Abstract
Purpose
Continuous monoculture leads to deterioration of soil microenvironment, which can severely threat the quality and efficiency of Angelica sinensis (A. sinensis), especially for seedlings production. However, little attention has been paid to investigate how continuous monocropping affects the growth of A. sinensis seedling, rhizosphere microbial populations, and nutrient status.
Methods
A field experiment consisting of two different planting patterns — raising A. sinensis seedlings in reclamation alpine uncultivated meadow (RW) and 1-year continuous monoculture (CC), was carried out at Min County, Gansu province, China.
Result
The results showed that compared with RW, the growth rate and valid quantity of A. sinensis seedlings were significantly reduced by 195.4% and 36.7% in CC, respectively. Continuous monocropping significantly increased the rhizosphere soil pH value during the growing season, ranging from 6.18 to 7.10, while reducing the content of SOM, total N, and available P and K. Glomalin, AMF spore densities, and the number of actinomycetes, ammonifiers, and azotobacter were also decreased by CC. The CC treatment significantly increased the abundance of fungi. The diversity and richness of bacteria in CC were lower than RW. Furthermore, the composition and structure of bacterial and fungal flora also changed and that the abundance of beneficial bacteria decreased, while the abundance of pathogens increased in CC. Thus, CC appeared to completely upend the relationship between soil nutrient availability and microbial activity.
Conclusion
The results illustrated that continuous monoculture led the flora of bacteria and fungi to changed dramatically, with the abundance of beneficial bacteria decreased and the abundance of harmful microbes, such as Lasiosphaeriaceae, Vishniacozyma, Myrmecridium, and Hypocreales, increased. The function of microbial population has changed from “beneficial bacteria dominated” to “harmful microbes dominant.” We concluded that continuous monoculture significantly reduced the growth and the efficiency of A. sinensis seedlings and deteriorated the rhizosphere soil microenvironment by increasing pH and decreasing nutrient availability, as well as altering the function of interactions between soil nutrients and microbial populations, thereby resulting in an unsuitable microenvironment for A. sinensis seedlings growth.
Similar content being viewed by others
Introduction
Angelica sinensis (Oliv.) Diels (A. sinensis) is an important medicinal plant, which is cultivated in Asia, Africa, and some areas of South America (Lu et al. 2020). This plant is also a typical geo-authentic medicinal herb, and its growth and active components are influenced by soil pH, rainfall, temperature, altitude, and other ecological factors (Xu et al. 2020; Katoh et al. 2011). In China, A. sinensis has been cultivated for about 2000 years, mainly grown in Gansu, Yunnan, Qinghai, Sichuan, and Shanxi (Zhu et al. 2021). Especially, the Minxian County, in Gansu province, is recognized as the region with most suitable climatic and soil conditions for the growth of A. sinensis (Zhang et al. 2021). The cultivation of A. sinensis in Minxian County was mainly divided into two processes: one to raise seedlings from June to October and next to harvest and store A. sinensis seedlings in sandy soil from November to March. The second process of planting is transplanting seedling to the field from April to October (Wang 2008). The first process is the key factor in determining the yield and quality of A. sinensis (Wang et al. 2018). Therefore, local farmers have gained more from the experience of cultivating A. sinensis seedlings and found that reclaiming alpine uncultivated meadow to raise A. sinensis seedlings is the current way to ensure high quality (Wu et al. 2009; Cai et al. 2013). Although this method of raising seedlings allows avoiding the problem of continuous monoculture, it could cause serious damage to turf ecology, ecological resources, and environment (Gong et al. 2015; Bai et al. 2019; Shang et al. 2021).
Deterioration of soil microenvironment is generally considered to be the main cause of the continuous cropping obstacles, including disorder of physiochemical properties, imbalance of soil microorganisms, and accumulation of autotoxic chemicals. The imbalance of soil nutrient utilization and the rhizosphere microbial population structure caused by continuous cropping may be the main reasons for the weakening of crop growth, yield, and quality. Imbalanced ammonia-oxidizing bacterial populations may lead to reduced growth of A. sinensis (Zhang et al. 2016). The rhizosphere fungal and bacterial diversity and core bacterial community were significantly affected by continuous cultivation of A. sinensis seedlings (An et al. 2020). Previously, we found that inoculation of beneficial microbial agents could improve the growth and quality of A. sinensis seedlings in continuous cropping (Zhu et al. 2017). It is well-known as mycorrhizal effect of arbuscular mycorrhizal fungi (AMF) for plant growth (Cabral et al. 2016; He et al. 2017), which have great potential in alleviating continuous cropping obstacles in the cultivation of geo-authentic medicinal herbs (He et al. 2012; Jiang and Wang 2012). However, the obstacles of continuous cropping in medicinal herbs mainly focused on the possible role of microflora only including bacteria and fungi in A. sinensis continuous cropping (An et al. 2020); there are few studies on the changes of soil microenvironment, such as bacteria, fungi and AMF, and soil physiochemical properties, and its comprehensive effect on the growth of medicinal herbs under field continuous cropping conditions. Therefore, it is urgent to elucidate the mechanism by which the rhizosphere microenvironment affects the growth of authentic A. sinensis seedlings under continuous monoculture conditions and to provide growers with effective guidelines for cultivating A. sinensis seedlings.
Herein, we hypothesized that continuous monoculture had a direct effect on the soil microenvironment, which then resulted in the inhibition of A. sinensis seedlings. A field experiment was therefore conducted to test this hypothesis, by comparing the strategy of raising A. sinensis seedlings in reclamation alpine uncultivated meadow (RW) with 1-year continuous monoculture (CC). Our main goals were (1) to investigate the effects of continuous cropping on A. sinensis seedlings growth and quality grades, (2) to analyze the changes of rhizosphere soil physical-chemical properties drove by continuous monoculture, and (3) to reveal the possible mechanism of rhizosphere soil microbial population succession dragged by continuous monoculture.
Materials and methods
Study site and sample collection
Experiments were performed at a tree-lined hillside shady slope (28°) in Wagougou, Min County, Gansu province, China (N 34° 15′ 50″, E104° 5′ 55″, elevation 2570 m). It represented a typical cool and semi-humid climate with an average annual temperature of 5.2–5.8 °C and annual average precipitation of 508.6–782.5 mm. The frost-free period of the whole year was about 120–125 days from June to October, and the soil type was chernozem.
Treatments for RW and CC were randomly distributed in ten 1 m × 5 m plots, and each treatment was replicated five times. The time of seedling cultivation was from June to October each year. The widely used cultivar A. sinensis (c.v. Hetuo) was chosen as the test material. Each plot was planted with 6 g/m2 seeds to obtain a sowing density of about 200 plants per m2. At harvest, A. sinensis seedlings of 1 m × 1 m were randomly sampled from each plot, the total number of seedlings, seedling weights were determined, and the seedling grades were further classified according to the criteria described in detail in Zhu et al. (2017). For each treatment, the rhizosphere soil and root system were collected from 5 plots and mixed thoroughly; 3 biological replicates were randomly collected at each treatment. There were 3 soil samples and 3 root system had been obtained for each treatment, and then, all of soil samples were divided into two subsamples for storage in icebox for transporting to the laboratory. One part was stored at 4 °C to determine the culturable microbial populations and physical and chemical parameters, and the other was stored at −80 °C for further analysis of high-throughput sequencing.
Culturable microbial populations of rhizosphere soil
The culturable microbial populations of bacteria, fungi, actinomycetes, ammonifiers, and azotobacter were determined by dilution plate coated method. Firstly, 1 g of fresh sample was diluted with 9 mL distilled water and mixed evenly, and then, 1 mL mixture was taken into 9 mL distilled water for gradient dilution. After dilutions were obtained, 0.1 mL appropriate concentration of dilution was taken into plate coated with the medium of Luria Bertani, Martin, modified Gao1, peptone agar, and modified Waxman 77, respectively. Then, each colony count was determined as log number per gram (fresh) soil (Zhu et al. 2018).
The AMF spore densities were extracted from rhizosphere soil by wet sieving decantation-sucrose centrifugation method. The number of spores was recorded under a Zeiss-50× stereomicroscope (Bi and Wu 2007) and expressed as the spore number per 10-g dry soil.
DNA extraction and high-throughput sequencing of rhizosphere soil
Genomic DNA was extracted from approximately 0.25 g of soil samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific Inc., USA) and kept at −80 °C prior to further use. The hypervariable region (V3-V4) of the bacterial 16S rRNA gene was amplified with primer pairs 338F and 806R (Table 1) (Liu et al. 2016), and the fungal internal transcribed spacer (ITS) region was amplified with the primer pairs ITS1F and ITS2R (Table 1). PCR was performed on an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA). The PCR reaction mixture includes 4 μL of 5 × Fast Pfu buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL each primer (5 μM), 0.4 μL Fast Pfu polymerase, 10 ng of template DNA, and ddH2O to a final volume of 20 μL. PCR amplification cycling conditions (Wang et al. 2022a) were as follows: initial denaturation at 95 °C for 3 min, followed by 27 and 35 (27 for primer 338F_806R and 35 for primer ITS1F_ITS2R) cycles of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s, and a final extension at 72 °C for 10 min, and end at 4 °C. All samples were amplified in triplicate. The PCR products were extracted from 2% agarose gels, and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to manufacturer’s instructions and quantified using Quantus™ Fluorometer (Promega, USA). The purified amplification products were quantitatively mixed, and paired-end sequencing was performed with a MiSeq PE300 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Physical and chemical indices of rhizosphere soil
Soil organic matter (SOM) was determined by the H2SO4-K2Cr2O7 oxidation method (Wu and Chen 2016). Total nitrogen (total N) of rhizosphere soil was determined by Kjeldahl method (Zhang et al. 2015). Available phosphorus (available P) was determined by ultraviolet spectrophotometry. A total of 2.5 g of dry soil sample was leached with 50 mL 0.5 mol L−1 sodium bicarbonate solution shaking for 30 min at 150 rpm and filtered with phosphorus-free filter paper. Then, 10 mL of filtrate was taken, and 5 mL of color developing solution (ascorbic acid solution) was added to the filtrate for 30-min color reaction. After the filtrate was colored, 10 mL of distilled water was added, and the OD value of reaction solution was estimated at 880 nm by an eight-cell-UV spectrophotometer (TU-1950, Beijing spectral analysis). Available potassium (available K) was determined by flame photometry method (Dai and Li 1997).
The contents of easily extractable glomalin (EEG) and total glomalin (TG) were measured by ultraviolet spectrophotometry (Janos et al. 2008). EEG was extracted from 1 g of ground dry-sieved soil with 8 mL of 20 mmol L−1 citrate solution (pH 7.0) at 121 ° for 30 min. TG was obtained from 1 g of ground dry-sieved soil with 8 mL of 50 mmol L−1citrate solution (pH 8.0) at 121 °C for 60 min, and the extraction was repeated twice. The extraction solution was centrifuged at 10,000 rpm for 15 min, and the supernatant was collected and pooled after each glomalin extraction cycle. Then, 0.5 mL supernatant was added to 5 mL of Coomassie brilliant blue G-250 stain. Finally, the content of glomalin was estimated using an eight-cell-UV spectrophotometer at 595 nm. In order to calculate the contents of EEG and TG, a standard curve was prepared with the bovine serum albumin (BSA) standard solution at the same time.
Root colonization rate
After removing the attached soil, root samples were cut into 10–20 mm segments and put into FAA fixed solution. The root sample was submerged into 8% potassium hydroxide solution in 90 °C water bath for 40 min and washed with fresh water for three times. Then, the root samples were immersed in 2% hydrochloric acid solution for 5 min, stained with 0.05% trypan blue solution at 90 °C for 40 min, and finally washed by lactic acid (Liu and Chen 2007). Root colonization was detected under microscope. The colonization rate was scored according to the amount and density of AMF hyphae in root of A. sinensis seedling.
Bioinformatic and statistical analysis
Raw FASTQ files were de-multiplexed using an in-house perl script and then quality filtered by fastp version 0.19.6 (Chen et al. 2018) and merged by FLASH version 1.2.7 (Tanja et al. 2011) with the following criteria: reads with any ambiguous bases or with average quality scores lower than 20 were discarded using a 50-bp sliding window. The resulting quality sequences were classified into the same OTUs (operational taxonomic units) using UPARSE (v. 7.1) with a 97% similarity cutoff value (Edgar 2013). The most abundant sequence for each OTU was selected as a representative sequence. The OTU table was manually filtered, i.e., chloroplast sequences in all samples were removed. To minimize the effects of sequencing depth on alpha and beta diversity measure, the number of gene sequences from each sample was rarefied, which still yielded an average good’s coverage of 97.00%, respectively. In this study, based on the OTUs information, the OTUs Venn analysis of biological communities under different treatments and the statistical analyses of unique and common OTUs were performed with Mothur v1.30.1 (Schloss et al. 2009) in Majorbio Cloud platform (https://cloud.majorbio.com). The data of A. sinensis seedlings growth, root colonization rate, culturable microbial populations, and physical and chemical indices of rhizosphere soil were analyzed by one-way analysis of variance (ANOVA) using the SPSS 22.0 (IBM, USA) software for Windows (Song et al. 2018). Student’s t-test was used in ANOVA for each parameter include A. sinensis seedlings growth and soil physiochemical and microenvironment parameters in RW and CC. The differences between RW and CC treatment were examined using least significant differences (LSD ≤ 0.05). Multiples comparison analysis was used to test differences among A. sinensis seedlings growth and soil microenvironments parameters. Bivariate correlation analysis was performed in the correlation analysis of soil microenvironments parameters, and the correlation is expressed by Pearson’s correlation coefficient. The effects of continuous monoculture (CME, %) were expressed by the value of each parameter (Kong et al. 2021) and were calculated using the following formula:
Where CME is the effect of continuous monoculture index, SAi is the mean value of CC index, and SAck is the mean value of RW index. Figures were drawn by the Origin Pro 2019 software (Origin Lab Corporation, USA).
Results
Response of seedlings growth and grades of A. sinensis to RW and CC treatments
The CC treatment inhibited A. sinensis seedlings growth and reduced quality grades (Fig. 1). Compared to RW, CC treatment reduced seedling weight per plant by 195.4% (Fig. 1A), and the index of seedling number, which reflects if the soils are suitable for seed emergence, was also significantly decreased by 36.7% (Fig. 1B). Similarly, CC treatment also reduced both the top grade (33.4% in RW to 31.8% in CC) and second-grade seedlings (32.9% in RW to 27.3% in CC) (Fig. 1C). The results indicated that continuous monoculture was detrimental to the quantity and quality of available A. sinensis seedlings.
Comparisons of the growth and grade proportion of A. sinensis seedlings between RW and CC. Significant differences were determined using least significant differences (LSD ≤ 0.05) of the Student’s t-test after one-way ANOVA, n = 5, (*P < 0.05; ***P < 0.001). A Seedling weight in RW and CC. B Seedling numbers in RW and CC. C Grade proportion of seedling in RW and CC
Changes of culturable microbial populations of rhizosphere soil between RW and CC treatments
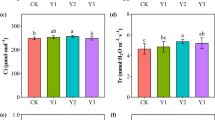
Culturable microbial counts were affected by RW and CC treatments (Fig. 2). Bacteria counts were significantly increased in CC compared to RW, and the same trend appeared in fungi counts. However, the counts of actinomycetes, ammonifiers, and azotobacter were significantly decreased in RW, indicating the weakening function of microorganisms in the continuous monoculture land. The results demonstrated that continuous monoculture affected the culturable microbial populations and weakened the quantity of beneficial functional bacteria in rhizosphere soil.
Changes of bacterial and fungal diversity of rhizosphere soil between RW and CC treatments
In this study, we employed molecular biology methods to further identify the bacterial and fungal diversity changed driven by continuous monoculture. The results demonstrated an obviously differences between RW and CC (Fig. 3). There were 26 phyla, 69 classes, 167 orders, 257 families, 405 genera, and 784 species with a total bacterial OTUs of 1389 and 6 phyla, 21 classes, 47 orders, 92 families, 142 genera, and 182 species with a total fungal OTUs of 255. The common and unique OTUs showed that 634 bacterial OTUs were shared by RW and CC with 361 bacterial OTUs of CC and 394 bacterial OTUs of RW (Fig. 3A), while 121 fungal OTUs were shared by RW and CC with 80 fungal OTUs of CC and 54 fungal OTUs of RW (Fig. 3B). It can be argued that rhizosphere soil bacterial and fungal diversities were dramatically effected by continuous monoculture.
Venn plots and relative abundance of bacteria and fungi in RW and CC, n = 3. A Venn plot of bacterial OTUs between RW and CC, B Venn plot of fungal OTUs between RW and CC, C relative abundance of bacteria community at genus level in RW and CC (others < 0.01), and D relative abundance of fungi community at genus level in RW and CC (others < 0.02)
The relative abundances of beneficial bacteria Pseudomonas and Lactococcus in genus with 17.92% and 14.84% in RW were sharply decreased to 0.87% and 7.28% in CC, while those of harmful bacteria Flavobacterium and Comamonas in genus with only 0.98% and 0.29% in RW were sharply increased to 8.10% and 7.18% in CC (Fig. 3C). On the other hand, the relative abundances of beneficial fungi Alternaria, Mortierella, and Ceratubasidium in genus with 28.72%, 18.57%, and 8.08% in RW were sharply decreased to 10.29%, 10.23%, and 0.54% in CC, while those of harmful fungi Lasiosphaeriaceae, Vishniacozyma, Myrmecridium, and Hypocreales in genus with only 2.44%,1.33%,0.19%, and 0.00% in RW were sharply increased to 11.93%, 6.95%, 7.58%, and 2.05% in CC (Fig. 3D). The results suggested that bacterial and fungal communities and functions were dramatically changed by continuous monoculture.
Differences of AMF and related indices between RW and CC treatments
Rhizosphere soil AMF spore densities and related indices, such as root colonization rate, TG, and EEG, were significantly reduced by continuous monoculture (Fig. 4). The AMF spore densities were 37.8% higher in RW (41.1 g−1 dry soil) than in CC (Fig. 4A). Correspondingly, CC treatment reduced root colonization rate by 16.8%, from 96.7% in RW to 82.8% in CC (Fig. 4B). The contents of TG and EEG were significantly reduced by 135.9% and 143.1% in CC treatment respectively (Fig. 4C and D). The results indicated that continuous monoculture negatively affected mycorrhiza formation and soil AMF spore densities, resulting in lower TG and EEG, and finally soil degradation.
Comparisons of the rhizosphere AMF and their related parameters in A. sinensis seedlings between RW and CC. Significant differences were determined using least significant differences (LSD ≤ 0.05) of the Student’s t-test after one-way ANOVA, n = 3, (*P < 0.05; **P < 0.01). A Spore densities in rhizosphere soil of seedlings in RW and CC. B Root colonization rate of seedlings in RW and CC. C Total extractable glomalin in rhizosphere soil of seedlings in RW and CC. D Easily extractable glomalin in rhizosphere soil of seedlings in RW and CC
Effects of rhizosphere soil pH value and nutrients under continuous monoculture
Rhizosphere soil pH value and soil nutrients were significantly changed by 1-year continuous monoculture (Table 2), with pH value 7.1 in CC compared to 6.18 in RW. Compared to RW, CC treatment reduced SOM by 10.2%, total N by 99.5%, available P by 45.3 %, and available K by 143%. The results suggested that continuous monoculture led to the increase of pH value, which was accompanied with the reduction in soil nutrients.
Correlations of rhizosphere soil parameters under continuous monoculture
The correlations between physicochemical parameters and microbes could reflect the function of microbes in driving nutrient turnover. In this study (Table 3), there were no significant correlations between six nutrients (SOM, total N, available K, available P, EEG, and TG) and six kinds of microorganisms in RW treatment, while EEG was significant negative relations with fungi and ammonifiers in CC. The correlations of fungi and SOM were derived from positive in RW to negative in CC, and the same with correlations with EEG, but the contrary correlation to available P, which indicated that fungi might play more dominant function in CC. Ammonifiers had the similar correlations to fungi. Actinomycetes and azotobacteria had the same correlation trends with total N, available K, and TG: continuous monoculture enhanced the positive correlations between the two microbial populations and total N, from 0.164 and 0.137 in RW to 0.805 and 0.718 in CC respectively, but obviously decreased the positive correlations of the two microbial populations with available P and TG. The positive correlations of AMF spore densities with total N and available K were elevated by continuous monoculture, from −0.680 to −0.948 in RW to 0.929 and 0.786 in CC respectively, while the positive correlations with SOM were sharply reduced from 0.953 in RW to −0.867 in CC. The results suggested that continuous monoculture induced changes in the microenvironments and affected the nutrients cycling by microorganisms.
The pH value directly affected soil culturable microbial populations and functions. As shown in Table 3, pH value was significantly positive correlation with bacteria and significantly negative correlation with fungi, ammonifiers, and AMF spore densities both in RW and CC. The negative correlations of pH value with AMF spore densities were declined by continuous monoculture from −0.842 in RW to −0.327 in CC. However, continuous monoculture completely subverted the correlation coefficients of pH value with actinomycetes and azotobacteria, which were significant positive correlations with 1.000 and 0.999 in RW and negative correlations with −0.976 and −0.996 in CC. The results indicated that changes in culturable microbial populations were induced by continuous monoculture, which result in the soil pH value alteration from 6.18 in RW to 7.10 in CC (Table 2). Consequently, the continuous monoculture changed the soil pH value significantly, and the changes of culturable microbial populations were more derived from the changes of soil pH value.
Microorganisms and their synergies were the key bio-stimulants to keep soil healthy and accelerated soil nutrients turnover. The correlation between microbial populations was further analyzed (Table 4). Continuous monoculture completely subverted the correlation coefficients of bacteria with fungi, ammonifiers, azotobacteria, and AMF spore densities, which were significant positive correlations with 0.998, 1.000, and 0.850 in RW and negative correlations with −0.998, −0.999, and −0.324 in CC. The correlations of fungi with actinomycetes and azotobacteria were changed dramatically by continuous monoculture too, with the negative coefficients of −0.995 and −0.992 in RW alternating to the significant positive that is 0.987 and 1.000 in CC respectively and the same trends in correlation between actinomycetes and AMF spore densities. The negative correlation between ammonifiers and azotobacteria with −0.997 in RW was converted into significant positive correlations with 0.998 in CC and similar to the correlation between azotobacteria and AMF spore densities. The results demonstrated that continuous monoculture had reshaped the relationships among functional microbial populations.
Principal component analysis and treatment average effect of rhizosphere soil parameters
Biplot of treatments with principal component analysis (PCA) of rhizosphere parameters was further designed to show the effects of continuous monoculture (Fig. 5A). Two principal components accounted for 100% of the variations with 99.85% by first axis and 0.15% on the second axis, which could fully represent the original information. The analysis of PCA showed that the parameters of AMF spore densities and available K were far away from the PC1, while the other rhizosphere soil variables distributed on or near the PC1, which indicated continuous monoculture, dramatically changed these indices. Furthermore, the parameters of pH value, culturable microbial populations, and available P overlapped together, which suggested that there was close interplay between these parameters. The treatments of RW and CC distributed in the fourth and first quadrants, and the angle between RW and CC was obtuse, indicating that CC had a negative impact on rhizosphere soil parameters of A. sinensis seedlings compared to RW. Consequently, CC had great influence in the rhizosphere soil of A. sinensis seedlings.
Principal component analysis (PCA) of rhizosphere parameters and continuous monoculture effect in A. sinensis seedlings between RW and CC, n = 3. SOM, soil organic matter; total N, total nitrogen; available P, available phosphorus; available K, available potassium; EEG, easily extractable glomalin; TG, total extractable glomalin; root C, root colonization rate; AMF SD, AMF spore densities. A principal component analysis (PCA) of rhizosphere parameters in A. sinensis seedlings between RW and CC. B Continuous monoculture effect (CME) of rhizosphere parameters in A. sinensis seedlings between RW and CC
The continuous monoculture effect (CME) was to evaluate the influence effects of rhizosphere soil parameters of continuous monoculture (Fig. 5B). Culturable bacteria and fungi populations, OTUs of fungi and pH value, were positive effects by CC, with the coefficients of 24.69%, 7.53%, 14.89%, and 14.86%, respectively. Positive effects of culturable fungi in sync with OTUs of fungi indicated that fungi could play a dominant role under CC. The effects of culturable actinomycetes and azotobacteria populations, total nitrogen and available potassium, and EEG and TG were sharply reduced by CC, with the coefficients of 41.49%, 47.94%, 49.87%, 58.92%, 58.88%, and 57.59%, respectively. The high negative coefficients indicate that CC disrupted the rhizosphere physiochemical properties and microbial balance of A. sinensis seedlings.
Discussion
Previous studies have shown that continuous monoculture can severely inhibit the growth of medicinal plants and reduce the yield and medicinal active ingredients (Sui et al. 2019; Song et al. 2018; Zhang et al. 2018). Continuous monoculture significantly inhibited the growth of Salvia miltiorrhiza or even led to the failure of harvest, as well as the dramatic reductions (up to 35%) in the active component of dihydrotanshinone (Liu et al. 2013). Results from 4 years of Salvia miltiorrhiza continuous monoculture showed that yield and concentration of active compounds were decreased year by year with the extension of continuous monoculture time (Liu et al. 2020a). Raising medicinal plant seedlings had a further negative impact on the yield and quality of typical geo-authentic medicinal herb, so high-quality medicinal seedlings play the key role in the efficient production of medical plants. Our observations showed that the seedling weight and seedling number, which reflected the health quality and effectiveness of A. sinensis seedlings, were significantly reduced by 195.4% and 36.7% under 1-year continuous monoculture land (Fig. 1A and B); correspondingly, the high-quality seedlings declined sharply (Fig. 1C), which obviously affected the efficiency of raising A. sinensis seedlings. Our findings were consistent with previous research that number and health index of surviving seedlings were significantly decreased under continuous monoculture of Panax quinquefolius L. (Chen et al. 2012). These results demonstrated that the practice of continuous monoculture reduced the quality and efficiency of typical geo-authentic medicinal herb seedlings.
Soil microenvironments, such as disorder of soil physiochemical properties and microbial imbalance, are the major causes for continuous cropping obstacles (Zhao et al. 2018). Previous studies suggested that the functions of microbial populations were changed by continuous monoculture from “bacteria-dominated type” to “fungi-dominated type,” with the raise of fungi count and the decline of actinomyces counts (Xiong et al. 2014; Sun et al. 2014). Our investigations were in part consistent with previous results that continuous monoculture significantly increased fungi count and significantly decreased actinomycetes, ammonifiers, and azotobacteria counts (Fig. 2). Compared with RW, rhizosphere soil nutrients, SOM, total N, and available P and K of CC were significantly decreased (Table 2), and the correlations of microbial populations and nutrients in CC were dramatically altered (Table 3). The results suggested that the practice of 1-year continuous monoculture had dramatically caused A. sinensis seedlings rhizosphere soil nutrients declined and the microbial populations disturbed.
Soil degradation is usually accompanied by overexploitation of nutrients such as SOM, nitrogen, and phosphorus, changes in soil pH value, and imbalance of microbial populations (Huang et al. 2019; Liu et al. 2020b). Our results showed that continuous monoculture deteriorated soil quality by increasing pH value and reducing the nutrients (Table 2). Correlation analysis results showed that pH value was significantly negative with AMF spore densities (Table 3), suggesting that elevated pH value reduced root colonization (Fig. 3B), and has also the significantly negative correlations with culturable fungi and ammonifiers and positive correlations with culturable bacteria. Moreover, the correlations among five culturable microbial populations and rhisphere soil nutrient parameters were also dramatically changed from RW to CC (Table 4). The results indicated that elevated pH value not only modulated rhizosphere soil microbial population but also altered the functions of interplay between nutrients and microbial populations (Tables 3 and 4). The bacterial and fungi diversities analysis results showed that the abundances of harmful microbes were increased under CC (Fig. 3), meaning that healthy microbial balance was broken down. With the deteriorating microbial community diversity and function, soil quality under continuous monoculture became degradated and unhealthy (Sun et al. 2021), resulting in the reduction of plant yield and quality (Zhang and Zhang 2008; Li et al. 2021).
Biochemical factors of rhizosphere are the microenvironment for the survival of microfloral population and shape the microbial populations and functions. The pH value, which is easily regulated by rhizosphere microenvironments, is one of key biochemical factors to impact the microbial populations and functions. Root exudates as a nutrient play an important role for rhizosphere microbial recruitment (Reinhold-Hurek et al. 2015). As rhizosphere exudates contain lots of organic acids, which have the ability to buffer the rhizosphere pH value (Peter and Mark 1993; Zhang et al. 2017), the soil pH value could be altered with the amount of rhizosphere exudates changing (Li et al. 2022). The smaller A. sinensis seedlings in CC reduced the content of rhizosphere exudates, which could lead to rhizosphere microbial proliferation disturbed and pH value increased. Moreover, soil pH value was the important drivers for the bacterial and fungal community composition, and changes in soil pH cause change in microbial groups (An et al. 2020; Liu et al. 2022). The dramatically degenerated microfloral populations in this study could be attributed to in the combined effects of the nutrients decreased and the pH value increased in CC.
Microfloral population types were also driven by the changes of soil nutrients and pH value, especially soluble carbon from root exudates, which are the carbon sources for most of microbial growth (Yao et al. 2006). The soil organic matter (SOM) was significantly decreased in CC (Table 1), indicating the total amount of carbon sources available to microorganisms was reduced. The reduction of SOM could cause the reduction of the number of microorganisms (Bonner et al. 2020). Continuous cropping also caused excessive consumption of soil nutrients (Yang et al. 2014; Wang et al. 2013). In this study, the contents of soil organic matter (SOM), total nitrogen, available phosphorus, and available potassium were significantly reduced in CC (Table 1), and the depletion of nutrients could lead to the reduction of the soil microbial populations (Martina et al. 2003; Wang et al. 2021). Besides, the OTU and taxa of microorganisms were also impacted by the depletion of nutrients in soil, which were more likely to cause the instability of the bacterial community (Wang et al. 2022b), and then led to the bacterial OTU reduced and fungal OTU increased significantly. The results further illustrated that continuous monoculture induced the microbial quantity and populations of rhizosphere degenerated (Fig. 5B).
What’s more, the dominant genera Pseudomonas was the common soil beneficial bacteria with the function of alleviating diseases in plants (Liu et al. 2020a, b; Peng et al. 2014; Qiu et al. 2021) and had the function of promoting the growth and quality of A. sinensis (Xie et al. 2022), sharply decreased from 17.92 in RW to 0.87% in CC (Fig. 3C). While Flavobacterium, which is usually regarded as plant pathogen (Mou et al. 2021), dramatically increased from 0.98% in RW to 8.10% in CC (Fig. 3C). The results suggested that both diversity and abundance of beneficial bacteria were decreased by continuous monoculture. Alternaria is known as probiotic fungal with the functions of inhibiting pathogenic microbes and secreting metabolites to promote plant growth (Qiu et al. 2021), Mortierella has the functions of dissolving soil phosphorus and providing nitrogen nutrition to promote plant growth and disease resistances (Tan et al. 2022; Kanse et al. 2015), and Ceratubasidium not only increases the plant chlorophyll contents but also secretes IAA to improve plant growth (Chen et al. 2011). However, the abundances of Alternaria, Mortierella, and Ceratubasidium were sharply decreased by continuous monoculture in this study. The abundances of Lasiosphaeriaceae, Vishniacozyma, Myrmecridium, and Hypocreales, which were considered as pathogen fungi (Meng et al. 2021; Sun et al. 2022; Yu et al. 2022; Sa et al. 2017; Bao et al. 2021), were dramatically increased by CC treatment. These findings were in consistent with previous results (Tan et al. 2022; Liu et al. 2020b). The bacterial and fungal diversities analysis provided critical evidences that the practice of continuous monoculture not only reduced the richness and diversity of bacteria and increased the richness and diversity of fungi (Figs. 3 and 5B) but also changed the compositions and structures of bacteria and fungi from beneficial type to harmful type with plants, which supported the conclusion of continuous monoculture driving the soil microbes from “beneficial bacteria dominated” to “harmful microbes dominant.”
The arbuscular mycorrhizal fungi can establish symbiosis with most of terrestrial plants, helping plant to absorb nutrients from soil to plant and to transport photosynthetic carbon from plant aboveground to rhizosphere soils (Cabral et al. 2016; He et al. 2017). Photosynthetic carbon resources, which are easily utilized by soil microorganisms, seem to be much faster by the mycorrhizal pathway than that of root exudates pathway (Kaiser et al. 2014). AMF could provide as much as 15% soil carbon resources by forming network of extramatrical mycorrhizal hyphae and producing glomalin-related soil protein (He et al. 2020). Thus, AMF is the important soil healthy indicator by acting as bio-regulators, bio-protectors, and bio-fertilizers (Rehman et al. 2022). Our results showed that AMF spore densities and root colonization rate were significantly declined in CC. The key glomalin parameters of EEG and TG were also decreased in CC. We also found that even though AMF spore densities could not directly affect EEG and TG content, which was far from PC1 (Fig. 5A) and no significant correlation between AMF spore densities and glomalin (Table 3), it was shown that root colonization rate almost overlapped with SOM in PCA (Fig. 5A), suggesting that AMF and their product glomalin had potential applications in maintaining soil health and restoring degraded land (Singh et al. 2020). Furthermore, our study showed that continuous monoculture significantly decreased TG and EEG content, not only because it reduced the AMF spore densities and root colonization (Fig. 4) but also because the poorer growth A. sinensis seedlings were (Fig. 1), the less AMF hyphae distributed in rhizosphere soil. The results suggested that the AMF extrametrical hyphal growth was negatively impacted (Jiang et al. 2020; He et al. 2020), which lead to less glomalin secreted. Consequently, the symbiotic relationship between AMF and A. sinensis seedlings was weakened, which led to the poor growth A. sinensis seedlings under CC (Chen et al. 2022; Wang et al. 2022a). These results demonstrated that continuous monoculture caused the degradation of A. sinensis seedling production fields, partly by negative regulating the activity of AMF (Fig. 5B), reducing the symbiotic relationship between AMF and the host, and affecting the supply of rhizosphere carbon resources, so as to cause the degradation of bacterial and fungal populations and functions.
Conclusion
Consistent with our hypotheses, continuous monoculture caused the rhizosphere soil microenvironments degradation and finally reduced the efficiency and effectiveness of available A. sinensis seedlings. Compared to raising A. sinensis seedlings in reclamation wasteland, continuous monoculture significantly inhibited the growth and available quantity of A. sinensis seedlings owing to soil microenvironments changed. With the contents of soil nutrients and glomalin decrease, the culturable microbial populations, bacterial and fungal richness, and diversities changed dramatically, and the microbial abundance and function developed from beneficial type to harmful type with plants. Enhanced soil pH value due to continuous monoculture significantly changed the correlations with five culturable microbial populations. Moreover, interplays of microbe-soil nutrient and microbe to microbe were also dramatically altered with culturable microbial populations change causing by continuous monoculture. These findings suggested that continuous monoculture could deteriorate microenvironments by enhancing soil pH value and reducing SOM, total N, available P and K, beneficial culturable microbial populations, and AMF spore densities, thus leading to the failure of A. sinensis seedlings raising in continuous monoculture land. Consequently, continuous monoculture problems could be effectively alleviated by application of microbial agents or organic and microbial fertilizer to regulate the rhizosphere microenvironments.
Availability of data and materials
The datasets are available from the corresponding author.
References
An ZG, Guo FX, Chen Y et al (2020) Rhizosphere bacterial and fungal communities during the growth of Angelica sinensis seedlings cultivated in an alpine uncultivated meadow soil. Peer J 8:e8541. https://doi.org/10.7717/peerj.8541
Bai G, Guo FX, Chen Y et al (2019) Differences in physiological resistance traits of Angelica sinensis seedlings from uncultivated and cultivated fields in Min County. Acta Pratacul Sin 28(11):86–95. https://doi.org/10.11686/cyxb2019292
Bao LM, Ding YF, Wei YL et al (2021) Analysis on the composition and diversity of fungi community in the continuous cropping and fallow soil of Panax notoginseng. J Chin Med Mater 44(01):7–12. https://doi.org/10.13863/j.issn1001-4454.2021.01.002
Bi YL, Wu WY (2007) Dyeing method-a kind of the method for mycorrhizal spore density quick improved measurement. Energy Environ Prot 21(2):9–11. https://doi.org/10.3969/j.issn.1006-2007.02.003
Bonner M, Allen D, Brackin R et al (2020) Tropical rainforest restoration plantations are slow to restore the soil biological and organic carbon characteristics of old growth rainforest. Microb Ecol 79:432–442. https://doi.org/10.1007/s00248-019-01414-7
Cabral C, Ravnskov S, Tringovska I et al (2016) Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticumaestivum L.) subjected to heat-stress. Plant Soil 408(1/2):385–399. https://doi.org/10.1007/s11104-016-2942-x
Cai ZP, Wang GX, Wang HX (2013) Effects of different sowing dates on greenhouse seedlings of Angelica sinense. J Zhe Jiang Agric Sci 12:1586–1589. https://doi.org/10.16178/j.issn.0528-9017.2013.12.028
Chen BB, Wang M, Hu YL et al (2011) Preliminary study on promoting effects of endophytic fungi to growth of Rehmannia glutinosa. China J Chin Materia Med 36(9):1137. https://doi.org/10.4268/cjcmm20110906
Chen J, Zhang XS, Yang JX et al (2012) Effect of continuous cropping and soil treatment on rhizosphere fungal community of Panaxquinquefolium. China J Chin Materia Med 37(23):3531–3535. https://doi.org/10.4268/cjcmm20122305
Chen KL, Huang G, Li YK et al (2022) Illumina miseq sequencing reveals correlations among fruit ingredients, environmental factors, and AMF communities in three Lycium barbarum producing regions of China. Microbiol Spectr. https://doi.org/10.1128/spectrum.02293-21
Chen S, Zhou Y, Chen Y et al (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34(17):i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Dai Z, Li M (1997) Study on determination methods for available potassium in upland field. Acta Pedol Sin 34:336–343. https://doi.org/10.11913/PSJ.2095-0837.2021.60643
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998. https://doi.org/10.1038/nmeth.2604
Gong LY, Cheng LL, Lu Q (2015) Analysis of the definition, classification and ecological function of wasteland. J Nat Resour 12(30):1969–1981. https://doi.org/10.11849/zrzyxb.2015.12.001
He JD, Chi GG, Zou YN et al (2020) Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl Soil Ecol 154. https://doi.org/10.1016/j.apsoil.2020.103592
He SB, Guo LX, Li J et al (2017) Advances in arbuscular mycorrhizal fungi and legumes symbiosis research. Acta Pratacul Sin 26(01):187–194. https://doi.org/10.11686/cyxb2016228
He XL, Wang YL, Zhao LL (2012) AM fungal genetic diversity in seven medicinal plant rhizospheres in Anguo city of Hebei province. Chin J Eco-Agric 20(2):144–150. https://doi.org/10.3724/SP.J.1011.2012.00144
Huang L, Riggins CW, Rodriguez-Zas S et al (2019) Long-term N fertilization imbalances potential N acquisition and transformations by soil microbes. Sci Total Environ 691:562–571. https://doi.org/10.1016/j.scitotenv.2019.07.154
Janos DP, Garamszegi S, Beltran B (2008) Glomalin extraction and measurement. Soil Biol Biochem 40(3):728–739. https://doi.org/10.1016/j.soilbio.2007.10.007
Jiang FY, Zhang L, Zhou JC et al (2020) Arbuscular mycorrhizal fungi enhance mineralization of organic phosphorus (P) by carrying bacteria along their extraradical hyphae. New Phytol:nph.17081. https://doi.org/10.1111/nph.17081
Jiang P, Wang MY (2012) Colonization rate and diversity of AM fungi in the rhizosphere of seven medicinal plants in XiaMen. Acta Ecol Sin 32(13):4043–4051. https://doi.org/10.5846/stxb201106030745
Kaiser C, Kilburn MR, Clode PL et al (2014) Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol 205:1537–1551. https://doi.org/10.1111/nph.13138
Kanse OS, Whitelaw-Weckert M, Kadam TA et al (2015) Phosphate solubilization by stress-tolerant soil fungus Talaromyces funiculosus SLS8 isolated from the Neem rhizosphere. Ann Microbiol 65(1):85–93. https://doi.org/10.1007/s13213-014-0839-6
Katoh A, Fukuda S, Fukusaki E et al (2011) Systems biology in a commercial quality study of the Japanese Angelica radix: toward an understanding of traditional medicinal plants. Am J Chin Med 39(4):757–777. https://doi.org/10.1142/S0192415X11009172
Kong T, Liu ZW, Shen HO et al (2021) Influence of apple-soybean intercropping on soil nutrient and microbial biomass distribution and effect in sandy land of northwest Liaoning province. Chin J Ecol 40(02):340–351. https://doi.org/10.13292/j.1000-4890.202102.029
Li BZ, Zhang QQ, Chen YH et al (2021) Different crop rotation systems change the rhizosphere bacterial community structure of Astragalusmembranaceus (Fisch) Bge. var. mongholicus (Bge.) Hsiao. Appl Soil Ecol 166:104003. https://doi.org/10.1016/j.apsoil.2021.104003
Li WN, Luo YM, Huang ZY et al (2022) Effects of mixed young plantations of Parashorea chinensis on soil microbial functional diversity and carbon source utilization. Chin J Plant Ecol 46. https://doi.org/10.17521/cjpe.2021-0296
Liu C, Zhao D, Ma W et al (2016) Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl Microbiol Biotechnol 100(3):1421–1426. https://doi.org/10.1007/s00253-015-7039-6
Liu HY, Niu M, Zhu S et al (2020a) Effect study of continuous monoculture on the quality of Salvia miltiorrhiza bge roots. Biomed Res Int 2020:4284385. https://doi.org/10.1155/2020/4284385
Liu JL, Wang QQ, Ku YL et al (2022) Precipitation and soil pH drive the soil microbial spatial patterns in the Robinia pseudoacacia forests at the regional scale. Catena 212. https://doi.org/10.1016/j.catena.2022.106120
Liu RJ, Chen YL (2007) Mycorrhizology. The Science Publishing Company, Beijing
Liu SP, Wei JM, Li Z et al (2020b) Effect of continuous cropping on structure of microbial community in Aconitum carmichaeli Debx rhizosphere soil. Chin J Microecol 32(05):520–526. https://doi.org/10.13381/j.cnki.cjm.202005005
Liu W, Zhang L, Zhang YY et al (2013) Influence of continuous cropping years on yield and compounds in Salvia miltiorrhizaf. Alba. China J Chin Materia Med 38(24):4252–4256. https://doi.org/10.4268/cjcmm20132412
Lu CC, Liu M, Shang WR et al (2020) Knowledge mapping of Angelica sinensis (Oliv.) Diels (Danggui) research: a scientometric study. Front Pharmacol 11:294. https://doi.org/10.3389/fphar.2020.00294
Martina SG, Juliet B, Jules NP et al (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Microbiology 69:1800–1809. https://doi.org/10.1128/AEM.69.3.1800-1809.2003
Meng PP, Feng H, Chen W et al (2021) Community structure and diversity of root-associated fungi of Catalpa bungei seedlings and grafted seedlings. Mycosystema 40(8):1965–1979. https://doi.org/10.13346/j.mycosystema.210039
Mou D, Zeng ZY, Zhong WL et al (2021) Bacterial community structure and function of Cordyceps cicadae microecosystem in Guiyang. Acta Microbiol Sin 61(02):469–481. https://doi.org/10.13343/j.cnki.wsxb.20200540
Peng SM, Wang BL, Xu JZ et al (2014) Effect of different treatment on endophytic bacterial communities in continuous cropping of Chrysanthemum morifoliu. China J Chin Materia Med 39(24):4763–4768. https://doi.org/10.4268/cjcmm20142413
Peter MA, Mark AA (1993) Nutrient cycling in forests. New Phytol 124:4. https://doi.org/10.1111/j.1469-8137.1993.tb03847.x
Qiu H, Chen JY, Lai YZ et al (2021) Relationships between endophytic structure, rhizosphere soil properties and aconite alkaloids accumulations in Aconitum carmichaelii Debx. Plant Sci J 39(6):643–653. https://doi.org/10.11913/PSJ.2095-0837.2021.60643
Rehman MMU, Zhu Y, Abrar M et al (2022) Moisture and period dependent interactive effects of plant growth promoting rhizobacteria and AM fungus on water use and yield formation in dryland wheat. Soil Plant. https://doi.org/10.1007/s11104-022-05641-9
Reinhold-Hurek B, Bunger W, Burbano CS et al (2015) Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol 53(1):403–424. https://doi.org/10.1146/annurev-phyto-082712-102342
Sa RL, Yang HS, Tai JC et al (2017) Effect of maize straw returning on soil fungal diversity in saline alkali Soil. Chin J Soil Sci 48(4):937–942. https://doi.org/10.19336/j.cnki.trtb.2017.04.24
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537. https://doi.org/10.1128/AEM.01541-09
Shang HS, Cao SQ, Zhang M et al (2021) Effect of soil fumigation with dazomet 98% GR on Angelica seedling in Minxian, Gansu province. Plant Prot 47(06):347–352. https://doi.org/10.16688/j.zwbh.2020419
Singh S, Malhotra S, Mukherjee P et al (2020) Peroxidases from an invasive mesquite species for management and restoration of fertility of phenolic-contaminated soil. J Environ Manag 256:109908. https://doi.org/10.1016/j.jenvman.2019.109908
Song XH, Pan Y, Li LY et al (2018) Composition and diversity of rhizosphere fungal community in CoptischinensisFranch. Continuous cropping fields. PLoS One 13(3). https://doi.org/10.1371/journal.pone.0193811
Sui J, Ji C, Wang X et al (2019) A plant growth-promoting bacterium alters the microbial community of continuous cropping poplar trees’rhizosphere. J Appl Microbiol 126:1209–1220. https://doi.org/10.1111/jam.14194
Sun J, Zhang Q, Zhou J et al (2014) Illumina amplicon sequencing of 16S rRNA tag reveals bacterial community development in the rhizosphere of apple nurseries at a replant disease site and a new planting site. PLoS One 9(10):e111744. https://doi.org/10.1371/journal.pone.0111744
Sun KN, Fu L, Song Y et al (2021) Effects of continuous cucumber cropping on crop quality and soil fungal community. Environ Monit Assess 193:436. https://doi.org/10.1007/s10661-021-09136-5
Sun Y, Wang L, Zhao L et al (2022) Effects of compound microbial fertilizer on apple replanted diseases and fungal community structure in rhizosphere soil. Acta Phytopathol Sin 52(02):256–268. https://doi.org/10.13926/j.cnki.apps.000743
Tan ZT, Li AD, Yang R et al (2022) Effects on community structure of soil fungi in Passiflora edulis continuous cropping. Mol Plant Breed 20(04):1373–1382. https://doi.org/10.13271/j.mpb.020.001373
Tanja M, Steven et al (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Wang F, Li SG, Xu FH et al (2013) The research progress on mechanism of continuous cropping obstacle. Soil Fertil Sci 5:6–13. https://doi.org/10.11838/sfsc.20130502
Wang F, Zhang L, Zhou J et al (2022a) Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant Soil. https://doi.org/10.1007/s11104-022-05621-z
Wang GX, Mi YW, Cai ZP et al (2018) Effects of breeding model on yield and quality traits of Danggui. Acta Chin Med Pharmacol 46(3):28–30. https://doi.org/10.19664/j.cnki.1002-2392.180075
Wang N (2008) Develop trends and yield of greenhouse raise seeding and transplant of Angelica sinensis. Gansu Agric Univ. https://doi.org/10.7666/d.y1333173
Wang QC, Zheng Y, Song G et al (2021) Impacts of simulated nitrogen and phosphorus depositions on soil microbial biomass and soil nutrients along two secondary succession stages in a subtropical forest. Acta Ecol Sin 41(15):6245–6256. https://doi.org/10.5846/stxb202007241944
Wang YT, Xie YZ, Ma HB et al (2022b) Responses of soil microbial communities and networks to precipitation change in a typical steppe ecosystem of the loess plateau. Microorganisms 10(4):817. https://doi.org/10.3390/microorganisms10040817
Wu Y, Chen J (2016) The potassium dichromate volumetric method for the determination of soil organic matter-heating method. China High Technol Ent:11–12. https://doi.org/10.12677/MEng.2017.44036
Wu YA, Lin HM, Liu XR et al (2009) Inhibition of matrix seedling raising in winter on premature bolting of Angelica sinensis. Chin Tradit Herb Drugs 3:456–462. https://doi.org/10.3321/j.issn:0253-2670.2009.03.036
Xie TP, Liu N, Liu YM et al (2022) Effects of chemical fertilizer reduction and application of plant growth regulators from traditional Chinese medicine on the quality and its bacterial community in rhizosphere soil. Biotechnol Bull 38(03):79–91. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2021-0467
Xiong W, Zhao QY, Zhao J et al (2014) Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb Ecol 70:209–218. https://doi.org/10.1007/s00248-014-0516-0
Xu X, Zhu T, Shi T et al (2020) Quality suitability regionalization analysis of Angelica sinensis in Gansu, China. PLoS One 15(12):e0243750. https://doi.org/10.19540/j.cnki.cjcmm.20210903.102
Yang J, Xie YZ, Wu XD et al (2014) Stoichiometry characteristics of plant and soil in alfalfa grassland with different growing years. Acta Pratacul Sin 23(2):340–345. https://doi.org/10.11686/cyxb20140240
Yao HY, Jiao XD, Wu FZ et al (2006) Effects of continuous cucumber cropping and alternative rotations under protected cultivation on soil microbial community diversity. Plant Soil 284:195–203. https://doi.org/10.1007/s11104-006-0023-2
Yu XJ, Chen Q, Shi WC et al (2022) Interactions between phosphorus availability and microbes in a wheat–maize double cropping system: a reduced fertilization scheme. J Integr Agric 21(03):840–854. https://doi.org/10.1016/S2095-3119(20)63599-7
Zhang JF, He LP, Wu YG et al (2018) Comparative protromic analysis of Pogostemoncablin leaves after continuous cropping. Protein Expr Purif 152:13–22. https://doi.org/10.1016/S2095-3119(20)63599-7
Zhang P, Wang XL, Ma X et al (2021) The correlations between the quality of min dang gui and the organic elementsin soil of Dang gui genuine producing areas Min xian. West J Tradit Chin Med 34(11):65–72. https://doi.org/10.12174/j.issn.2096-9600.2021.11.13
Zhang RF, Vivanco JM, Shen QR (2017) The unseen rhizosphere root-soil-microbe interactions for crop production. Curr Opin Microbiol 37:8–14. https://doi.org/10.1016/j.mib.2017.03.008
Zhang W, Fu Y, Li JF et al (2015) Comparative study on Kjeldahl method and dumas combustion method for total nitrogen measurement in soil. Chin Agric Sci Bull 31:172–175 doi: CNKI:SUN:ZNTB.0.2015-35-029
Zhang XH, Lang DY, Zhang EH (2016) Effects of soil sterilization on growth of Angelica sinensis plant and soil microbial populations in a continuous mono-cropping soil. Int J Agric Biol 18:458–463. https://doi.org/10.17957/IJAB/15.0118
Zhang XH, Zhang EH (2008) Effect of various rotation systems on yield of Angelica sinensis and microbial populations in its rhizosphere. Chin Tradit Herb Drugs 2(39):267–269. https://doi.org/10.3969/j.issn.1006-8759.2007
Zhao Q, Xiong W, Xing Y et al (2018) Long-term coffee monoculture alters soil chemical properties and microbial communities. Sci Rep 8:6116. https://doi.org/10.1038/s41598-018-24537-2
Zhu L, Yan H, Zhou GS et al (2021) Insights into the mechanism of the effects of rhizosphere microorganisms on the quality of authentic Angelica sinensis under different soil microenvironments. BMC Plant Biol 21:285. https://doi.org/10.1186/s12870-021-03047-w
Zhu Y, Chen YL, Gong XF et al (2018) Plastic film mulching improved rhizosphere microbes and yield of rainfed spring wheat. Agric For Meteorol 263:130–136. https://doi.org/10.1016/j.agrformet.2018.08.015
Zhu Y, Peng YN, Gong XF et al (2017) Effects of different microbial agents on growth of Angelica sinensis and microorganism population and nutrients of rhizosphere soil. Chin J Appl Environ Biol 23(03):511–519. https://doi.org/10.3724/SP.J.1145.2016.06013
Acknowledgements
Thanks to Professor Baoluo Ma for his valuable revision opinions and careful revision work. And then, thanks to our colleagues Jun Zhang and Kunyuan Ma for their help in the field experiment.
Funding
This work was supported by Outstanding Youth Fund of the Gansu Academy of Sciences (2019QN-02) and Science and Technology Plan Funding of Gansu province (21JR1RA349).
Author information
Authors and Affiliations
Contributions
XG, measurement and analysis of data, chart making and paper writing, etc. YZ, design of experiment and manuscript revision. YP, performed the field experiments. ZG, conduct field experiments and experimental field management. JZ, contact with text site and research coordination. HY, experimental guidance. ZW, field test coordination. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This paper does not involve human and animal experiments, so it does not need ethical approval and informed consent.
Consent for publication
All the authors agree to publish the paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, X., Zhu, Y., Peng, Y. et al. Insights into the deriving of rhizosphere microenvironments and its effects on the growth of authentic Angelica sinensis seedlings under continuous monoculture. Ann Microbiol 72, 34 (2022). https://doi.org/10.1186/s13213-022-01692-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-022-01692-6