Abstract
Parkinson's disease (PD) is the second most predominant neurodegenerative disease worldwide. It is recognized clinically by severe complications in motor function caused by progressive degeneration of dopaminergic neurons (DAn) and dopamine depletion. As the current standard of treatment is focused on alleviating symptoms through Levodopa, developing neuroprotective techniques is critical for adopting a more pathology-oriented therapeutic approach. Regenerative cell therapy has provided us with an unrivalled platform for evaluating potentially effective novel methods for treating neurodegenerative illnesses over the last two decades. Mesenchymal stem cells (MSCs) are most promising, as they can differentiate into dopaminergic neurons and produce neurotrophic substances. The precise process by which stem cells repair neuronal injury is unknown, and MSC-derived exosomes are suggested to be responsible for a significant portion of such effects. The present review discusses the application of mesenchymal stem cells and MSC-derived exosomes in PD treatment.
Similar content being viewed by others
Introduction
PD occurs as a result of dopamine depletion, loss of neurons in substantia nigra (SN), and Lewy body build-up in other brain areas [1,2,3]. Patients with PD experience bradykinesia, stiffness, tremors at rest and unsteady gait as their motor abilities deteriorate [2, 3]. Deep brain stimulation or therapies to increase DA levels by administering a DA precursor (Levodopa) are the currently available therapy options for PD. However, L-DOPA therapy has little effect on the progression of PD, and its efficacy decreases as the disease progresses. At the same time, undesirable symptoms such as dyskinesia might also occur [4,5,6]. In vitro studies have shown that stem cells can differentiate into DAn [7,8,9,10,11,12] and accelerate the recovery of injured DAn [13,14,15,16,17].
Stem cells can be classified according to their origin: hematopoietic stem cells, neural stem cells, epithelial stem cells, skin stem cells, mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and neural stem cells (NSCs) [18]. MSCs can be originated from different sources, including adult bone marrow, adipose tissue, peripheral blood and various neonatal birth-associated tissues and they can also be induced in vitro to differentiate into osteoblasts, chondrocytes, adipocytes, and other cell types [19]. MSCs are multipotent non-hematopoietic cells with certain advantages over others. MSCs have a minimal immunogenicity, no risk of teratoma, and no ethical issues. Another advantage is their potential for individualized therapy, as MSCs can be obtained from the same patients without triggering immunological reactions. Furthermore, MSCs have a low risk of tumorigenesis after transplantation into humans or animals [18]. However, recent investigations have suggested that the paracrine actions of MSCs are mediated by the formation and release of extracellular vesicles (EVs) [20]. Exosomes are a subtype of EVs that range from 30 to 150 nm in size and are secreted by all living organisms cells [21, 22]. MSCs and their exosomes have been used in several clinical studies around the world to treat a variety of diseases, such as bone and cartilage pathologies[23], diabetes, cardiovascular disease, immune-related and neurological problems, as well as diseases of the lungs, kidneys, and liver [24,25,26]. This therapeutic technique may be beneficial for presently incurable diseases. Still, important uncertainties remain about the administration routes, ideal MSC dosage, best engraftment period, and the destiny of the cells being infused [27]. In the present article, we will focus on the therapeutic potential of MSCs and their exosomes in treating PD.
Pathophysiology of PD
PD is a degenerative disease that affects 0.3% of the population and causes progressive impairment in motor function [28]. Misfolded proteins, such as a-synuclein, as a possible pathologic agent, accumulate abnormally in the brain [29], causing Lewy body dementia, PD dementia, multisystemic atrophy and PD.
Toxic a-synuclein oligomers and protofibrils [30] can be transmitted from one cell to another in a prion-like way [31]. This is thought to facilitate the pathogenesis of PD, as a-synuclein oligomers are known to spread from the basal to the neocortical parts of the brain [32]. In addition to a-synuclein build-up, an amyloid-beta and tau containing a-synuclein coaggregation were recently discovered [33,34,35]. Specific mechanisms underlying neuronal degeneration in PD are yet to be identified. However, infectious agents [36], pesticides [37], heavy metals [38], and rural life [39] have been recognized as risk factors for PD.
Although environmental risk factors for Parkinson's disease have attracted a lot of attention, the role of genetic variables in determining the possibility of having the disease is becoming more widely realized. Although familial types of PD account for less than 10% of all cases, the identification of multiple genes (e.g., parkin, DJ1[a parkin-associated protein involved with oxidative stress], α-synuclein, UCHL1 [ubiquitin carboxy-terminal hydrolase L1], and PINK1 [putative serine-threonine kinase]) that cause early onset PD has provided vital information about possible pathological pathways [40]. Many other genes have been linked to the parkinsonian phenotype; however, neurological testing frequently reveals other signs such as ataxia, dystonia, or dementia [41].
The pathogenesis of PD is influenced by cell metabolism and protein clearance. Degeneration of noradrenergic neurons in the locus coeruleus could be associated with depression and dementia [42]. Depression can also be caused by the degeneration of serotonergic neurons in the median raphe and raphe obscurus [43].
Modulatory effects of MSCs on PD
Recently, dysregulation of the autophagy system has been identified in the brains of PD patients and animal models of the condition, suggesting a potential role for autophagy in PD [44]. In PD models, MSCs have been demonstrated to improve a-syn clearance and regulate autophagy-lysosomal activity [45]. MSCs may activate autophagy signaling through upregulation of Beclin-1 [46], a key positive regulator of mammalian autophagy. The secretome of MSCs has been found to contain numerous components associated with autophagy signaling in cell-based experiments through induction of autophagy-related genes, including beclin-1 (BCEN1), Gamma-aminobutyric acid receptor-associated protein-like 1 (GABARAPL1) and Autophagy related 12 (ATG12). The secretome of MSCs drives PI3K/Akt activation and modulates different signaling pathways to improve nutrient absorption, cell growth, metabolism, and proliferation [47, 48].
According to several investigations, MSCs exhibit immunomodulatory effects after infiltrating to injury sites in response to particular chemotactic recruitment [49] and releasing numerous growth and immunoregulatory factors, so they can alleviate inflammation and improve tissue healing [50]. Therefore, MSC-based cell therapy has been used to modulate inflammation and accommodate tissue regeneration in treating many neuroinflammatory and neurodegenerative illnesses such as Parkinson's disease [51].
MSCs are also believed to have immunoregulatory effects since they might induce inflammation when the immune system is underactive and suppress inflammation when the immune system is overactive. This process is often referred to as the immune system's "sensor and switcher" [52]. In animal models of epilepsy and PD, MSC therapy increased anti-inflammatory cytokine levels like Transforming growth factor-beta1 (TGF-β1), Prostaglandin E2 (PGE2), Hepatocyte growth factor (HGF), Indoleamine 2,3 dioxygenase (IDO), Nitric oxide (NO), interleukin 4 (IL-4) and interleukin 10 (IL-10), while decreasing pro-inflammatory cytokine levels (such as interleukin-6 (IL-6), Interleukin-1beta (IL-1β), Tumor necrosis factor-alpha (TNF-α)) in the brain and blood[53,54,55,56]. Consistent with these findings, it has been proposed that TNF promotes the immunosuppression capacity of MSCs by increasing the expression of TNF-α stimulated gene/protein-6 (TSG-6) to limit microglial activation (56) effectively. More importantly, an investigation found that MSCs might alter local microglia from a pro-inflammatory state to an anti-inflammatory state via the CX3CL1/CX3CR1 signaling axis [57].
On the other hand, some therapeutic effects appear to be dependent on MSC-released neurotrophic factors (Glial cell-derived neurotrophic factor (GDNF), Nerve growth factor (NGF), Brain-derived neurotrophic factor (BDNF)), which can inhibit DA neurons apoptosis and enhance neurogenesis by secreting proangiogenic and mitotic factors like Vascular endothelial growth factor (VEGF) and, fibroblast growth factor 2 (FGF2) [58, 59].
Preclinical studies of MSC therapy on PD
Bone marrow-derived MSCs
Recently Bouchez et al. used rat bone marrow mesenchymal stem cells (BMSCs) to treat PD in animal models. In this study, rat BMSCs were administered to the same location in adult rats that had been injured by unilateral striatal injection of 6-hydroxydopamine (6-OHDA) [15]. The frequency of amphetamine-induced rotations, caused by the pre-synaptic positive regulatory effect of amphetamine on synaptic concentrations of dopamine, was dramatically reduced in transplanted rats compared to the control group. This test evaluate the motor impairment induced by lesions in PD disease [60].The level of dopaminergic biomarkers in nerve endings and cell bodies was largely recovered. This effect was attributed to the survival of dopaminergic neurons and budding from the remaining nigrostriatal fibers.
In a 6-OHDA animal model, intracarotid injection of rat BMSCs was tested by Cerri et al. [61]. While there was no change in the progression of 6-OHDA-induced lesion or motor impairment, there was a gradual normalization of the pathologic reaction to apomorphine treatment. The authors stated that arterial administration of BMSCs might result in functional compensatory modifications in the nigrostriatal pathway, but neuroprotective effects remain unknown.
Intravenous (IV) delivery of rat BMSCs was investigated in another animal model of PD created by subcutaneous (SC) administrations of rotenone and ovariectomy [62]. Ahmed et al. demonstrated that BMSCs were capable of spreading in the damaged brain and resulted in a significant drop in serum TGF-1 concentrations, as well as an elevation in nestin gene expression and brain tyrosine hydroxylase (TH). DA levels in the brain and serum BDNF were likewise raised. The anti-inflammatory, immunomodulatory, and neurotrophic actions of BMSCs are thought to be responsible for normalizing these indicators. The striatum's histologic integrity was also preserved in the brain slices [62].
To achieve functional benefits, the appropriate dosage and delivery rate will be better identified in future investigations. Intranasal administration of murine BMSCs was also evaluated in a rat model created by intraperitoneal (IP) rotenone administrations [63]. Histological analysis in various brain areas confirmed the successful survival of MSCs. Nigral dopaminergic neurons and striatal TH-positive fibers were retained considerably. Furthermore, neurobehavioral testing revealed that MSC treatment could slow the gradual rotenone-induced loss of locomotor capabilities [57].
Adipose tissue-derived MSCs
In another study by Schwerk et al., seven days after the medial forebrain bundle was entirely damaged by administrations of 6-OHDA, autologous adipose tissue-derived mesenchymal stem cells (AD-MSCs) were grafted into the substantia nigra of rats [64]. Compared to untreated animals, enhanced subventricular zone (SVZ) neurogenesis was observed three days following transplantation. This observation is intriguing because impairment of the SVZ-olfactory bulb pathway is believed to be the etiology of hyposmia in PD patients. Transplanted AD-MSCs were predominantly seen in the SN area and the adjacent arachnoid mater. Some cells with endothelial characteristics were also discovered adjacent to arteries. This line of differentiation could be effective in amending vascular changes (Table 1).
In their prospective study [54], Schwerk et al. reported the presence of AD-MSCs near the arachnoid mater and the SN, where they expressed pericyte and endothelial biomarkers. Improved memory performance, upregulation of systemic anti-inflammatory cytokines, conserved levels of dopamine, and enhanced neurogenesis in hippocampus and SVZ were attained following transplantation of AD-MSCs. However, improvements in cognitive functions were not matched by gains in motor abilities.
Human dental pulp-derived MSCs
Human dental pulp stem cells (h-DPMSCs) have also yielded promising results, according to Chun et al. experiments in vitro [68]. It has been shown that DPMSCs can develop into neural lines when cultivated in specific conditions, and an increase in TH expression was also found in some of these cells.
Human conjunctiva-derived MSCs
Another in vivo study on the rat model of PD by Forouzandeh et al. found that MSCs obtained from human conjunctiva (CJ-MSCs) exhibited protective effects against PD sequelae and nerve induction of cells owing to their capability to release dopamine. On the other hand, CJ-MSC microencapsulation leads to an even more significant protective impact of CJ-MSCs [65].
Clinical trials of MSC therapy on PD
Bone marrow-derived MSCs
Autologous BMSCs were grafted into the SVZ of seven patients with severe PD in a prospective, uncontrolled, pilot study by Venkataramana in 2010 [69]. This brain area was selected because it possesses the greatest amount of NSCs participating in neurogenesis. The Unified PD Rating Scale (UPDRS), Schwab and England (S&E), and Hoehn and Yahr (H&Y) scales were used to assess clinical circumstances throughout a 10–36 month period. Symptoms like facial expression, gait, and freezing periods were also reported to have improved subjectively. Furthermore, in two cases, the L-DOPA doses were dramatically lowered. Although the particular mechanisms are unknown, this experiment has validated the procedure's safety, as none of the patients experienced clinical exacerbation until the completion of the monitoring period. Furthermore, there were no aberrant parenchymal alterations on cerebral magnetic resonance imaging [69].
Furthermore, modified MSCs that had been differentiated into dopaminergic cells were directly inserted into the femoral artery that supplies the substantia nigra in a recent trial on 53 PD patients [70]. The findings demonstrate that intra-arterial autologous BMSCs transplantation is a safe and effective therapy and eliminates the tumorigenesis and immunological reactions risks. This is supported by the fact that none of the patients treated with this method developed severe adverse complications, and as the study suggests, all participants were able to leave the hospital the day after receiving MSC infusion.
In another pilot study by Canesi et al., five patients with progressive supranuclear palsy, an uncommon and severe form of parkinsonism, were given bone marrow-derived MSCs by administration into the cerebral arteries [71]. All treated patients survived a year after cell infusion, except one, who died nine months later for causes unrelated to cell administration or progression of the disease (accidental fall). During the one-year follow-up, motor function assessment scales in all treated individuals remained steady for at least six months. In late 2021, Schiess et al. confirmed the safety and tolerance of intravenous administration of bone marrow-derived MSCs on 20 subjects with mild to moderate PD, who exhibited reduced UPDRS scores by the end of a one-year follow-up interval [72].
Human umbilical cord-derived MSCs
Ever since Venkataramana in 2010 [69], several other investigations have been conducted to confirm the efficacy of MSC therapy on PD using the scales mentioned above. In 2011, Qiu et al. performed human umbilical cord-derived MSC (hUC-MSC) transplantation on 8 patients with PD, observing a clinical improvement in UPDRS within a month from transplantation [73]. Three years later, in 2014, Wang et al. enrolled 15 patients with PD (H&Y staging: 3 – 5) into an experimental program on hUC-MSC transplantation, demonstrating a significant decreased in UPDRS within a month after the intervention [74]. In 2016, similar results were reported by a limited trial on intravenous transplantation of allograft hUC-MSCs in 5 PD cases, that led to reduced disease severity in 3 out of the 5 patients, based on UPDRS, in three months [75]. More recently, in 2020, Boika et al. explored the efficacy of autologous MSC transplantation on 12 PD patients. The therapy's effectiveness was assessed one and three months after the transplant. UPDRS was used to determine the severity of motor symptoms. In the post-transplant period, they discovered a statistically significant reduction in the severity of motor and nonmotor complaints in the study participants [76].
Adipose tissue-derived MSCs
Most recently, in early 2022, Shigematsu et al. treated 3 PD patients with 5 – 6 repeated infusions of autologous adipose tissue-derived MSCs, denoting a significant clinical improvement in terms of UPDRS in all three subjects [77].
The summary of clinical trials of MSC therapy for PD is presented in Table 2.
Exosomes; Act as MSC paracrine mediators
Definition of exosome
Exosomes are EVs derived from the endosomal complex [78]. EVs are divided into three categories based on their size, cargo, and origin: exosomes, microvesicles, and apoptotic bodies [79, 80]. Among EVs, exosomes are the smallest group; they have a size of 30 to 150 nm and can be extracted by centrifugation from all body fluids such as breast milk, blood, urine, amniotic and synovial fluid, ascites, and pleural effusions [81]. Exosomes are secreted by most cell lines, including neurons, immune cells, epithelial and endothelial cells [82], and have cytosolic substances and phospholipid bilayer that are similar to those of the donor cells [83]. In addition to creating stability and durability of the membrane, the exosome lipid bilayer membrane has a role in other activities such as absorption and fusion by the target cells. Phosphatidylserine (PS), for example, is involved in exosome sprouting and merging due to its enhanced flexibility [84]. Lysosomal-associated membrane protein (LAMP), tetraspanins (CD81, CD82, CD9, CD63), GTPases, major histocompatibility complex-I and II (MHC I-II), CD13, intercellular adhesion molecule-1 (ICAM-1), fusion proteins such as tumor susceptibility gene 101 protein (TSG101), annexin, integrin, heat shock protein 90 (HSC90) and HSC70 are other molecules found abundantly in exosomal membranes [22, 85,86,87]. These cytosolic-loaded membrane proteins function as biomarkers for a variety of clinical diseases. Exosomes include a specific collection of protein groups from intracellular domains such as the plasma membrane, endosomal pathway, and cytoplasm [78]. Messenger RNAs (mRNA) and microRNAs (miRNA) are also found in exosomes and can carry genetic information to target cells [88].
Exosomal nucleic acids
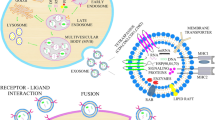
As explained previously, nucleic acids like miRNAs constitute a significant component of exosomes [89]. Several disorders, including PD, have been shown to involve alterations in gene expression, particularly at the miRNA stage [90]. MiRNAs generated from exosomes have also been identified as possible diagnostic markers and targeted therapeutics (Fig. 1).
(1) Exosomes are separated from cell culture and bodily fluids using a variety of techniques. (2) They contain a series of Rab proteins, tetraspanins, heat shock proteins, intercellular adhesion molecule (ICAM-1), endosome-associated proteins (TSG101), and nucleic acids such as mRNAs and mRNAs, according to fluorescence-activated cell sorting (FACS), mass spectrometry analyses, and Western blot
MiRNAs are a type of non-coding RNA that plays a crucial role in gene expression regulation. MiRNAs often interact with the 3′ untranslated region (3′-UTR) of target mRNAs to cause mRNA degradation and translational inhibition. However, miRNAs have been shown to interact with other areas such as the 5′-UTR, coding sequences, and gene promoters. MiRNAs can also initiate translation or regulate transcription under certain situations [91].
In animal cells, miRNAs are generated in two phases, beginning with primary miRNAs and continuing with Drosha/DGCR8 RNase in the nuclei and Dicer RNase in the cytoplasm [92]. It was discovered that Dicer deficiencies in the midbrain rats might result in progressive destruction of DAn. Also, post-mortem cerebral examination revealed DAn loss and Lewy body formation when the DiGeorge syndrome critical region 8 (DGCR8) gene was eliminated [93, 94]. Exosomes seem to assist exogenous miRNA distribution to target cells. Regardless, the processes of target absorption should be thoroughly investigated [95].
MSC-derived exosome characteristics
Having established the definition of exosomes as nanoscopic particles derived from MSCs, one might conclude that they are, in effect, the mediators of MSC functions in a paracrine fashion [96]. Similar to other exosomes, MSCs-derived exosomes transport a complex cargo of lipids, proteins, and nucleic acids. MSC-derived exosomes are particularly suitable for this role due to their high enzyme content [97]. This is because many enzymes' catalytic activity is controlled by feedback mechanisms influenced by the tissue microenvironment. By supplying catalytically active enzymes to support tissue homeostasis, MSC-derived exosomes might be able to restore normal tissue function to some extent, though, such speculations should be validated by prospective investigations.
The activity of an enzyme is proportional to the relative concentrations of its substrate and product, and it stops when the concentrations of its substrate and product are in equilibrium. Because an injury-induced change in the relative concentrations of substrate and product is expected to be proportional to the degree of the injury, exosomes might construct a measurable biological response proportionate to the severity of the injury. In contrast, improving the damage restores substrate and product balance, resulting in the termination of enzyme activity. As a result, the enzyme-centric nature of exosomes reduces the possibility of over-or under-dosing [98].
Exosomes produced from MSCs have also been found to contain cytokines and growth factors such as IL-6, IL-10, HGF and TGFβ-1; all of them have a role in regulating the immune system [99]. Comparable quantities of extracellular matrix metalloproteinase inducer (EMMPRIN), Matrix Metalloproteinase-9 (MMP-9) and VEGF have been found in MSC-derived exosomes; all of which are important in inducing angiogenesis, which may be essential for tissue repair [100]. Many miRNAs have been discovered in MSC-derived exosomes, and they are thought to be involved in physiological and pathological pathways like organism development, epigenetic regulation, immunoregulation (miR-155 and miR-146) [101], carcinogenesis, and tumor growth [102].
MSC-exosomes have been studied for their immunomodulatory characteristics [103]. Exosomes produced by BM-derived MSCs can effectively treat chronic graft-versus-host disease (cGVHD) in mice by suppressing CD4+ T cell activation and infiltration, lowering pro-inflammatory cytokine production, enhancing the formation of IL-10-expressing Treg cells, and suppressing Th17 cells [104]. In animal models of type 1 diabetes (T1D) and experimental autoimmune uveoretinitis, EVs derived from human multipotent stromal cells decrease autoimmunity. EVs inhibited antigen-presenting cell (APCs) activation and suppressed the growth of T helper 1 and T helper 17 cells, as well as increasing the immunosuppressive cytokine IL-10 [105].
BMSCs-derived exosomes increases Treg proliferation and immunosuppressive capacity in asthmatic patients' peripheral blood mononuclear cells (PBMCs) via up-regulation suppressive cytokines TGF-1 and IL-10 [106]. hUC-MSC-derived exosomal MiR-181c have an important role in anti-inflammatory activities in a burnt rat model via inhibiting the TLR-4 signaling cascade [107]. Furthermore, MSC-derived exosomes suppressed complement-mediated destruction of sheep red blood cells in a CD59-dependent way, implying that CD59 on the exosome membrane might reduce complement activation and hinder the generation of the membrane attack complex [97]. These findings demonstrate MSC-derived exosomes' immunomodulatory activity and support their potential to restore immunological homeostasis in a tissue (Fig. 2).
(1) Secretion of α-syn can occur via secretory lysosomes (exocytosis), microvesicle shedding, or multivesicular bodies, with the second and third methods including the release of α-syn into exosomes. α-Syn can be eliminated from the extracellular space by proteolysis. One of the tiny molecules implicated in the proteolytic degradation of aggregated α-syn would be MMP2, a factor generated from MSCs. MMP-2 produced from MSCs breaks freshly formed amyloid fibrils, resulting in a considerable decrease in the quantities of insoluble and oligomeric α-syn. (2) Furthermore, tiny compounds generated from MSCs alter PI3K/Akt signaling, which ultimately regulates multiple downstream targets to increase autophagy. Upregulation of PI3K/AKT promotes autophagy via regulating the expression of autophagy-related genes such as BECN1, ATG, and GABARAPL1. As a result, autophagic flux upregulation by MSC derived small molecules increases the clearance of harmful α-syn aggregates and so plays a vital role in maintaining α-syn homeostasis in the PD-related milieu. (3) MSC interactions with immune system cells, with primary signaling pathways revealed. (4) MSCs secrete neurotrophic factors like as BDNF, NGF, and FGF-2, which interact with injured axons and cause axonal regrowth. 5) When activated by pro-inflammatory mediators, MSCs release paracrine factors such as TSG-6 and IL-4. Paracrine factors stimulate M2 macrophage polarization, resulting in an elevated Th2 response
Exosomes’ advantages in PD
There is currently no accurate diagnostic method for PD. Exosomes play two role in PD: diagnostic markers and therapeutic agents. Elevated leucine-rich repeat kinase 2 (LRRK2) mutations in urine have recently been linked to idiopathic PD and the intensity of cognitive impairment [108,109,110]. Another research revealed that the concentration of the L1 cell adhesion molecule (L1-CAM) was much more in PD patients and that it was linked with a-synuclein level and tau concentrations in cerebrospinal fluid (CSF) [111,112,113,114]. The expression patterns of mRNA and miRNA in PD exosomes act as a diagnostic method for the disease. For treatment, exosomes generated from DPSC have been discovered to prevent 80% of dopamine neuron death in PD models [115]. Also, a recent study on a PD model demonstrated that exosomes produced by MSCs could retain human brain microvascular endothelial cells (HBMECs) in a transcriptionally active mode, which may be favorable for angiogenesis [116]. In conclusion, the use of exosomes in the treatment of PD is still in its initial phases, but they are largely applied in diagnosis. Although no reliable exosome-based diagnostic tool is currently available for PD.
Challenges of stem cell therapy
MSCs source
The primary source of stem cells is a critical subject to address in order to minimize an immunogenic reaction, especially if persistent immunosuppressive therapy is to be prevented. Because of the minimal risk of rejection, autologous stem cells would be ideal for grafting. However, one negative aspect of autologous transplants is that grafted cells may keep the genetic abnormalities or risk factors resulting from the onset of PD [117]. Though, regardless of the potential immune reactions that might ensue transplantation, isolation of MSCs from candidate donors, based on the source tissue, can be a laborious task. For instance, less than 0.01% of the cells residing in bone marrow are MSCs, and this astonishingly low prevalence makes their isolation considerably difficult [118], particularly when one considers the fact that bone marrow aspiration is an invasive and painful procedure, and not all donors would opt to participate in such procedure. In this sense, adipose tissue might provide a better source for extraction of MSCs, as it is not as deeply located as the bone marrow, is more abundantly distributed throughout the body and can be accessed through less invasive methods [119].
Graft location
Several methods are currently available, either experimentally or practically, for transplantation of MSCs. In a more general sense, MSCs can be administrated either systemically or locally. Systemic transplantation is achieved via intravenous/intra-arterial infusion or inhalation of MSCs. While systemic transplantation allows MSC-based treatment of pathologies affecting the entire body, local transplantation aims to alleviate symptoms associated with illnesses that originate from certain organs, and is mostly performed through intramuscular or direct tissue injection. Each of these are limited in their own field [120].
The dopaminergic neurons are predominantly depleted in PD in the substantia nigra pars compacta (SNpc). These neurons send dopamine to the receiving cells via the putamen and caudate nuclei. As a result, several SNpc cell therapy trials in animals transplant dopaminergic neurons ectopically into striatums [121, 122]. However, since the human neural network is significantly larger than rat brains, the size of neuronal extensions may still be insufficient to stimulate the striatum if the cells are transplanted in the SNpc. As a result, transplanting into the putamen, where dopaminergic neurons finally innervate, is still the best option.
Graft cell count
Several studies have demonstrated that stem cell-derived dopaminergic transplants persist in animals after graft for at least 2 years. Still, fetal-derived dopaminergic transplants have persisted in human hosts for more than a decade. Several investigations have found that a grafting could be a suitable method for the long-term survival and functioning of transplants [122,123,124]. In the case of dosing, the average dose of each MSC therapy session in experimental animal models is usually 50 million cells per every kg of weight. Though, the baseline dose in human studies is 1–2 million cells/kg and rarely exceeds 12 million MSCs/kg [125]. According to a 2020 systematic review by Katab et al., intravenous infusion of MSCs is the most commonly preferred route of transplantation practiced in 43% of all clinical trials on MSC therapy, with every single dose per patient containing a median count of 100 million MSCs. Intravenous infusion of as many MSCs has often been deemed as safe and effective, rarely resulting in adverse effects beyond transient fever [126].
Removing the risk of neoplasia
When implanted into patients, PSCs resistant to differentiation stimuli poses an increased neoplasia risk [127]. Multiple techniques for mitigating this danger have been developed, broadly classified as removing stem cells from ultimate cultures and purifying the target cell types. For example, undifferentiated PSCs exhibit distinct cell surface biomarkers [128]. Although this "negative-sorting" technique has effectively removed undifferentiated PSCs in a mixed culture [129].
Moreover, in order to make stem cells more clinically reliable, the process by which stem cells function in experimental animals should be properly known, and patients would be able to use stem cell therapy instead of expensive drug treatment when faced with organ failure [130]. Also, bioengineering methods, such as loading MSCs with oncolytic viruses and recombinant genes, can be employed to address the challenges mentioned earlier and provide more effective and predictable MSC therapies [131,132,133].
Advantages of MSCs over other stem cell lines
PD is a devastating neurological condition that affects millions of individuals worldwide; yet, the molecular and cellular pathways that cause it are still unknown. Despite improvements in Parkinson's disease research, current pharmaceutical options improve PD patients' quality of life but do not prevent PD progression or boost dopaminergic neuron viability. Because of their capacity to promote dopaminergic neurons viability, stimulate neurogenesis, reduce neuroinflammation, and enhance functional recovery in the in vivo models, cell therapy with MSCs and their derived exosomes has recently been proposed as an efficient treatment method for a variety of neurodegenerative disorders, including PD.
In any case, investigations involving MSC and PD point to the possibility that these cells could be used as a standard treatment in the coming years. The ability of MSC to differentiate into diverse types of neurons shows that MSC can replace damaged cells not just in the basal nuclei and nigrostriatal pathway but also in other parts of the parenchyma whose degradation leads to the cognitive and behavioural symptoms of PD [134]. However, this is not the only advantage of MSCs. In fact, these cells are inert in terms of immunogenicity, as their progeny do not include immune cells, in contrast to hematopoietic stem cells (HSCs), for example. In addition to this, MSCs can be safely suppressed when required by immune cells, a capability that minimizes their potential hazards to considerable extents. Owing to their lineage, MSCs have also been shown to facilitate engraftment of other stem cells, indicating that they are quite good housekeepers. This is further bolstered by the fact that these cells can be maintained for prolonged periods of time in their initial state without transformation [135]. Else, MSCs bring about salutary alterations in their surrounding environment by releasing an extensive secretome including extracellular vesicles, especially miRNA-containing exosomes that participate in intercellular signal transduction, which has been found to confer neurorestorative effects in the case of ischemic stroke [136]. By secreting these exosomes, MSCs facilitate cell proliferation and angiogenesis, while repressing apoptosis, all of which are of high therapeutic value in degenerative diseases, such as PD [137].
Conclusions
Although stem cells continue to be an invaluable treatment modality for neural regenerative medicine, their therapeutic use is limited by the possibility of immune reactivity, tumorigenesis, and insufficient differentiation, as well as non-specific targeting and the inability to cross physiological and biological barriers. MSC-derived exosomes not only have therapeutic characteristics similar to their parent cells, but they also have the ability to avoid whole-cell post-transplant adverse events due to their potential to pass physiological barriers, migrate and reside in brain lesion sites, have a high safety profile, and no cases of immune response and rejection have been reported. Furthermore, exosomes cannot turn into pre-malignant cells. In animal models, the exchange of genetic material, such as miRNA, via exosomes can enhance neurogenesis, reduce neuroinflammation, and promote functional recovery. Indeed, miRNAs have acquired prominence in the PD research area, not only for their role in PD pathogenesis but also as a promising window for usage as biomarkers or potential therapeutic agents for PD treatment.
Nonetheless, one should not overlook the experimental nature of such therapeutic interventions, as the majority of clinical trials concerned with MSC therapy, particularly in the case of PD, have had few participants, and the results obtained from these initiatives cannot necessarily be generalized to everyday medical care. In addition to this, isolation and transplantation of MSCs require resource-demanding methods that are usually costly, and there is always a risk of failure, even if low, that need to be considered.
Availability of data and materials
The data supporting the conclusions of this article are all online.
Abbreviations
- PD:
-
Parkinson's disease
- DAn:
-
Dopaminergic neurons
- SN:
-
Substantia nigra
- MSCs:
-
Mesenchymal stem cells
- ESCs:
-
Embryonic stem cells
- iPSCs:
-
Induced pluripotent stem cells
- NSCs:
-
Neural stem cells
- EVs:
-
Extracellular vesicles
- UCHL1:
-
Ubiquitin carboxy-terminal hydrolase L1
- PINK1:
-
Putative serine-threonine kinase
- BCEN1:
-
Beclin 1 gene
- GABARAPL1:
-
Gamma-aminobutyric acid receptor-associated protein-like 1
- ATG12:
-
Autophagy-related gene 12
- TGF-β1:
-
Transforming growth factor-beta 1
- PGE2:
-
Prostaglandin E2
- HGF:
-
Hepatocyte growth factor
- IDO:
-
Indoleamine 2,3 dioxygenase
- NO:
-
Nitric oxide
- IL-4:
-
Interleukin 4
- IL-10:
-
Interleukin 10
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor-alpha
- GDNF:
-
Glial cell-derived neurotrophic factor
- NGF:
-
Nerve growth factor
- BDNF:
-
Brain-derived neurotrophic factor
- VEGF:
-
Vascular endothelial growth factor
- FGF2:
-
Fibroblast growth factor 2
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- 6-OHDA:
-
6-Hydroxydopamine
- ASCs:
-
Adipose stem cells
- SVZ:
-
Subventricular zone
- TH:
-
Tyrosine hydroxylase
- DPSC:
-
Human dental pulp stem cells
- CJ-MSCs:
-
Human conjunctiva mesenchymal stem cells
- UPDRS:
-
Unified PD Rating Scale
- S&E:
-
Schwab and England
- H&Y:
-
Hoehn and Yahr
- LAMP:
-
Lysosomal-associated membrane protein
- MHC I,II:
-
Major histocompatibility complex I, II
- ICAM-1:
-
Intercellular adhesion molecule-1
- TSG101:
-
Tumor susceptibility gene 101 protein
- HSC90:
-
Heat shock protein 90
- HSC70:
-
Heat shock protein 70
- mRNA:
-
Messenger RNA
- miRNA:
-
MicroRNA
- 3′ UTR:
-
3′ untranslated region
- DGCR8:
-
DiGeorge syndrome critical region 8
- APCs:
-
Antigen-presenting cells
- DCs:
-
Dendritic cells
- LRRK2:
-
Leucine-rich repeat kinase 2
- L1-CAM:
-
L1 cell adhesion molecule
- CSF:
-
Cerebrospinal fluid
- HBMECs:
-
Human brain microvascular endothelial cells
- SNpc:
-
Substantia nigra pars compacta
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- MMP-9:
-
Matrix metalloproteinase-9
- PBMCs:
-
Peripheral blood mononuclear cells
References
Gao L-l, Wu T. The study of brain functional connectivity in Parkinson’s disease. Transl Neurodegener. 2016;5(1):1–7.
Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–66.
Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14(5):518–31.
Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci. 2019;226:77–90.
Chang CY, Ting HC, Liu CA, Su HL, Chiou TW, Lin SZ, Harn HJ, Ho TJ. Induced pluripotent stem cell (iPSC)-based neurodegenerative disease models for phenotype recapitulation and drug screening. Molecules. 2020;25(8):2000.
Trombetta-Lima M, Sabogal-Guáqueta AM, Dolga AM. Mitochondrial dysfunction in neurodegenerative diseases: a focus on iPSC-derived neuronal models. Cell Calcium. 2021;94:102362.
Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25(11):2797–808.
Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008;17(3):547–54.
Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11:25.
Taran R, Mamidi MK, Singh G, Dutta S, Parhar IS, John JP, Bhonde R, Pal R, Das AK. In vitro and in vivo neurogenic potential of mesenchymal stem cells isolated from different sources. J Biosci. 2014;39(1):157–69.
Zhang P, Xia N, Reijo Pera RA. Directed dopaminergic neuron differentiation from human pluripotent stem cells. J Vis Exp. 2014;91:51737.
Chuang J-H, Tung L-C, Lin Y. Neural differentiation from embryonic stem cells in vitro: an overview of the signaling pathways. World J Stem Cells. 2015;7(2):437–47.
Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J Neurosci. 2006;26(48):12497–511.
Shintani A, Nakao N, Kakishita K, Itakura T. Protection of dopamine neurons by bone marrow stromal cells. Brain Res. 2007;1186:48–55.
Bouchez G, Sensebé L, Vourc’h P, Garreau L, Bodard S, Rico A, Guilloteau D, Charbord P, Besnard JC, Chalon S. Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem Int. 2008;52(7):1332–42.
Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Neurochem. 2008;107(1):141–51.
Glavaski-Joksimovic A, Virag T, Chang QA, West NC, Mangatu TA, McGrogan MP, Dugich-Djordjevic M, Bohn MC. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 2009;18(7):801–14.
Gugliandolo A, Bramanti P, Mazzon E. Mesenchymal stem cell therapy in Parkinson’s disease animal models. Curr Res Transl Med. 2017;65(2):51–60.
Molina ER, Smith BT, Shah SR, Shin H, Mikos AG. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J Control Release. 2015;219:107–18.
Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359.
Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3:228.
György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–64.
Malekpour K, Hazrati A, Zahar M, Markov A, Zekiy AO, Navashenaq JG, Roshangar L, Ahmadi M. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep. 2021. https://doi.org/10.1007/s12015-021-10185-z.
Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S, Wang Z, Xu Q. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol. 2020;81:106030.
Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–48.
Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG, Tian X. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11:590470.
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16.
de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–35.
Goedert M, Jakes R, Crowther R, Spillantini MG. Parkinson’s disease, dementia with lewy bodies, and multiple system atrophy as α-synucleinopathies. Methods Mol Med. 2001;62:33–59.
Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48.
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015;112(38):E5308-5317.
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211.
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(21):12245–50.
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62(4):389–97.
Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30(21):7281–9.
Takahashi M, Yamada T. Viral etiology for Parkinson’s disease–a possible role of influenza A virus infection. Jpn J Infect Dis. 1999;52(3):89–98.
Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22(16):7006–15.
Mizuno Y, Hattori N, Kitada T, Matsumine H, Mori H, Shimura H, Kubo S, Kobayashi H, Asakawa S, Minoshima S, et al. Familial Parkinson’s disease Alpha-synuclein and parkin. Adv Neurol. 2001;86:13–21.
Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res. 2001;86(2):122–7.
Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(1):a008888.
Dawson TM, Dawson VL. Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J Clin Invest. 2003;111(2):145–51.
German DC, Manaye KF, White CL 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32(5):667–76.
Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14(3):153–97.
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(4):a009357.
Park HJ, Shin JY, Kim HN, Oh SH, Lee PH. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a parkinsonian model. Neurobiol Aging. 2014;35(8):1920–8.
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10(1):32–44.
Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83–97.
Shanware NP, Bray K, Abraham RT. The PI3K, metabolic, and autophagy networks: interactive partners in cellular health and disease. Annu Rev Pharmacol Toxicol. 2013;53:89–106.
Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576–8.
Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy. 2006;8(6):559–61.
Glavaski-Joksimovic A, Bohn MC. Mesenchymal stem cells and neuroregeneration in Parkinson’s disease. Exp Neurol. 2013;247:25–38.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, Kim HO, Lee PH. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57(1):13–23.
Schwerk A, Altschüler J, Roch M, Gossen M, Winter C, Berg J, Kurtz A, Akyüz L, Steiner B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen Med. 2015;10(4):431–46.
Leal MM, Costa-Ferro ZS, Souza BS, Azevedo CM, Carvalho TM, Kaneto CM, Carvalho RH, Dos Santos RR, Soares MB. Early transplantation of bone marrow mononuclear cells promotes neuroprotection and modulation of inflammation after status epilepticus in mice by paracrine mechanisms. Neurochem Res. 2014;39(2):259–68.
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7(1):e2062.
Giunti D, Parodi B, Usai C, Vergani L, Casazza S, Bruzzone S, Mancardi G, Uccelli A. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. 2012;30(9):2044–53.
Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7(1):131.
d’Angelo M, Cimini A, Castelli V. Insights into the effects of mesenchymal stem cell-derived secretome in Parkinson’s disease. Int J Mol Sci. 2020;21(15):5241.
Björklund A, Dunnett SB. The amphetamine induced rotation test: a re-assessment of its use as a tool to monitor motor impairment and functional recovery in rodent models of Parkinson’s disease. J Parkinsons Dis. 2019;9(1):17–29.
Cerri S, Greco R, Levandis G, Ghezzi C, Mangione AS, Fuzzati-Armentero MT, Bonizzi A, Avanzini MA, Maccario R, Blandini F. Intracarotid infusion of mesenchymal stem cells in an animal model of Parkinson’s disease, focusing on cell distribution and neuroprotective and behavioral effects. Stem Cells Transl Med. 2015;4(9):1073–85.
Ahmed HH, Salem AM, Atta HM, Eskandar EF, Farrag AR, Ghazy MA, Salem NA, Aglan HA. Updates in the pathophysiological mechanisms of Parkinson’s disease: emerging role of bone marrow mesenchymal stem cells. World J Stem Cells. 2016;8(3):106–17.
Salama M, Sobh M, Emam M, Abdalla A, Sabry D, El-Gamal M, Lotfy A, El-Husseiny M, Sobh M, Shalash A, et al. Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson’s disease. Exp Ther Med. 2017;13(3):976–82.
Schwerk A, Altschüler J, Roch M, Gossen M, Winter C, Berg J, Kurtz A, Steiner B. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy. 2015;17(2):199–214.
Forouzandeh M, Bigdeli MR, Mostafavi H, Nadri S, Eskandari M. Therapeutic potentials of human microfluidic encapsulated conjunctival mesenchymal stem cells on the rat model of Parkinson’s disease. Exp Mol Pathol. 2021;123:104703.
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24(3):781–92.
Xiong N, Cao X, Zhang Z, Huang J, Chen C, Zhang Z, Jia M, Xiong J, Liang Z, Sun S, et al. Long-term efficacy and safety of human umbilical cord mesenchymal stromal cells in rotenone-induced hemiparkinsonian rats. Biol Blood Marrow Transplant. 2010;16(11):1519–29.
Chun SY, Soker S, Jang YJ, Kwon TG, Yoo ES. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. J Korean Med Sci. 2016;31(2):171–7.
Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62–70.
Brazzini A, Cantella R, Cruz ADI, Yupanqui J, León C, Jorquiera T, Brazzini M, Ortega M, Saenz LN. Intraarterial autologous implantation of adult stem cells for patients with parkinson disease. J Vasc Interv Radiol. 2010;21(4):443–51.
Canesi M, Giordano R, Lazzari L, Isalberti M, Isaias IU, Benti R, Rampini P, Marotta G, Colombo A, Cereda E, et al. Finding a new therapeutic approach for no-option Parkinsonisms: mesenchymal stromal cells for progressive supranuclear palsy. J Transl Med. 2016;14(1):127.
Schiess M, Suescun J, Doursout MF, Adams C, Green C, Saltarrelli JG, Savitz S, Ellmore TM. Allogeneic bone marrow-derived mesenchymal stem cell safety in idiopathic Parkinson’s disease. Mov Disord. 2021;36(8):1834.
Qiu Y, Wang Z, Lu HS. Umbilical cord mesenchymal stem cell transplantation for treatment of Parkinson’s disease in 8 cases. Chin J Tissue Eng Res. 2011;15(36):6836.
Wang Y, Zhao XL, Zhang JY, Tan J. Therapeutic applications of umbilical cord mesenchymal stem cells in Parkinson’s disease. Chin J Tissue Eng Res. 2014;18(6):937.
Wang WT, Gu P, Qiu FC, Zhang LN, Zhang ZX, Xie BC, Dong C, Han R, Liu HM, Yan BY. Intravenous transplantation of allograft huc-msc was more effective than subarachnoid transplantation of bm-mscs in patients with parkinson’s syndrome and secondary parkinson’s syndrome. J Biomater Tissue Eng. 2016;6(2):164.
Boika A, Aleinikava N, Chyzhyk V, Zafranskaya M, Nizheharodava D, Ponomarev V. Mesenchymal stem cells in Parkinson’s disease: motor and nonmotor symptoms in the early posttransplant period. Surg Neurol Int. 2020;11:380.
Shigematsu K, Komori N, Tahara K, Yamagishi H. Repeated infusion of autologous adipose tissue-derived stem cells for Parkinson’s disease. Acta Neurol Scand. 2022;145(1):122.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727.
Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23.
Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–99.
Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17(3):160–72.
van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12(7):1182–92.
Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, De Medina P, Monsarrat B, Perret B, Silvente-Poirot S, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51(8):2105–20.
Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteom. 2010;73(10):1907–20.
Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182.
Gho YS, Lee C. Emergent properties of extracellular vesicles: a holistic approach to decode the complexity of intercellular communication networks. Mol Biosyst. 2017;13(7):1291–6.
McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7.
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8(28):45200–12.
Sonntag KC. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010;1338:48–57.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402.
Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803(11):1231–43.
Butcher NJ, Kiehl TR, Hazrati LN, Chow EW, Rogaeva E, Lang AE, Bassett AS. Association between early-onset Parkinson disease and 22q11.2 deletion syndrome: identification of a novel genetic form of Parkinson disease and its clinical implications. JAMA Neurol. 2013;70(11):1359–66.
Pang X, Hogan EM, Casserly A, Gao G, Gardner PD, Tapper AR. Dicer expression is essential for adult midbrain dopaminergic neuron maintenance and survival. Mol Cell Neurosci. 2014;58:22–8.
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–32.
Suh N, Subramanyam D, Lee MY. Molecular signatures of secretomes from mesenchymal stem cells: therapeutic benefits. Mol Cell Toxicol. 2017;13(2):133–41.
Lai RC, Yeo RWY, Tan SS, Zhang B, Yin Y, Sze NSK, Choo A, Lim SK. Mesenchymal stem cell exosomes: the future MSC-based therapy? In: Chase LG, Vemuri MC, editors. Mesenchymal stem cell therapy. Totowa: Humana Press; 2013. p. 39–61.
Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–8.
Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol. 2016;4:83.
Vrijsen KR, Maring JA, Chamuleau SA, Verhage V, Mol EA, Deddens JC, Metz CH, Lodder K, van Eeuwijk EC, van Dommelen SM, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater. 2016;5(19):2555–65.
Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6:24120.
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63.
You L, Mao L, Wei J, Jin S, Yang C, Liu H, Zhu L, Qian W. The crosstalk between autophagic and endo-/exosomal pathways in antigen processing for MHC presentation in anticancer T cell immune responses. J Hematol Oncol. 2017;10(1):1–9.
Lai P, Chen X, Guo L, Wang Y, Liu X, Liu Y, Zhou T, Huang T, Geng S, Luo C, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11(1):135.
Shigemoto-Kuroda T, Oh JY, Kim D-K, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8(5):1214–25.
Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363(1):114–20.
Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82.
Ho DH, Yi S, Seo H, Son I, Seol W. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res Int. 2014;2014:704678.
Fraser KB, Moehle MS, Alcalay RN, West AB. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology. 2016;86(11):994–9.
Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N, West AB. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov Disord. 2016;31(10):1543–50.
Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, Yang L, Stewart T, Zheng D, Aro P, et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement. 2016;12(11):1125–31.
Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of α-synuclein aggregation by exosomes. J Biol Chem. 2015;290(5):2969–82.
Bliederhaeuser C, Grozdanov V, Speidel A, Zondler L, Ruf WP, Bayer H, Kiechle M, Feiler MS, Freischmidt A, Brenner D, et al. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 2016;131(3):379–91.
Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B, Schneider A. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2016;139(Pt 2):481–94.
Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17(7):932–9.
Xue C, Li X, Ba L, Zhang M, Yang Y, Gao Y, Sun Z, Han Q, Zhao RC. MSC-derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson’s disease. Aging Dis. 2021;12(5):1211–22.
Fan Y, Winanto, Ng SY. Replacing what’s lost: a new era of stem cell therapy for Parkinson’s disease. Transl Neurodegener. 2020;9:2.
Yang Y-HK, Ogando CR, Wang See C, Chang T-Y, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9(1):131.
Najar M, Melki R, Khalife F, Lagneaux L, Bouhtit F, Moussa Agha D, Fahmi H, Lewalle P, Fayyad-Kazan M, Merimi M. Therapeutic mesenchymal stem/stromal cells: value, challenges and optimization. Front Cell Dev Biol. 2022;9:716853.
Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645.
Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Björklund A, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15(5):653–65.
Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548(7669):592–6.
Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinsons Dis. 2011;1(4):395–412.
Rath A, Klein A, Papazoglou A, Pruszak J, Garcia J, Krause M, Maciaczyk J, Dunnett SB, Nikkhah G. Survival and functional restoration of human fetal ventral mesencephalon following transplantation in a rat model of Parkinson’s disease. Cell Transplant. 2013;22(7):1281–93.
Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33.
Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004–2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9(1):17–27.
Liu CC, Ma DL, Yan TD, Fan X, Poon Z, Poon LF, Goh SA, Rozen SG, Hwang WY, Tergaonkar V, et al. Distinct responses of stem cells to telomere uncapping: a potential strategy to improve the safety of cell therapy. Stem Cells. 2016;34(10):2471–84.
Brimble SN, Sherrer ES, Uhl EW, Wang E, Kelly S, Merrill AH Jr, Robins AJ, Schulz TC. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25(1):54–62.
Fong CY, Peh GS, Gauthaman K, Bongso A. Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS). Stem Cell Rev Rep. 2009;5(1):72–80.
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68.
Ko IK, Kean TJ, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30(22):3702–10.
Zhang TY, Huang B, Yuan ZY, Hu YL, Tabata Y, Gao JQ. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine. 2014;10(1):257–67.
Martinez-Quintanilla J, He D, Wakimoto H, Alemany R, Shah K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol Ther. 2015;23(1):108–18.
Hernández R, Jiménez-Luna C, Perales-Adán J, Perazzoli G, Melguizo C, Prados J. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol Ther. 2020;28(1):34.
El-Badri NS. The mesenchymal stem cell advantage. Stem Cells Dev. 2006;15(4):473–4.
Chen M, Shen YJ, Yang R, Song JK, Li L, Du GH. Research progress on the neurorestorative benefits of mesenchymal stem cell exosomes for the treatment of ischemic stroke. Yaoxue Xuebao. 2020;55(10):2306–13.
Qin S, Baolan S, Xiaoqing Y, Yuquan Z. Mesenchymal stem cell-derived exosomes carrying miRNAs in tissue repair and treatment of related diseases: application and advantages. Chin J Tissue Eng Res. 2021;25(31):5040–5.
Acknowledgements
Not applicable.
Funding
We want to thank Stem Cell Research Center of Tabriz University of Medical Sciences for supporting this study (Grant numbers: 67004 and 67006).
Author information
Authors and Affiliations
Contributions
RMH and MS wrote the manuscript, SA-A and HV cooperated in design and manuscript writing, AH and FJN edited the final version of the manuscript, SS and HRY drew the schematic figure and tables, MA designed and supervised the study and correspondence during the paper submission. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Heris, R.M., Shirvaliloo, M., Abbaspour-Aghdam, S. et al. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem Cell Res Ther 13, 371 (2022). https://doi.org/10.1186/s13287-022-03050-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03050-4