Abstract
Background
Incidence of myocarditis following messenger RNA coronavirus disease 2019 vaccination has been widely described, but this clinical scenario after adenoviral vector coronavirus disease 2019 vaccination has only been rarely reported. In addition, a few case reports of thyroiditis after adenoviral vector coronavirus disease 2019 vaccination have been published.
Case presentation
A 55-year-old Thai woman presented with palpitation without neck pain 14 days after receiving AstraZeneca coronavirus disease 2019 vaccination. Electrocardiography revealed sinus tachycardia. Her blood tests showed elevation of cardiac troponin and free triiodothyronine with suppressed serum thyroid stimulating hormone, reflecting a hyperthyroid status. Evidence of myocardial inflammation and necrosis from cardiac magnetic resonance imaging supported the diagnosis of recent myocarditis. Laboratory results and imaging findings were consistent with thyroiditis. After 3 weeks of symptomatic treatment, her symptom and blood tests had returned to normal.
Conclusions
This case demonstrates that the adenoviral vector coronavirus disease 2019 vaccine could possibly cause myocarditis and painless thyroiditis. Clinicians should have a high index of suspicion and promptly evaluate these conditions, despite minimal symptoms.
Similar content being viewed by others
Background
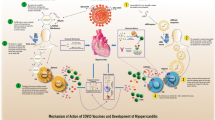
Since the emergence of the coronavirus disease 2019 (COVID-19)/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, effective vaccines against COVID-19 have been developed [1]. The AstraZeneca COVID-19 vaccine (ChAdOx1-S/nCoV-19 [recombinant], AZD1222), a replication-deficient adenoviral vector vaccine that contains the SARS-CoV-2 spike protein gene, triggers the immune system to generate an immune response against the coronavirus and retain that information in memory immune cells [2, 3]. This vaccine was used for mass vaccination nationally and globally. In Thailand, more than 40 million doses have already been given. The most well-publicized adverse reactions are vaccine-induced thrombosis and thrombocytopenia [4]. However, there are a few published reports regarding thyroiditis and myocarditis following exposure to the AstraZeneca COVID-19 vaccine.
We report the case of a patient who developed concomitant myocarditis and painless thyroiditis after receiving the AstraZeneca COVID-19 vaccine.
Case presentation
A 55-year-old Thai woman presented with palpitation 14 days after receiving the second dose of the AstraZeneca COVID-19 vaccination. She had no history of fever, dyspnea, chest pain, neck pain, or weight loss. Her past medical histories were essential hypertension and hypercholesterolemia. She had no history of thyroid disease. On admission, she had tachycardia with a heart rate of 120/min. Other examinations including respiratory rate, body temperature, and thyroid gland were unremarkable. Twelve-lead electrocardiography (ECG) revealed sinus tachycardia without evidence of ST–T segment change. Plain chest radiography showed a normal cardiothoracic ratio and pulmonary vasculature. High-sensitivity cardiac troponin I was 2007.5 ng/L (normal < 15.6 ng/L). Thyroid function tests showed elevated serum free triiodothyronine of 7.37 pg/mL (normal 1.60–4.00 pg/mL), normal serum free thyroxine of 1.08 ng/dL (normal 0.70–1.48 ng/dL) with suppressed serum thyroid stimulating hormone (TSH) of 0.113 uIU/mL (normal 0.350–4.940 uIU/mL). Thyroid peroxidase, thyroglobulin, and TSH receptor antibodies were negative (Table 1). High-sensitivity C-reactive protein (4.09 mg/L) and erythrocyte sedimentation rate (11 mm/h) were normal. Nasopharyngeal swab polymerase chain reaction tests for respiratory viruses including COVID-19 were negative. Thyroid ultrasound revealed normal thyroid gland size, homogeneous parenchyma without increased Doppler flow. Iodine-131 uptake study showed very low thyroid uptake (3.95% and 4.39% at 3 and 4 hours, respectively), consistent with thyroiditis. Transthoracic echocardiography (TTE) showed normal biventricular size and function. Cardiac magnetic resonance imaging (CMR) demonstrated basal inferoseptal segment hypokinesia on steady-state free precession (SSFP) cine images (see Additional file 1) with evidence of myocardial edema on T2 mapping (64 ms, normal myocardium 41 ms), myocardial hyperemia on early gadolinium enhancement images, as well as myocardial necrosis on delayed gadolinium enhancement images, native T1 mapping (1511 ms, normal myocardium 1229 ms), and postcontrast T1 mapping (320 ms, normal myocardium 536 ms) (Figs. 1, 2). CMR findings were suggestive of recent myocarditis according to the Lake Louise criteria. Endomyocardial biopsy was omitted because of mild symptoms. After 3 weeks of symptomatic treatment with low-dose beta-blocker (propranolol 30 mg/day) and exercise restriction, her symptom and blood tests had returned to normal (Table 1).
Cardiac magnetic resonance imaging with conventional techniques showed multiple signs of acute focal myocarditis at the basal inferoseptal segment of the left ventricle (arrows). A Steady-state free precession (SSFP) cine image in basal short-axis (SAX) view demonstrating regional wall motion abnormality. B Early gadolinium enhancement image in basal SAX view revealing hyperenhancement, a sign of myocardial hyperemia. C, D Delayed gadolinium enhancement images in basal SAX and modified four-chamber views depicting hyperenhancement, a sign of myocardial necrosis and fibrosis
Discussion
Myocarditis and pericarditis are well-known potential adverse reactions after mRNA-1273 and BNT162b2 vaccine administration [5]. They are not widely recognized as possible adverse reactions of AstraZeneca COVID-19 vaccine, though the incidence of suspected myocarditis–pericarditis following mRNA and AstraZeneca COVID-19 vaccine from the vaccine adverse event reporting system was similar (1.6–5.0 versus 2.0–3.7 per million doses, respectively) [6]. To date, only a few cases of myocarditis following exposure to the AstraZeneca COVID-19 vaccine have been published [7, 8]. Our patient age was not common for mRNA-induced myocarditis, but the typical age of AstraZeneca COVID-19 vaccine-related myocarditis has not previously been concluded. Vaccine-related myocarditis usually presented with mild symptoms, which resolved spontaneously with conservative treatment [7, 9]. A severe form presented with cardiogenic shock and need for hemodynamic support had been scarcely reported [8, 10]. Chest pain is the most common presentation; unlike other reports, our case presented with palpitation, which could be a manifestation of myocarditis, thyroiditis, or both [9]. CMR is recommended, in addition to ECG, cardiac markers, and TTE, for myocarditis with minimal symptoms due to noninvasiveness and trustworthy tissue characterization ability [11]. This case highlights the significance of CMR for diagnosis of myocarditis, particularly when the presentation is mild and TTE findings are negative.
Post-vaccination thyroiditis, a well-knwon autoimmune/inflammatory syndrome induced by adjuvants (ASIA), has been reported after various types of vaccine, including all COVID-19 vaccine platforms. This condition predominantly affects women in an age range from 26 to 75 years [12,13,14]. Symptoms, typically mild and self-resolving without specific treatment, can occur 4–21 days after vaccination. Most patients have neck pain at the onset, while only one case of painless thyroiditis, as in our patient, has been reported [13]. At the onset of presentation, patients can have hyperthyroid (most common), hypothyroid, or euthyroid status [13]. Differential diagnoses of post-vaccination thyrotoxicosis included Graves’ disease, and co-occurrence of subacute thyroiditis and Graves’ disease was also reported [15]. Thyroid antibodies and iodine-131 uptake should be investigated to clarify the etiology of thyrotoxicosis.
AstraZeneca COVID-19 vaccine contains recombinant replication-deficient chimpanzee adenovirus vector encoding the SARS CoV-2 spike protein. Possible mechanisms for post-vaccination myocarditis and thyroiditis are molecular mimicry between SARS CoV-2 spike protein and self-antigens (myocyte protein and thyroid peroxidase) as well as triggering of preexisting dysregulated immune pathways [9, 16, 17]. However, the entire mechanism is unclear.
To our knowledge, this is the first case report of concomitant myocarditis and painless thyroiditis following AstraZeneca COVID-19 vaccine administration. The true association cannot be established, although we demonstrate the temporal relationships between vaccine and these conditions.
Conclusion
This case demonstrates that the adenoviral vector COVID-19 vaccine could possibly cause myocarditis and painless thyroiditis. Clinicians should have a high index of suspicion and promptly evaluate these conditions. Given a low incidence and minimal symptoms in most cases, the COVID-19 vaccine is recommended in the pandemic situation because the benefit from the vaccine greatly outweighs the risks.
Availability of data and materials
The data for this case report are located at King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Abbreviations
- CMR:
-
Cardiac magnetic resonance imaging
- COVID-19:
-
Coronavirus disease 2019
- ECG:
-
Electrocardiography
- GRE:
-
Spoiled gradient echo
- SAX:
-
Short-axis
- SSFP:
-
Steady-state free precession
- TTE:
-
Transthoracic echocardiography
- TSH:
-
Thyroid stimulating hormone
References
Vaccine for COVID-19. Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html. Accessed 4 Dec 2021.
Vaxzevria (previously COVID-19 Vaccine AstraZeneca). https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca#productinformation-section. Accessed 6 December 2021.
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93. https://doi.org/10.1016/S0140-6736(20)32466-1.
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101. https://doi.org/10.1056/NEJMoa2104840.
Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39(29):3790–3. https://doi.org/10.1016/j.vaccine.2021.05.087.
Pepe S, Gregory AT, Denniss AR. Myocarditis, pericarditis and cardiomyopathy after COVID-19 vaccination. Heart Lung Circ. 2021;30(10):1425–9. https://doi.org/10.1016/j.hlc.2021.07.011.
Hung YP, Sun KS. A case of myopericarditis with pleuritis following AstraZeneca COVID-19 vaccination. QJM. 2021. https://doi.org/10.1093/qjmed/hcab278.
Rodríguez RM, Herraiz ATI, González MJG. Cardiogenic shock due to acute myocarditis following AZD1222 vaccine administration: a case report. IJCR. 2021;5:235. https://doi.org/10.28933/ijcr-2021-07-2605.
Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–84. https://doi.org/10.1161/CIRCULATIONAHA.121.056135.
Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med. 2021;385(14):1332–4. https://doi.org/10.1056/NEJMc2109975.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2013. https://doi.org/10.1093/eurheartj/eht210.
Oyibo SO. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19). Cureus. 2021;13(6): e16045. https://doi.org/10.7759/cureus.16045.
Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metab Open. 2021;12: 100136. https://doi.org/10.1016/j.metop.2021.100136.
Ratnayake GM, Dworakowska D, Grossman AB. Can COVID-19 immunisation cause subacute thyroiditis? Clin Endocrinol (Oxf). 2021. https://doi.org/10.1111/cen.14555.
Lee KA, Kim YJ, Jin HY. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine. 2021;74(3):470–2. https://doi.org/10.1007/s12020-021-02898-5.
Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323–6. https://doi.org/10.1161/CIRCRESAHA.121.318902.
Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11: 617089. https://doi.org/10.3389/fimmu.2020.617089.
Acknowledgements
Not applicable.
Funding
There was no financial support for this work.
Author information
Authors and Affiliations
Contributions
AM participated in the conception of the work, data acquisition, and drafting of the manuscript. NT participated in the conception of the work, figure preparation, editing, and revising the manuscript. MT contributed to image acquisition and interpretation. PC participated in image interpretation and figure preparation. PS helped in editing and revising the manuscript. SP contributed to the conception of the work, editing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee approval was waived. The patient signed an informed consent form.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. SSFP cine image in basal short-axis (SAX) view demonstrating regional wall motion abnormality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marsukjai, A., Theerasuwipakorn, N., Tumkosit, M. et al. Concomitant myocarditis and painless thyroiditis after AstraZeneca coronavirus disease 2019 vaccination: a case report. J Med Case Reports 16, 212 (2022). https://doi.org/10.1186/s13256-022-03438-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03438-z