Abstract
In the present study, soil bacterial and fungal communities across vanilla continuous cropping time-series fields were assessed through deep pyrosequencing of 16S ribosomal RNA (rRNA) genes and internal transcribed spacer (ITS) regions. The results demonstrated that the long-term monoculture of vanilla significantly altered soil microbial communities. Soil fungal diversity index increased with consecutive cropping years, whereas soil bacterial diversity was relatively stable. Bray-Curtis dissimilarity cluster and UniFrac-weighted principal coordinate analysis (PCoA) revealed that monoculture time was the major determinant for fungal community structure, but not for bacterial community structure. The relative abundances (RAs) of the Firmicutes, Actinobacteria, Bacteroidetes, and Basidiomycota phyla were depleted along the years of vanilla monoculture. Pearson correlations at the phyla level demonstrated that Actinobacteria, Armatimonadetes, Bacteroidetes, Verrucomicrobia, and Firmicutes had significant negative correlations with vanilla disease index (DI), while no significant correlation for fungal phyla was observed. In addition, the amount of the pathogen Fusarium oxysporum accumulated with increasing years and was significantly positively correlated with vanilla DI. By contrast, the abundance of beneficial bacteria, including Bradyrhizobium and Bacillus, significantly decreased over time. In sum, soil weakness and vanilla stem wilt disease after long-term continuous cropping can be attributed to the alteration of the soil microbial community membership and structure, i.e., the reduction of the beneficial microbes and the accumulation of the fungal pathogen.




Similar content being viewed by others
References
Shipton PJ (1977) Monoculture and soilborne plant pathogens. Annu Rev Phytopathol 15:387–407
Cao Y, Zhang Z, Ling N et al (2011) Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47:495–506
Feng Y, Motta AC, Reeves DW et al (2003) Soil microbial communities under conventional-till and no-till continuous cotton systems. Soil Biol Biochem 35:1693–1703
Lu L, Yin S, Liu X et al (2013) Fungal networks in yield-invigorating and -debilitating soils induced by prolonged potato monoculture. Soil Biol Biochem 65:186–194
Mazzola M, Manici LM (2012) Apple replant disease: role of microbial ecology in cause and control. Annu Rev Phytopathol 50:45–65
Trout T, Ajwa H, Schneider S (2003) Fumigation and fallowing effects on replant problems in California peach. pp 77–1
Lake RJ, Falloon PG, Cook DWM (1993) Replant problem and chemical components of asparagus roots. N Z J Crop Hortic Sci 21:53–58
Minoo D, Jayakumar VN, Veena SS et al (2008) Genetic variations and interrelationships in Vanilla planifolia and few related species as expressed by RAPD polymorphism. Genet Resour Crop Evol 55:459–470
Thomas J, Suseela Bhai R, Vijayan AK (2003) Diseases and their management. Vanilla- Prince Spices 27–41
Pinaria AG, Liew ECY, Burgess LW (2010) Fusarium species associated with vanilla stem rot in Indonesia. Australas Plant Pathol 39:176–183
Radjacommare R, Usharani R, Samiyappan R (2007) Genotyping antibiotic producing fluorescent pseudomonads to select effective rhizobacteria for the management of major vanilla diseases. Ann Microbiol 57:163–170
Zhao Q, Wang H, Wang H et al (2012) Effects of planting period on vanilla physiological indices and rhizosphere soil microbial community structure. Chin J Trop Crop 33:1562–1567 (in chinese)
Van Bruggen AHC, Semenov AM (2000) In search of biological indicators for soil health and disease suppression. Appl Soil Ecol 15:13–24
Zhao J, Zhang R, Xue C et al (2014) Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb Ecol 67:443–453
Zhao J, Ni T, Li Y et al (2014) Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS ONE 9:e85301
van Elsas JD, Garbeva P, Salles J (2002) Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 13:29–40
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol 42:243–270
Klein E, Katan J, Minz D, et al. (2011) Soil suppressiveness against Fusarium crown and root rot of cucumber in organic-amended soil: Occurrence and possible mechanisms. Phytopathology. Amer Phytopathological Soc 3340 Pilot knob road, St Paul, MN 55121 USA, pp S92–S92
Qiu M, Zhang R, Xue C et al (2012) Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol Fertil Soils 48:807–816
Li X, Ding C, Zhang T, Wang X (2014) Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol Biochem 72:11–18
Chen M, Li X, Yang Q et al (2012) Soil eukaryotic microorganism succession as affected by continuous cropping of peanut - pathogenic and beneficial fungi were selected. PLoS ONE 7:e40659
Zhou X, Wu F (2012) Dynamics of the diversity of fungal and Fusarium communities during continuous cropping of cucumber in the greenhouse. FEMS Microbiol Ecol 80:469–478
Urashima Y, Sonoda T, Fujita Y, Uragami A (2012) Application of PCR-denaturing-gradient gel electrophoresis (DGGE) method to examine microbial community structure in asparagus fields with growth inhibition due to continuous cropping. Microbes Environ 27:43–48
Margulies M, Egholm M, Altman WE et al (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380
Shokralla S, Spall JL, Gibson JF, Hajibabaei M (2012) Next-generation sequencing technologies for environmental DNA research. Mol Ecol 21:1794–1805
Acosta-Martínez V, Dowd S, Sun Y, Allen V (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40:2762–2770
Fierer N, Ladau J, Clemente JC et al (2013) Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science 342:621–624
Li R, Khafipour E, Krause DO et al (2012) Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 7:e51897
Lin X, Feng Y, Zhang H et al (2012) Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing. Environ Sci Technol 46:5764–5771
Xu L, Ravnskov S, Larsen J et al (2012) Soil fungal community structure along a soil health gradient in pea fields examined using deep amplicon sequencing. Soil Biol Biochem 46:26–32
Li J-G, Ren G-D, Jia Z-J, Dong Y-H (2014) Composition and activity of rhizosphere microbial communities associated with healthy and diseased greenhouse tomatoes. Plant Soil 1–11
Ciavatta C, Govi M, Antisari LV, Sequi P (1991) Determination of organic carbon in aqueous extracts of soils and fertilizers. Commun Soil Sci Plant Anal 22:795–807
JianGuo L, Wei Z, YanBin L et al (2009) Effects of long-term continuous cropping system of cotton on soil physical-chemical properties and activities of soil enzyme in oasis in Xinjiang. Sci Agric Sin 42:725–733
Shen Z, Zhong S, Wang Y et al (2013) Induced soil microbial suppression of banana fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur J Soil Biol 57:1–8
Mehlich A (1978) New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese and zinc 1. Commun Soil Sci Plant Anal 9:477–492
Wei Z, Yang X, Yin S et al (2011) Efficacy of bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl Soil Ecol 48:152–159
Zhou J, Wu L, Deng Y et al (2011) Reproducibility and quantitation of amplicon sequencing-based detection. ISME J 5:1303–1313
Schoch CL, Seifert KA, Huhndorf S et al (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109:6241–6246
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 6:e27310
Nilsson RH, Veldre V, Hartmann M et al (2010) An open source software package for automated extraction of ITS1 and ITS2 from fungal ITS sequences for use in high-throughput community assays and molecular ecology. Fungal Ecol 3:284–287
Tedersoo L, Nilsson RH, Abarenkov K et al (2010) 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188:291–301
Huang X, Madan A (1999) CAP3: a DNA sequence assembly program. Genome Res 9:868–877
Abarenkov K, Henrik Nilsson R, Larsson K-H et al (2010) The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytol 186:281–285
Karsch-Mizrachi I, Nakamura Y, Cochrane G (2012) The international nucleotide sequence database collaboration. Nucleic Acids Res 40:D33–D37
Lozupone C, Hamady M, Knight R (2006) UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinforma 7:371
Liu X, Zhang J, Gu T et al (2014) Microbial community diversities and taxa abundances in soils along a seven-year gradient of potato monoculture using high throughput pyrosequencing approach. PLoS ONE 9:e86610
Li C, Li X, Kong W et al (2010) Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 330:423–433
Buée M, Reich M, Murat C et al (2009) 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol 184:449–456
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415
Mendes R, Kruijt M, de Bruijn I et al (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100
Li R, Fernando DG, de Kievit T, et al. (2011) 454-Pyrosequencing reveals the influence of organic and conventional farming systems on beneficial bacterial communities to enhance plant health. Phytopathology. Amer Phytopathological Soc 3340 Pilot knob road, St Paul, MN 55121 USA, pp S102–S102
Jayasekhar M, Manonmani K, Justin CGL (2008) Development of integrated biocontrol strategy for the management of stem rot disease (Fusarium oxysporum f. sp. Vanillae) of Vanilla. Agric Sci Dig Abstr 28:109–111
Zhang N, Wu K, He X et al (2011) A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil 344:87–97
Antoun H, Beauchamp CJ, Goussard N et al (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204:57–67
Wang B, Yuan J, Zhang J et al (2013) Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol Fertil Soils 49:435–446
Zhang S, Raza W, Yang X et al (2008) Control of Fusarium wilt disease of cucumber plants with the application of a bioorganic fertilizer. Biol Fertil Soils 44:1073–1080
Bhai RS, Thomas J (2000) Phytophthora rot–a new disease of vanilla (Vanilla planifolia Andrews) in India. J Spices Aromat Crop 9:73–75
Zeng H, Ho H, Zheng F-C (2009) A survey of Phytophthora species on Hainan Island of South China. J Phytopathol 157:33–39
Girvan MS, Bullimore J, Pretty JN et al (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl Environ Microbiol 69:1800–1809
Rosenzweig N, Tiedje JM, Quensen JF et al (2012) Microbial communities associated with potato common scab-suppressive soil determined by pyrosequencing analyses. Plant Dis 96:718–725
Acknowledgments
This study was financially supported by the 973 project (2015CB150500), Innovative Research Team Development Plan of the Ministry of Education of China (IRT1256), and National Natural Science Foundation of China (no. 31201683). We would like to thank Personal Biotechnology Company (Shanghai, China) for their help with the pyrosequencing experiments and Spice and Beverage Research Institute (Wanning, China) for huge help to us in vanilla planting.
Conflict of Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 14 kb)
Table S2
(DOCX 14 kb)
Table S3
(DOCX 15 kb)
Fig. S1
Average relative abundance (%) of bacterial and fungal phyla in 4 time-series soil from vanilla fields. The “others” comprised the rare bacterial/fungal phyla (RA <0.1 %). (GIF 4 kb)
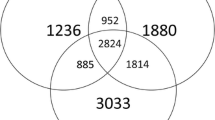
Fig. S2
Venn diagrams showed shared unique OTUs of bacteria and fungi in the soils from 4 time-series vanilla fields. “a”, “b”, “c” and “d” represent field with 1, 6, 11 or 21 years continuous cropping history, respectively. (GIF 3 kb)
Rights and permissions
About this article
Cite this article
Xiong, W., Zhao, Q., Zhao, J. et al. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb Ecol 70, 209–218 (2015). https://doi.org/10.1007/s00248-014-0516-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0516-0