):1–12.
Mossahebi-Mohammadi M, et al. FGF signaling pathway: a key regulator of stem cell pluripotency. Front Cell Dev Biol. 2020;8:79.
Yan L, et al. Peripheral blood monocytes from patients with HBV related decompensated liver cirrhosis can differentiate into functional hepatocytes. Am J Hematol. 2007;82(11):949–54.
Wang J, et al. Bone mesenchymal stem cells overexpressing FGF4 contribute to liver regeneration in an animal model of liver cirrhosis. Int J Clin Exp Med. 2015;8(8):12774.
Bonefeld K, Møller S. Insulin-like growth factor-I and the liver. Liver Int. 2011;31(7):911–9.
Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin. 2012;41(2):425–43.
Fiore EJ, et al. Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24(6):791–801.
Fiore E, et al. Involvement of hepatic macrophages in the antifibrotic effect of IGF-I-overexpressing mesenchymal stromal cells. Stem Cell Res Ther. 2016;7(1):1–14.
Rios P, Li X, Köhn M. Molecular mechanisms of the PRL phosphatases. FEBS J. 2013;280(2):505–24.
Peng Y, et al. The gene encoding human nuclear protein tyrosine phosphatase, PRL-1: cloning, chromosomal localization, and identification of an intron enhancer. J Biol Chem. 1998;273(27):17286–95.
Dumaual CM, et al. Cellular localization of PRL-1 and PRL-2 gene expression in normal adult human tissues. J Histochem Cytochem. 2006;54(12):1401–12.
Wang Y, Lazo JS. Metastasis-associated phosphatase PRL-2 regulates tumor cell migration and invasion. Oncogene. 2012;31(7):818–27.
Cates CA, et al. Prenylation of oncogenic humanPTPCAAXprotein tyrosine phosphatases. Cancer Lett. 1996;110(1–2):49–55.
Semba S, Mizuuchi E, Yokozaki H. Requirement of phosphatase of regenerating liver-3 for the nucleolar localization of nucleolin during the progression of colorectal carcinoma. Cancer Sci. 2010;101(10):2254–61.
Diamond RH, et al. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994;14(6):3752–62.
Luo Y, Liang F, Zhang Z-Y. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry. 2009;48(8):1838–46.
Diamond RH, et al. Expression of PRL-1 nuclear PTPase is associated with proliferation in liver but with differentiation in intestine. Am J Physiol Gastrointest Liver Physiol. 1996;271(1):G121–9.
Jiao Y, et al. Protein tyrosine phosphatase of liver regeneration-1 is required for normal timing of cell cycle progression during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2015;308(2):G85–91.
Kim JY, et al. Enhanced PRL-1 expression in placenta-derived mesenchymal stem cells accelerates hepatic function via mitochondrial dynamics in a cirrhotic rat model. Stem Cell Res Ther. 2020;11(1):1–17.
Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006;580(27):6289–94.
Kaneko N, et al. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39(1):1–16.
Dinarello CA. The role of the interleukin-1–receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343(10):732–4.
Baranova A, et al. Association of serum adipocytokines with hepatic steatosis and fibrosis in patients with chronic hepatitis C. Digestion. 2011;83(1–2):32–40.
Sun CC, et al. Interleukin-1 receptor antagonist (IL-1RA) prevents apoptosis in ex vivo expansion of human limbal epithelial cells cultivated on human amniotic membrane. Stem Cells. 2006;24(9):2130–9.
Sgroi A, et al. Interleukin-1 receptor antagonist modulates the early phase of liver regeneration after partial hepatectomy in mice. PLoS ONE. 2011;6(9):e25442.
Zheng Y-B, et al. Amniotic-fluid-derived mesenchymal stem cells overexpressing interleukin-1 receptor antagonist improve fulminant hepatic failure. PLoS ONE. 2012;7(7):e41392.
Watt AJ, Garrison WD, Duncan SA. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37(6):1249–53.
Parviz F, et al. Hepatocyte nuclear factor 4α controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet. 2003;34(3):292–6.
Lau HH, et al. The molecular functions of hepatocyte nuclear factors—in and beyond the liver. J Hepatol. 2018;68(5):1033–48.
Lu H. Crosstalk of HNF4α with extracellular and intracellular signaling pathways in the regulation of hepatic metabolism of drugs and lipids. Acta Pharm Sin B. 2016;6(5):393–408.
Ye Z, et al. Mesenchymal stem cells overexpressing hepatocyte nuclear factor-4 alpha alleviate liver injury by modulating anti-inflammatory functions in mice. Stem Cell Res Ther. 2019;10(1):1–10.
Yu Y, Zhang Q, Wu N, Xia L, Cao J, Xia Q, Zhao J, Zhang J, Hang H. HNF4α overexpression enhances the therapeutic potential of umbilical cord mesenchymal stem/stromal cells in mice with acute liver failure. FEBS Lett. 2022;596(24):3176–90.
Girard S, et al. Pro-inflammatory disequilibrium of theIL-1β/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide andhypoxia–ischemia. Cytokine. 2008;43(1):54–62.
Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology. 2004;43(suppl_3):iii2–9.
Tron K, et al. Upregulation of heme oxygenase-1 gene by turpentine oil-induced localized inflammation: involvement of interleukin-6. Lab Investig. 2005;85(3):376–87.
Ma H, et al. IL-1β siRNA adenovirus benefits liver regeneration by improving mesenchymal stem cells survival after acute liver failure. Ann Hepatol. 2016;15(2):260–70.
Su J, et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577(7791):566–71.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30.
Verrecchia F, Chu M-L, Mauviel A. Identification of novel TGF-β/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276(20):17058–62.
Verrecchia F, et al. Smad3/AP-1 interactions control transcriptional responses to TGF-β in a promoter-specific manner. Oncogene. 2001;20(26):3332–40.
Dennler S, et al. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17(11):3091–100.
Hua X, et al. Synergistic cooperation of TFE3 and smad proteins in TGF-β-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev. 1998;12(19):3084–95.
Margadant C, Sonnenberg A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11(2):97–105.
Dadlani H, et al. Smad and p38 MAP kinase-mediated signaling of proteoglycan synthesis in vascular smooth muscle. J Biol Chem. 2008;283(12):7844–52.
Romaris M, Bassols A, David G. Effect of transforming growth factor-β 1 and basic fibroblast growth factor on the expression of cell surface proteoglycans in human lung fibroblasts. Enhanced glycanation and fibronectin-binding of CD44 proteoglycan, and down-regulation of glypican. Biochem J. 1995;310(1):73–81.
Yuan W, Varga J. Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem. 2001;276(42):38502–10.
Chen Y, et al. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62(4):1149–59.
Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–91.
Wu SP, et al. Smad7-overexpressing rat BMSCs inhibit the fibrosis of hepatic stellate cells by regulating the TGF-β1/Smad signaling pathway. Exp Ther Med. 2017;14(3):2568–76.
Su D-N, Wu S-P, Xu S-Z. Mesenchymal stem cell-based Smad7 gene therapy for experimental liver cirrhosis. Stem Cell Res Ther. 2020;11(1):1–11.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33.
Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–87.
Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4.
John K, et al. MicroRNAs play a role in spontaneous recovery from acute liver failure. Hepatology. 2014;60(4):1346–55.
Tsay H-C, et al. Hepatocyte-specific suppression of microRNA-221-3p mitigates liver fibrosis. J Hepatol. 2019;70(4):722–34.
Qu Y, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491–502.
Liu Y, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140–50.
Lou G, et al. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21(11):2963–73.
Fan X-L, et al. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771–94.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NA designed the review study and contributed to writing the manuscript draft. FS, MS, ZM, and MD searched the literature and contributed to writing the manuscript. LT contributed to literature search and edited the manuscript. All authors have confirmed the final version of the manuscript and are accountable for the contents of all parts of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sani, F., Sani, M., Moayedfard, Z. et al. Potential advantages of genetically modified mesenchymal stem cells in the treatment of acute and chronic liver diseases. Stem Cell Res Ther 14, 138 (2023). https://doi.org/10.1186/s13287-023-03364-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03364-x