Abstract
Background
Brachiopods and molluscs are lophotrochozoans with hard external shells which are often believed to have evolved convergently. While palaeontological data indicate that both groups are descended from biomineralising Cambrian ancestors, the closest relatives of brachiopods, phoronids and bryozoans, are mineralised to a much lower extent and are comparatively poorly represented in the Palaeozoic fossil record. Although brachiopod and mollusc shells are structurally analogous, genomic and proteomic evidence indicates that their formation involves a complement of conserved, orthologous genes. Here, we study a set of genes comprised of 3 homeodomain transcription factors, one signalling molecule and 6 structural proteins which are implicated in mollusc and brachiopod shell formation, search for their orthologs in transcriptomes or genomes of brachiopods, phoronids and bryozoans, and present expression patterns of 8 of the genes in postmetamorphic juveniles of the rhynchonelliform brachiopod T. transversa.
Results
Transcriptome and genome searches for the 10 target genes in the brachiopods Terebratalia transversa, Lingula anatina, Novocrania anomala, the bryozoans Bugula neritina and Membranipora membranacea, and the phoronids Phoronis australis and Phoronopsis harmeri resulted in the recovery of orthologs of the majority of the genes in all taxa. While the full complement of genes was present in all brachiopods with a single exception in L. anatina, a bloc of four genes could consistently not be retrieved from bryozoans and phoronids. The genes engrailed, distal-less, ferritin, perlucin, sp1 and sp2 were shown to be expressed in the biomineralising mantle margin of T. transversa juveniles.
Conclusions
The gene expression patterns we recovered indicate that while mineralised shells in brachiopods and molluscs are structurally analogous, their formation builds on a homologous process that involves a conserved complement of orthologous genes. Losses of some of the genes related to biomineralisation in bryozoans and phoronids indicate that loss of the capacity to form mineralised structures occurred already in the phoronid–bryozoan stem group and supports the idea that mineralised skeletons evolved secondarily in some of the bryozoan subclades.
Similar content being viewed by others
Background
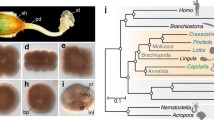
Biomineralisation was an evolutionary innovation of major importance to early animals [1, 2], its significance evident both from the vast amounts of skeletal taxa preserved as fossils and the ubiquity of hard tissues in present ecosystems. Beginning with a surge of small shelly fossils in terminal Ediacaran strata [3, 4] the evolution of skeletons accelerated during the Cambrian radiations [5,6,7,8], and today a wide array of mineralised structures of differing chemical compositions are distributed across animal phylogeny. Many fascinating configurations of such hard parts can be found in the Lophotrochozoa, a subclade of Spiralia which includes brachiopods, annelids, molluscs, and several other invertebrate groups [9,10,11,12,13,14]. By the Early Cambrian; most lophotrochozoan crown groups had diverged and some had evolved biomineralised parts [4, 7, 8, 15,16,17], leaving behind a trail of skeletal fossils [3, 7, 8] from an evolutionary arms race which culminated in the emergence of extensively mineralised taxa, such as brachiopods and molluscs [17,18,19,20,21]. While brachiopod shells are superficially similar to the shells of bivalve molluscs, molecular data [12, 22,23,24] do not support the idea that brachiopods and molluscs have a close relationship within Lophotrochozoa. Rather, the favoured view is that the morphological similarities between brachiopod and mollusc shells are an example of homoplasy, the structures having arisen independently in an instance of convergent evolution [8]. In fact, even the shells and sclerites of different mollusc classes are considered likely to be independently derived structures [8, 25,26,27] due to differences in form, formation, and composition. Within Lophotrochozoa, the closest relatives of brachiopods are instead the worm-like phoronids and the sessile bryozoans [28], and these three groups together form the clade Lophophorata [12, 29,30,31]. Mineralised tissues are not evenly distributed among the lophophorates; no extant phoronids and only 2 out of 5 major clades of bryozoans are skeletal [32, 33], while even the earliest known brachiopods had sclerites or shells [15, 18,19,20,21]. Loss of biomineralised tissues seems to be a recurring evolutionary theme among lophotrochozoans (Fig. 1A): the putative phoronid stem group was mineralised [8, 15, 34, 35], although extant phoronids are not, and similar losses seem to have occurred in annelids [36] and entoprocts [37]. Three clades of brachiopods survive to the present; the Rhynchonelliformea, Craniiformea and Linguliformea, with the latter two being most closely related [38, 39]. In the extant rhynchonelliform brachiopod Terebratalia transversa Sowerby 1846, shell formation begins shortly after its three-lobed lecithotrophic larva has settled on appropriate substrate and given rise to a juvenile which resembles a miniature version of the adult animal [40]. After metamorphosis, a thin, unmineralised and non-articulating bivalved shell known as the protegulum envelops the juvenile [40]. Mineralisation of the protegulum begins shortly after metamorphosis is completed and takes place periodically [40] along the anterior and lateral sides of the structure. At the edge of the mantle epithelium (Fig. 1B) lies the periostracal slot, where the shell is secreted. Lobate cells located beneath the periostracal slot produce the organic layer of the periostracum, while vesicular cells above them secrete calcite crystals in what is known as the ‘primary mineralisation’ [41, 42]. Combined with shell material produced by the outer mantle epithelium in a ‘secondary mineralisation’, this eventually results in a calcareous shell marked by concentric growth lines [41, 42], which articulates at a teeth-and-socket hinge structure [41].
A Cladogram depicting lophotrochozoan interrelationships [23, 43, 44] (in green) with Rouphozoa [45] as outgroup (in black) with an overview of mineralisation capacities. Taxa marked with an obelisk are Cambrian stem groups of their respective sister clades. B Detailed morphology of the mantle margin which excretes the shell of the brachial valve. Lobate cells (pink) secrete the organic portion of the periostracum, while vesicular cells (green) produce the primary layer of the inorganic shell and the outer epithelial cells of the mantle (purple) secrete the secondary layer of the inorganic shell. EC outer mantle epithelial cell, LC lobate cell, PO periostracum, PS periostracal slot, SF fibre of the secondary shell layer, VC vesicular cell. Drawing after Stricker and Reed 1985 [40]
By merit of being the best-studied extensively mineralised lophotrochozoan taxon, molluscs provide a useful reference point for the investigation of hard tissue formation in brachiopods. While the last common ancestor of the two groups was likely weakly mineralised at most [8], mollusc shell formation does have similarities to that of brachiopods [40, 42, 47, 48], and mounting evidence suggests that the two groups may share some of the underlying genetic architecture required for biomineralisation [48,49,50,51,52,53]. A conserved ‘biomineralisation toolkit’ has been proposed by several authors [1, 8, 27, 54,55,56,57,58,59], possibly encompassing not only molluscs, brachiopods and other lophotrochozoans but all bilaterians. This idea implies that while structurally analogous, the formation of shells in brachiopods and molluscs builds on a complement of orthologous genes, and has support from genomics- [49, 51, 57] and proteomics-based studies [53]. Recent single-cell transcriptomic data from the bivalve Dreissena have supported the notion of a biomineralisation toolkit but also showed enrichment of numerous lineage specific transcripts in the shell field, indicating rapid evolution of shell matrix proteins in molluscs [56]. Here, we have examined which of a handful of select mollusc genes related to biomineralisation are present in the transcriptomes or genomes of various lophophorate taxa and studied the expression patterns of the same genes in postmetamorphic juveniles of T. transversa. Our primary aims were to answer whether phoronids and non-mineralised bryozoans could have lost parts of their biomineralisation-related gene complement, and whether the genes would be expressed in the T. transversa mantle margin, where biomineralisation occurs [40,41,42]. We targeted 10 genes with a known function in the biomineralisation of molluscs and/or brachiopods, including the three homeodomain transcription factors engrailed (en) [48, 56, 59,60,61,62,63,64,65], distal-less (dlx) [66, 67], goosecoid (gsc) [68], the decapentaplegic ortholog bmp2-4 [59, 64, 66, 69, 70], the matrix protein genes ferritin [66, 71,72,73], calmodulin [50] and perlucin [50, 74, 75], a mantle peroxidase [48, 76, 77], and two supposedly brachiopod-specific shell-associated genes [53] originally designated as F10023803 and R20087389 but herein dubbed shell protein 1 and 2 (abbreviated as sp1 and sp2).
Results
The lophophorate biomineralisation gene complement and gene orthology
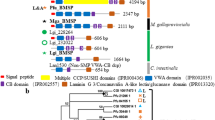
Our search for biomineralization-related genes in the transcriptomes and genomes of lophophorates resulted in the identification of several putative orthologs, the homology of which were tested using phylogenetic analyses (Additional file 1: Figs. S1–S6). Brachiopods have retained orthologs of nearly all the targeted biomineralisation genes (Fig. 2), with the exception for an apparent loss of the specific calmodulin orthologue in L. anatina. Most of the genes are also identifiable in transcriptome or genome sequences from phoronids and bryozoans, with the notable exception of perlucin and mpox, in addition to the putatively brachiopod-specific genes sp1 and sp2. Although sequences with similarity to en were present in the bryozoan transcriptomes, they did not form a clade with en sequences from other taxa (Additional file 1: Fig. S1). The seeming absence of some genes here signifies lack of an ortholog to the reference sequence, and does not indicate definite absence of other, closely related genes (for instance, several calmodulin-related genes are present in the L. anatina genome [49], but the specific calmodulin ortholog investigated here is not). In addition, since we did not have access to genomes for all taxa and, therefore, only searched the transcriptomes of B. neritina, M. membranacea, N. anomala and P. harmeri, it is possible that some genes are present in the genome but were not expressed at the time of transcriptome generation.
Expression patterns of biomineralisation genes in T. transversa
In-situ hybridisation of the genes yielded a range of expression patterns in various morphological features (Fig. 3A) of 2- and 6-day-old juveniles of T. transversa, with most being expressed in varying degrees in the mantle margin, around the pedicle opening or in the area of the future hinges of the shell. Of the three homeodomain transcription factors, en was expressed exclusively in the mantle margin (black arrowhead, Fig. 3B). dlx was expressed in the mantle margin (black arrowheads, Fig. 3D, E) and lophophore (red arrowheads, Fig. 3D, E), while gsc was exclusively expressed in the lophophore (red arrowheads, Fig. 3F, G). The primary expression domains of bmp2-4 were in the developing hinges of the protegulum (white arrowheads, Fig. 3H, I) and the lophophore (red arrowheads, Fig. 3H, I). In addition, in juveniles at 6 dpm there was expression in the area of the future pedicle opening (blue arrowhead, Fig. 3I). ferritin was universally and abundantly expressed throughout the juvenile tissues to the point where overstaining became apparent minutes after starting the colour-producing reaction but seemed particularly prominent in the mantle margin (black arrowhead, Fig. 3C) and in an area posterior to the lophophore. perlucin, sp1 and sp2 were primarily expressed in the mantle margin (black arrowheads, Fig. 3J–O), with an additional sp1 expression domain located in the outer epithelial cells of the mantle in juveniles at 2 dpm (yellow arrowhead and insert, Fig. 3L).
A Morphological features of a T. transversa juvenile at 2 dpm, interpreted after Stricker & Reed [40] and Gąsiorowski & Hejnol [46]. B–O Expression patterns of targeted genes. Gene names are indicated in the white bars above the images. For each gene, the left picture depicts a juvenile at 2 dpm, and the right picture a juvenile at 6 dpm. Data could not be retrieved for en and ferritin at 2 dpm. The insert in panel L provides a magnified view of expression within the outer mantle epithelium cells. Black arrowheads indicate expression in the mantle margin, yellow arrowheads in the mantle epithelium, red arrowheads expression in the lophophore, white arrowheads expression in the hinges and blue arrowheads expression in the pedicle opening. Scale bars represent 50 μm (insert in panel L 20 μm). CS chaetal sac, LO lophophore rudiment, MC mantle cavity, MM mantle margin, PE pedicle, PR protegulum, HI future hinge
Discussion
A shared brachiopod-mollusc complement of genes involved in biomineralisation
The gene expression patterns we observed (Fig. 4) indicate that the two main groups of lophotrochozoan biomineralisers, brachiopods and molluscs, base their shell formation on a shared and conserved genetic foundation.
Of the studied genes, en is arguably the most implicated in shell development in both groups [48, 66] and as demonstrated here en was clearly and exclusively expressed in the T. transversa mantle margin, which indicates its involvement in shell formation due to the vicinity of CaCO3-excreting vesicular cells. dlx was also predominately expressed in the mantle margin, with an additional expression domain in the lophophore rudiment. Although dlx could not be confirmed to have biomineralising functions in L. anatina [48], the expression pattern herein corresponds well with the reported involvement of this gene in gastropod shell formation [66]. In accordance with our expression patterns in T. transversa, dlx is involved in lophophore formation in L. anatina [48], and the gene has been reported to be part of an “head appendage” genetic program [78] which agrees well with the idea that lophophores are homologous to the head of other bilaterians. Meanwhile, gsc was exclusively expressed in the developing lophophore and does not seem involved in T. transversa biomineralisation. Aside from its role in gastropod shell field formation, the gene is generally known to be one of the head patterning’ homeodomain transcription factors [79, 80] with a specific role of patterning sensory cells around the mouth, agreeing well with the aforementioned link between lophophores and bilaterian heads [38, 46, 49, 81]. bmp2-4 was primarily expressed in the region of the lophophore, the developing hinge structure and the pedicle opening, the two latter of which are not close to the mantle margin but are areas which undergo structural modifications involving secondary biomineralisation of the pre-existing larval protegulum [40, 42]. The bmp2-4 ortholog in Saccostrea kegaki is likewise expressed in the hinges of the shell and seemingly important for hinge formation [64], and this similar expression pattern could represent a convergently evolved co-option of the gene for hinge formation. Lophophore expression of bmp2-4 in the lophophore agrees with another proposed function of this gene family; the formation of the head, appendages and other outgrowths of the body [78, 82]. The two shell matrix protein genes ferritin and perlucin were united by a clear expression in the mantle margin, but while this was the exclusive expression domain for perlucin, ferritin was broadly expressed also in other tissues. The highly specific mantle margin expression of perlucin adds weight to the argument for a shared brachiopod–mollusc biomineralisation gene complement, while the broad expression of ferritin could be explained by the wide range of physiological functions ferritins are known to carry [83]. Orthologues of sp1 and sp2, the genes which were previously described as shell forming proteins from the rhynchonelliformean brachiopod Magellania [53] are also present in the transcriptomes or genomes of Terebratalia, Novocrania and Lingula but are absent in the transcriptomes or genomes of phoronids and bryozoans, indicating that the genes are brachiopod-specific and evolved in the common ancestor of all brachiopods after the split from other lophophorates. Alignment of the protein sequences of the sp1 and sp2 genes (Additional file 1: Fig. S7) suggests that both proteins are homologous; however, they are divergently distributed among particular brachiopod clades. In Lingula, sp2 was present but sp1 absent, and in Novocrania we identified 2 paralogous sequences corresponding to sp2. Like in Magellania, both sp1 and sp2 could be identified in Terebratalia, suggesting that sp1 may be a rhynchonelliform innovation. Both sp1 and sp2 were distinguished by mantle margin expression in T. transversa juveniles, indicating a conserved function among brachiopods. The expression of sp1 in the outer epithelial cells of the mantle in 2- but not 6-dpm juveniles of T. transversa corresponds well with what would be expected of a gene involved in the progressive secondary mineralisation of the protegulum, which is finished shortly after metamorphosis [40]. In a broader context, our results support the notion of structurally analogous but genetically homologous shells in brachiopods and molluscs, an idea reinforced by both genomics- [49, 51, 57] and proteomics- [53] based studies. In addition to the genes investigated here, pou3, which regulates expression of shell matrix proteins in molluscs [54, 84] is also expressed in the mantle margin of T. transversa [85], further supporting idea of a common molecular control of the biomineralisation in both groups. It should be noted that the observed similarities could also reflect independent co-option of genes, and that it is seldom an easy task to separate evolutionary conservation from co-option. While our findings support similar and shared functions for a broad set of functionally diverse genes and lend support to the claim of brachiopod and mollusc biomineralisation being a homologous process in at least some respect, further studies on the interactions of these genes and whether they form similar networks in both lineages are needed to conclusively demonstrate the existence and scope of this homology. Until then, the possibility that some, all or none of the studied genes form conserved networks, while others have been independently co-opted persists. Looking beyond the genes, the fossil record indicates that both molluscs [7, 8, 17, 86] and brachiopods [15, 18,19,20,21] have had the capacity for biomineralisation for a very long time, although their stem groups bore variations of sclerites rather than fully formed shells. Likely, their common ancestor had a genetic capacity for biomineralisation which was initially used to mineralise an organic skeleton [8] before giving rise to bona fide brachiopod and mollusc shells through convergent evolution.
Implications for the evolution of lophophorate biomineralisation
It is not surprising that of the lophophorate clades, phoronids and bryozoans seemingly retain the fewest orthologs of the putative biomineralisation-related genes (Fig. 2). It is worth noting that four of the tested genes which are present in the phoronid and bryozoan genomes or transcriptomes (en, dlx, gsc and dmp2-4) are not strictly biomineralisation-specific, but play multiple functions during the development of various lophophorates as has been shown in several studies [48, 87,88,89]. When it comes to the more biomineralisation-specific genes, phoronids and bryozoans are united not only by the lack of tentatively brachiopod-specific sp1 and sp2 genes, but also of perlucin and mantle peroxidase which otherwise have orthologs in other bilaterians, including molluscs (Additional file 1: Figs. S5 and S6). In addition, phoronids and bryozoans both lack the Hox gene scr [38, 81, 90], which in brachiopods is specifically expressed in the shell forming epithelium [46, 49, 91]. The loss of scr has previously been hypothesised to be related to the reduction of the biomineralisation capacities in phoronids [38, 81]. The similar pattern of loss in bryozoans and phoronids agrees well with recent studies that indicate a sister-group relationship between the two [31, 43, 44, 92, 93]. The bryozoan stem group was unmineralised and originated in the early Cambrian, as indicated by recent fossil discoveries [94], while the crown group did not appear until at least in the Early Ordovician by which time the Stenolaemata had secondarily evolved a mineralised skeleton [95]. The notion of an unmineralised origin of bryozoans has further support [33], with the morphologically disparate skeletons of stenolaemates and cheilostomates likely being secondarily evolved as indicated by their phylogenetic distribution (Fig. 5). The sister relationship and similar pattern of loss of biomineralisation genes in phoronids and bryozoans suggests that the capacity of forming hard tissues was lost before the two groups diverged from each other. Taking this scenario into account, we suggest that the Early Cambrian tommottiid Eccentrotheca helenia, a putative stem group phoronid [34, 35] which possessed a hard skeleton of loosely associated sclerites, likely represents an evolutionary stage predating loss of biomineralisation and should be rather considered as belonging to the phoronid–bryozoan stem group (Fig. 5).
Evolution of biomineralisation in lophophorates. Black parts of the tree indicate biomineralising lineages, while light blue indicates soft-bodied taxa. The ancestral lophophorate has been assumed to be biomineralising due to the similarity between brachiopod and mollusc biomineralisation-related genes. Bryozoan topology after Schwaha et al. [33], Xia et al. [95] and Zhang et al. [94]
Conclusions
Several genes involved in mollusc biomineralisation are expressed in the mantle margin and other biomineralising cell types in postmetamorphic juveniles of the rhynchonelliform brachiopod Terebratalia transversa. We suggest that the common ancestor of brachiopods and molluscs was able to form mineralised structures, for which it utilised a molecular machinery that has been at least partially retained in both lineages. This ancestral biomineralisation ability has been independently co-opted into formation of the shell-like external skeleton in molluscs and brachiopods, which evolved convergently as indicated by comparative morphology and the fossil record. This explains why the shells of both groups, despite being structurally analogous, show homology on the genetic level. Losses of some of the biomineralization-specific genes in bryozoans and phoronids indicate loss of the capacity to form mineralised structures in the phoronid–bryozoan stem group and supports the idea that mineralised skeletons evolved secondarily in some of the bryozoan subclades.
Methods
Specimen collection and preservation
Egg-bearing specimens of the rhynchonelliform brachiopod Terebratalia transversa were collected off San Juan Island, Washington State, USA. Animal husbandry and fertilisation of eggs was performed in vitro, and juveniles were fixed in 3.7% formaldehyde and stored in methanol at – 20 ℃.
Transcriptome searches and orthology assessment
A set of reference sequences for each gene of interest (Additional file 1: Table S1) was used to search the transcriptomes and genomes for orthologous sequences via BLAST. Transcriptomes of the bryozoans M. membranacea and B. neritina, the brachiopods T. transversa and N. anomala and the phoronid P. harmeri (available in-house) and published genomes of the brachiopod L. anatina (GenBank accession number: LFEI00000000.2) and the phoronid Phoronis australis (GenBank accession number: NMRA01000001.1) were also screened for orthologs using the reference sequences. Sequences retrieved from the transcriptomes and genomes were imported into the software CLC Main Workbench v. 7, translated to protein sequences, and aligned with related sequences from other taxa retrieved from the NCBI repository of protein sequences and other databases. Alignments of protein sequences (including the sp1 and sp2 genes, Additional file 1: Fig. S7) were trimmed using the software trimAl v1.1 [96] and imported into FastTree v. 2.1.10 [97] for phylogenetic reconstructions using the ML Model option ‘Le-Gascuel 2008’ and CAT approximation with 20 rate categories. The resulting approximately maximum-likelihood phylogenies (Additional file 1: Figs. S1–S6) were used to assess orthology of the lophophorate genes of interest. New sequences obtained and identified in this study were uploaded to GenBank (accession numbers ON868422–ON868458 and BK061549–BK061561).
Gene amplification, cloning and sequencing
Specific primers against the genes of interest were designed using the software MacVector v 11.1.2. Genes were amplified from cDNA libraries using PCR, verified by electrophoresis on a 1% agarose gel, extracted from the gel using a MinElute Gel Extraction Kit according to the manufacturer's instructions, and ligated into vectors. The vectors were heat-shocked into competent Escherichia coli cells which were left to proliferate for 24 h in 37 ℃. Bacterially amplified vectors were purified using a Qiagen Spin Miniprep Kit according to the manufacturer's instructions, and inserts were sequenced using the Sanger method.
Riboprobe synthesis, whole-mount in situ hybridisation and imaging
PCR was used to amplify genomic DNA from vectors, and amplicons were purified using a MinElute PCR Purification Kit according to the manufacturer's instructions. Resulting nucleic acid concentrations were measured using a NanoDrop device, while amplicon sizes were verified through electrophoresis on a 1% agarose gel. dUTP–digoxigenin-labelled RNA probes were synthesised according to the manufacturer's instructions (Roche, USA). WISH was performed in accordance with previously published protocols [46, 89, 98]. Following specimen digestion with proteinase K for 10 min, probes were allowed to hybridise with T. transversa juveniles at a concentration of 1 ng ⋅ μL−1 at 67 ℃ for 72 h. Anti-digoxigenin–AP antibodies were used to detect the probes, and they were visualised using nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate. Successfully hybridised and visualised specimens were transferred to cover slides, mounted in 70% glycerol and photographed using a Zeiss Axiocam camera connected to a Zeiss Axioscope Ax10 using Nomarski bright field optics.
Availability of data and materials
The data set of genetic sequences supporting the conclusions of this article is available in the GenBank repository (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers ON868422–ON868458 and BK061549–BK061561). All remaining data generated or analysed during this study are included in the article itself or its supplementary materials.
References
Gilbert PUPA, Bergmann KD, Boekelheide N, Tambutté S, Mass T, Marin F, Adkins JF, Erez J, Gilbert B, Knutson V, Cantine M, Hernández JO, Knoll AH. Biomineralization: integrating mechanism and evolutionary history. Sci Adv. 2022;8:10.
Knoll AH. Biomineralization and evolutionary history. Rev Mineral Geochem. 2003;54:329–56.
Conway Morris S, Fritz W. Shelly microfossils near the Precambrian–Cambrian boundary, Mackenzie Mountains, northwestern Canada. Nature. 1980;286:381–4.
Budd GE, Jackson ISC. Ecological innovations in the Cambrian and the origins of the crown group phyla. Philos Trans R Soc B. 2016;371:20150287.
Zhuravlev AY, Wood RA. The two phases of the Cambrian Explosion. Sci Rep. 2018;8:16656.
Vermeij GJ. The origin of skeletons. Palaios. 1989;4:585–9.
Kouchinsky A, Bengtson S, Runnegar B, Skovsted C, Steiner M, Vendrasco M. Chronology of early Cambrian biomineralization. Geol Mag. 2012;149:221–51.
Murdock DJE. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biol Rev. 2020;95:1372–92.
Mallatt J, Craig CW, Yoder MJ. Nearly complete rRNA genes assembled from across the metazoan animals: effects of more taxa, a structure-based alignment, and paired-sites evolutionary models on phylogeny reconstruction. Mol Phylogenet Evol. 2010;55:1–17.
Hejnol A. A twist in time-the evolution of spiral cleavage in the light of animal phylogeny. Integr Comp Biol. 2010;50:695–706.
Hejnol A, Obst M, Stamatakis A, Ott M, Rouse GW, Edgecombe GD, Martinez P, Baguñà J, Bailly X, Jondelius U, Wiens M, Müller WEG, Seaver E, Wheeler WC, Martindale MQ, Giribet G, Dunn CW. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc R Soc B. 2009;276:4261–70.
Halanych KM, Bacheller JD, Aguinaldo AMA, Liva SM, Hillis DM, Lake JA. Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science. 1995;267:1641–3.
Kocot KM. On 20 years of Lophotrochozoa. Org Divers Evol. 2016;16:329–43.
Bleidorn C. Recent progress in reconstructing lophotrochozoan (spiralian) phylogeny. Org Divers Evol. 2019;19:557–66.
Steiner M, Yang B, Hohl S, Li D, Donoghue P. Exceptionally preserved early Cambrian bilaterian developmental stages from Mongolia. Nat Commun. 2021;12:1037.
Qian Yi, Stefan B. Palaeontology and biostratigraphy of the Early Cambrian Meishucunian stage in Yunnan Province, South China. Foss Strata. 1989;24:1–156.
Li L, Zhang X, Yun H, Li G. Complex hierarchical microstructures of Cambrian mollusk Pelagiella: insight into early biomineralization and evolution. Sci Rep. 2017;7:1935.
Holmer LE, Skovsted CB, Brock GA, Valentine JL, Paterson JR. The Early Cambrian tommotiid Micrina, a sessile bivalved stem group brachiopod. Biol Let. 2008;4:724–8.
Holmer L, Skovsted C, Williams A. A stem group brachiopod from the Lower Cambrian: support for a Micrina (Halkieriid) ancestry. Palaeontology. 2003;45:875–82.
McMenamin MAS. Two new species of the Cambrian genus Mickwitzia. J Paleontol. 1992;66:173–82.
Liang Y, Holmer LE, Skovsted CB, Duan XL, Zhang ZF. Shell structure, ornamentation and affinity of the problematic early Cambrian brachiopod Heliomedusa orienta. Lethaia. 2020;53:574–87.
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sørensen MV, Haddock SHD, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9.
Laumer CE, Bekkouche N, Kerbl A, Goetz F, Neves RC, Sørensen MV, Kristensen RM, Hejnol A, Dunn CW, Giribet G, Worsaae K. Spiralian phylogeny informs the evolution of microscopic lineages. Curr Biol. 2015;25:2000–6.
Helmkampf M, Bruchhaus I, Hausdorf B. Phylogenomic analyses of lophophorates (brachiopods, phoronids and bryozoans) confirm the Lophotrochozoa concept. Proc R Soc B. 2008;275:1927–33.
Chen C, Copley JT, Linse K, Rogers AD, Sigwart J. How the mollusc got its scales: convergent evolution of the molluscan scleritome. Biol J Lin Soc. 2015;114:949–54.
Jackson DJ, McDougall C, Woodcroft B, Moase P, Rose RA, Kube M, Reinhardt R, Rokhsar DS, Montagnani C, Joubert C, Piquemal D, Degnan BM. Parallel evolution of nacre building gene sets in molluscs. Mol Biol Evol. 2010;27:591–608.
Varney RM, Speiser DI, McDougall C, Degnan BM, Kocot KM. The iron-responsive genome of the chiton Acanthopleura granulata. Genome Biol Evol. 2021;13:evaa263.
Cohen BL, Weydmann A. Molecular evidence that phoronids are a subtaxon of brachiopods (brachiopoda: phoronata) and that genetic divergence of metazoan phyla began long before the early Cambrian. Org Divers Evol. 2005;5:253–73.
Emig C. Un nouvel embranchement: les Lophophorates. J Zool Syst Evol Res. 1997;22:91–4.
Nesnidal MP, Helmkampf M, Meyer A, Witek A, Bruchhaus I, Ebersberger I, Hankeln T, Lieb B, Struck TH, Hausdorf B. New phylogenomic data support the monophyly of Lophophorata and an ectoproct-phoronid clade and indicate that Polyzoa and Kryptrochozoa are caused by systematic bias. BMC Evol Biol. 2013;13:253.
Temereva EN. Innervation of the lophophore suggests that the phoronid Phoronis ovalis is a link between phoronids and bryozoans. Sci Rep. 2017;7:14440.
Taylor PD, Vinn O, Wilson MA. Evolution of biomineralization in lophophorates. Spec Pap Palaeontol. 2010;84:317–33.
Schwaha TF, Ostrovsky AN, Wanninger A. Key novelties in the evolution of the aquatic colonial phylum Bryozoa: evidence from soft body morphology. Biol Rev. 2020;95:696–729.
Skovsted CB, Brock GA, Paterson JR, Holmer LE, Budd GE. The scleritome of Eccentrotheca from the Lower Cambrian of South Australia: lophophorate affinities and implications for tommotiid phylogeny. Geology. 2008;36:171–4.
Skovsted CB, Brock GA, Topper TP, Paterson JR, Holmer LE. Scleritome construction, biofacies, biostratigraphy and systematics of the tommotiid Eccentrotheca helenia sp. nov. from the Early Cambrian of South Australia. Palaeontology. 2011;54:253–86.
Vinther J, van Roy P, Briggs DEG. Machaeridians are Palaeozoic armoured annelids. Nature. 2008;451:185–8.
Zhang Z, Holmer LE, Skovsted CB, Brock GA, Budd GE, Fu D, Zhang X, Shu D, Han J, Wang H, Butler A, Li G. A sclerite-bearing stem group entoproct from the early Cambrian and its implications. Sci Rep. 2013;3:1066.
Luo YJ, Kanda M, Koyanagi R, Hisata K, Akiyama T, Sakamoto H, Sakamoto T, Satoh N. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nat Ecol Evol. 2018;2:141–51.
Kocot KM, Struck TH, Merkel J, Waits DS, Todt C, Brannock PM, Weese DA, Cannon JT, Moroz LL, Lieb B, Halanych KM. Phylogenomics of lophotrochozoa with consideration of systematic error. Syst Biol. 2017;66:256–82.
Stricker SA, Reed CG. The ontogeny of shell secretion in Terebratalia transversa (brachiopoda, articulata) II. Formation of the protegulum and juvenile shell. J Morphol. 1985;183:251–71.
Williams A. The calcareous shell of the Brachiopoda and its importance to their classification. Biol Rev. 1956;31:243–87.
Stricker SA, Reed CG. The protegulum and juvenile shell of a recent articulate brachiopod: patterns of growth and chemical composition. Lethaia. 1985;18:295–303.
Laumer CE, Fernández R, Lemer S, Combosch D, Kocot KM, Riesgo A, Andrade SCS, Sterrer W, Sørensen MV, Giribet G. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc R Soc B. 2019;286:20190831.
Zverkov OA, Mikhailov KV, Isaev SV, Rusin LY, Popova OV, Logacheva MD, Penin AA, Moroz LL, Panchin YV, Lyubetsky VA, Aleoshin VV. Dicyemida and Orthonectida: two stories of body plan simplification. Front Genet. 2019;10:443.
Struck TH, Wey-Fabrizius AR, Golombek A, Hering L, Weigert A, Bleidorn C, Klebow S, Iakovenko N, Hausdorf B, Petersen M, Kück P, Herlyn H, Hankeln T. Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Mol Biol Evol. 2014;31:1833–49.
Gąsiorowski L, Hejnol A. Hox gene expression in postmetamorphic juveniles of the brachiopod Terebratalia transversa. EvoDevo. 2019;10:1.
Timmermans LPM. Studies on shell formation in molluscs. Neth J Zool. 1969;19:417–523.
Shimizu K, Luo YJ, Satoh N, Endo K. Possible co-option of engrailed during brachiopod and mollusc shell development. Biol Lett. 2017;13:20170254.
Luo YJ, Takeuchi T, Koyanagi R, Yamada L, Kanda M, Khalturina M, Fujie M, Yamasaki S, Endo K, Satoh N. The Lingula genome provides insights into brachiopod evolution and the origin of phosphate biomineralization. Nat Commun. 2015;6:8301.
Jackson DJ, McDougall C, Green K, Simpson F, Wörheide G, Degnan BM. A rapidly evolving secretome builds and patterns a sea shell. BMC Biol. 2006;4:40.
Sun J, Chen C, Miyamoto N, Li R, Sigwart JD, Xu T, Sun Y, Wong WC, Ip JCH, Zhang W, Lan Y, Bissessur D, Watsuji T, Watanabe HK, Takaki Y, Ikeo K, Fujii N, Yoshitake K, Qiu JW, Takai K, Qian PY. The scaly-foot snail genome and implications for the origins of biomineralised armour. Nat Commun. 2020;11:1657.
Chirat R, Moulton DE, Goriely A. Mechanical basis of morphogenesis and convergent evolution of spiny seashells. Proc Natl Acad Sci. 2013;110:6015–20.
Jackson DJ, Mann K, Häussermann V, Schilhabel MB, Lüter C, Griesshaber E, Schmahl W, Wörheide G. The Magellania venosa biomineralizing proteome: a window into brachiopod shell evolution. Genome Biol Evol. 2015;7:1349–62.
Zhang R, Xie L, Yan Z. Biomineralization mechanism of the pearl oyster, Pinctada fucata. 1st ed. Singapore: Springer; 2019.
Yarra T, Blaxter M, Clark MS. A bivalve biomineralization toolbox. Mol Biol Evol. 2021;38:4043–55.
Salamanca-Díaz DA, Ritschard EA, Schmidbaur H, Wanninger A. Comparative single-cell transcriptomics reveals novel genes involved in bivalve embryonic shell formation and questions ontogenetic homology of molluscan shell types. Front Cell Dev Biol. 2022;10:883755.
McDougall C, Degnan BM. The evolution of mollusc shells. Wiley Interdiscip Rev Dev Biol. 2018;7:e313.
Livingston BT, Killian CE, Wilt F, Cameron A, Landrum MJ, Ermolaeva O, Sapojnikov V, Maglott DR, Buchanan AM, Ettensohn CA. A genome-wide analysis of biomineralization-related proteins in the sea urchin Strongylocentrotus purpuratus. Dev Biol. 2006;300:335–48.
Kocot KM, Aguilera F, McDougall C, Jackson DJ, Degnan BM. Sea shell diversity and rapidly evolving secretomes: insights into the evolution of biomineralization. Front Zool. 2016;13:23.
Jacobs DK, Wray CG, Wedeen CJ, Kostriken R, Desalle R, Staton JL, Gates RD, Lindberg DR. Molluscan engrailed expression, serial organization, and shell evolution. Evol Dev. 2000;2:340–7.
Moshel SM, Levine M, Collier JR. Shell differentiation and engrailed expression in the Ilyanassa embryo. Dev Genes Evol. 1998;208:135–41.
Nederbragt AJ, van Loon AE, Dictus WJAG. Expression of Patella vulgata orthologs of engrailed and dpp-BMP2/4 in adjacent domains during molluscan shell development suggests a conserved compartment boundary mechanism. Dev Biol. 2002;246:341–55.
Wanninger A, Haszprunar G. The expression of an engrailed protein during embryonic shell formation of the tusk-shell, Antalis entalis (Mollusca, Scaphopoda). Evol Dev. 2001;3:312–21.
Kin K, Kakoi S, Wada H. A novel role for dpp in the shaping of bivalve shells revealed in a conserved molluscan developmental program. Dev Biol. 2009;329:152–66.
Vellutini BC, Hejnol A. Expression of segment polarity genes in brachiopods supports a non-segmental ancestral role of engrailed for bilaterians. Sci Rep. 2016;6:32387.
Jackson DJ, Wörheide G, Degnan BM. Dynamic expression of ancient and novel molluscan shell genes during ecological transitions. BMC Evol Biol. 2007;7:160.
Zhang H, Zhao M, Yi X, Ou Z, Li Y, Shi Y, He M. Characterization of the distal-less homologue gene, PfDlx, involved in regulating the expression of Pif in the pearl oyster, Pinctada fucata. Comp Biochem Physiol B: Biochem Mol Biol. 2017;212:51–8.
Lartilot N, le Gouar M, Adoutte A. Expression pattern of fork head and goosecoid homologues in the mollusc Patella vulgata supports the ancestry of the anterior mesendoderm across Bilateria. Dev Genes Evol. 2002;212:551–61.
Miyashita T, Hanashita T, Toriyama M, Takagi R, Akashika T, Higashikubo N. Gene cloning and biochemical characterization of the BMP-2 of Pinctada fucata. Biosci Biotechnol Biochem. 2008;72:37–47.
Lambert JD, Johnson AB, Hudson CN, Chan A. Dpp/BMP2-4 mediates signaling from the D-quadrant organizer in a spiralian embryo. Curr Biol. 2016;26:2003–10.
Hashimoto N, Kurita Y, Wada H. Developmental role of dpp in the gastropod shell plate and co-option of the dpp signaling pathway in the evolution of the operculum. Dev Biol. 2012;366:367–73.
Salinas-Clarot K, Gutiérrez AP, Núñez-Acuña G, Gallardo-Escárate C. Molecular characterization and gene expression of ferritin in red abalone (Haliotis rufescens). Fish Shellfish Immunol. 2011;30:430–3.
de Zoysa M, Lee J. Two ferritin subunits from disk abalone (Haliotis discus discus): cloning, characterization and expression analysis. Fish Shellfish Immunol. 2007;23:624–35.
Dodenhof T, Dietz F, Franken S, Grunwald I, Kelm S. Splice variants of Perlucin from Haliotis laevigata modulate the crystallisation of CaCO3. PLoS ONE. 2014;9:e97126.
Wang N, Lee YH, Lee J. Recombinant perlucin nucleates the growth of calcium carbonate crystals: molecular cloning and characterization of perlucin from disk abalone, Haliotis discus discus. Comp Biochem Physiol B: Biochem Mol Biol. 2008;149:354–61.
Engel J. A critical survey of biomineralization. 1st ed. Cham: Springer; 2017.
Hohagen J, Jackson DJ. An ancient process in a modern mollusc: early development of the shell in Lymnaea stagnalis. BMC Dev Biol. 2013;13:27.
Tarazona OA, Lopez DH, Slota LA, Cohn MJ. Evolution of limb development in cephalopod mollusks. Elife. 2019;8:e43828.
Bruon M, Sokol S, Bode HR. Cngsc, a homologue of goosecoid, participates in the patterning of the head, and is expressed in the organizer region of Hydra. Development. 1999;126:5245–54.
Yamada G, Mansouri A, Torres M, Stuart ET, Blum M, Schultz M, De Robertis EM, Gruss P. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121:2917–22.
Gąsiorowski L, Hejnol A. Hox gene expression during development of the phoronid Phoronopsis harmeri. EvoDevo. 2020;11:2.
Lyons DC, Perry KJ, Batzel G, Henry JQ. BMP signaling plays a role in anterior-neural/head development, but not organizer activity, in the gastropod Crepidula fornicata. Dev Biol. 2020;463:135–57.
Granick S. Structure and physiological functions of ferritin. Physiol Rev. 1951;31:489–511.
Gao J, Chen Y, Yang Y, Liang J, Xie J, Liu J, Li S, Zheng G, Xie L, Zhang R. The transcription factor Pf-POU3F4 regulates expression of the matrix protein genes Aspein and Prismalin-14 in pearl oyster (Pinctada fucata). FEBS J. 2016;283:1962–78.
Gąsiorowski L, Andrikou C, Janssen R, Bump P, Budd GE, Lowe CJ, Hejnol A. Molecular evidence for a single origin of ultrafiltration-based excretory organs. Curr Biol. 2021;31:3629-3638.e2.
Vinther J, Nielsen C. The Early Cambrian Halkieria is a mollusc. Zool Scr. 2005;34:81–9.
Vellutini BC, Martín-Durán JM, Hejnol A. Cleavage modification did not alter blastomere fates during bryozoan evolution. BMC Biol. 2017;15:33.
Andrikou C, Passamaneck YJ, Lowe CJ, Martindale MQ, Hejnol A. Molecular patterning during the development of Phoronopsis harmeri reveals similarities to rhynchonelliform brachiopods. EvoDevo. 2019;10:33.
Martín-Durán JM, Passamaneck YJ, Martindale MQ, Hejnol A. The developmental basis for the recurrent evolution of deuterostomy and protostomy. Nat Ecol Evol. 2017;1:5.
Passamaneck YJ, Halanych KM. Evidence from Hox genes that bryozoans are lophotrochozoans. Evol Dev. 2004;6:275–81.
Schiemann SM, Martín-Durán JM, Børve A, Vellutini BC, Passamaneck YJ, Hejnol A. Clustered brachiopod Hox genes are not expressed collinearly and are associated with lophotrochozoan novelties. Proc Natl Acad Sci. 2017;114:E1913–22.
Temereva EN. Myoanatomy of the phoronid Phoronis ovalis: functional and phylogenetic implications. Zoology. 2019;133:27–39.
Temereva EN. Myoanatomy of the lophophore in adult phoronids and the evolution of the phoronid lophophore. Biol Bull. 2019;237:270–82.
Zhang Z, Zhang Z, Ma J, Taylor PD, Strotz LC, Jacquet SM, Skovsted CB, Chen F, Han J, Brock GA. Fossil evidence unveils an early Cambrian origin for Bryozoa. Nature. 2021;599:251–5.
Xia FS, Zhang SG, Wang ZZ. The oldest bryozoans: new evidence from the late tremadocian (early ordovician) of East Yangtze Gorges in China. J Paleontol. 2007;81:1308–26.
Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–3.
Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490.
Hejnol A. In situ protocol for embryos and juveniles of Convolutriloba longifissura. Protocol Exch. 2008;201:101–12.
Acknowledgements
We are thankful to Ferenc Kagan for his input on some bioinformatic issues, and to Aina Børve for providing valuable assistance in the lab. The staff of UW Friday Harbor Laboratories and crew of the vessel “Centennial” are gratefully acknowledged for helping in collection of adult T. transversa. We would also like to thank all present and former members of the Hejnol lab who contributed to culturing and fixing T. transversa juveniles. Finally, we are thankful to the reviewers for their efforts and valuable comments, which helped improve the quality of the manuscript.
Funding
Open access funding provided by University of Bergen. Research was supported by the European Research Council Community’s Framework Program Horizon 2020 (2014–2020) ERC Grant agreement 648861 and the Norwegian Research Council FRIPRO Grant 815194 to AH.
Author information
Authors and Affiliations
Contributions
LG and JVW conceived the study, JVW conducted gene search and orthology assessments, performed in situ hybridisation, produced figures, and drafted the manuscript. LG, JVW and AH analysed data, and read, discussed, and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Studies of brachiopods do not require ethics approval or consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figure S1. Phylogenetic relationships of the homeodomain transcription factor proteins engrailed, goosecoid and distal-less in various metazoan taxa. Blue font denotes lophophorates. SH-like support values are indicated for select nodes. Figure S2. Phylogenetic relationships of the signalling molecule BMP2–4 to closely related proteins in various metazoan taxa. Blue font denotes lophophorates. SH-like support values are indicated for select nodes. Figure S3. Phylogenetic relationships of ferritins from various metazoan taxa to fungal orthologues. Blue font denotes lophophorates. SH-like support values are indicated for select nodes. Figure S4. Phylogenetic relationships of calmodulin to other calcium-binding proteins in various metazoan taxa. Blue font denotes lophophorates. SH-like support values are indicated for select nodes. Figure S5. Phylogenetic relationships of perlucin orthologues to other lectin-related proteins in various metazoan taxa. Blue font denotes lophophorates. SH-like support values are indicated for select nodes. Figure S6. Phylogenetic relationships of mpox (mantle peroxidases) to other peroxidase-related proteins in various metazoan taxa. Blue font denotes lophophorates. SH-like support values are indicated for select nodes. Figure S7. Alignment of the SP1 and SP2 protein sequences from four brachiopod species. Table S1. Reference sequences used to search genomes/transcriptomes for each gene of interest
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wernström, J.V., Gąsiorowski, L. & Hejnol, A. Brachiopod and mollusc biomineralisation is a conserved process that was lost in the phoronid–bryozoan stem lineage. EvoDevo 13, 17 (2022). https://doi.org/10.1186/s13227-022-00202-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13227-022-00202-8