Abstract
Background
This work aimed to investigate the potential pathways involved in the association between social and lifestyle factors, biomarkers of Alzheimer’s disease and related dementia (ADRD), and cognition.
Methods
The authors studied 2323 participants from the Memento study, a French nationwide clinical cohort. Social and lifestyle factors were education level, current household incomes, physical activity, leisure activities, and social network from which two continuous latent variables were computed: an early to midlife (EML) and a latelife (LL) indicator. Brain magnetic resonance imaging (MRI), lumbar puncture, and amyloid-positron emission tomography (PET) were used to define three latent variables: neurodegeneration, small vessel disease (SVD), and AD pathology. Cognitive function was defined as the underlying factor of a latent variable with four cognitive tests. Structural equation models were used to evaluate cross-sectional pathways between social and lifestyle factors and cognition.
Results
Participants’ mean age was 70.9 years old, 62% were women, 28% were apolipoprotein-ε4 carriers, and 59% had a Clinical Dementia Rating (CDR) score of 0.5. Higher early to midlife social indicator was only directly associated with better cognitive function (direct β = 0.364 (0.322; 0.405), with no indirect pathway through ADRD biomarkers (total β = 0.392 (0.351; 0.429)). In addition to a direct effect on cognition (direct β = 0.076 (0.033; 0.118)), the association between latelife lifestyle indicator and cognition was also mostly mediated by an indirect effect through lower neurodegeneration (indirect β = 0.066 (0.042; 0.090) and direct β = − 0.116 (− 0.153; − 0.079)), but not through AD pathology nor SVD.
Conclusions
Early to midlife social factors are directly associated with higher cognitive functions. Latelife lifestyle factors may help preserve cognitive functions through lower neurodegeneration.
Similar content being viewed by others

Introduction
Due to the continuous increase in life expectancy, a growing part of the population is expected to be at risk for severe cognitive impairment and age-related disorders such as Alzheimer’s disease and related dementias (ADRD), and there is an urgent need to accelerate research on ADRD prevention [1]. Several social and lifestyle factors, such as high educational level, higher socioeconomic status (SES), and engagement in stimulating activities (either mentally, physically, or socially), have been hypothesized to promote resilience against ADRD and cognitive decline [2,3,4,5,6,7,8]. However, the different mechanisms involved, as well as the role of ADRD biomarkers (i.e. amyloid-β and tau (AD biomarkers), cerebrovascular pathology, and neurodegeneration) in the association between these factors and cognitive function remain unclear. In addition, previous studies have often investigated social and lifestyle factors individually at one point in time, which may not entirely capture the interconnected nature of risk and protective factors against ADRD over the life course.
Results of studies investigating the impact of social and lifestyle factors on ADRD biomarkers have been mixed. Some have reported a lower level of amyloid plaques, tauopathies, neurodegeneration, or small vessel disease (SVD) pathology among individuals with more stimulating levels of social and lifestyle factors [9,10,11,12,13,14,15,16], while others showed no association between these factors and the level or change of AD biomarkers, brain volumes, or white matter hyperintensities (WMH) [9, 10, 16,17,18,19,20,21,22,23,24]. Mediation analysis using comprehensive disease burden measures is needed to improve our understanding of the different pathways linking social and lifestyle factors to cognitive functions. A few previous studies using a combination of factors have reported a mediated association between more stimulating levels of social and lifestyle factors and higher cognitive functions through better cerebrovascular health and lower neurodegeneration, but not through AD biomarkers such as amyloid-beta [13, 14, 18, 25].
The objective of our study was thus to investigate the associations between combined social and lifestyle factors at two different times over the life course (i.e. early to midlife and latelife respectively), multimodal ADRD biomarkers, and cognitive functions, through mediation analysis, in Memento, a French nationwide large clinical cohort.
Methods
Study sample
The Memento cohort is a prospective clinic-based study aiming at better understanding the natural history of ADRD and identifying new subtypes of the disease. Details of the study have been previously published [26]. The study sample was drawn from a defined population. Briefly, 2323 participants consulting within 28 French memory clinics and presenting with either isolated cognitive complaints or recently diagnosed mild cognitive impairment (MCI) were recruited from April 2011 to June 2014. MCI was defined as [1] performing 1 SD worse than the mean of the group with the same subject’s own age, sex and education level in one or more cognitive domains, this deviation being identified for the first time through cognitive tests performed recently (less than 6 months preceding screening phase), and [2] having a Clinical Dementia Rating (CDR) ≤ 0.5 and not being demented. Participants were examined at baseline and followed every 6 to 12 months up to 5 years. Baseline data collection during the face-to-face interview included socio-demographic characteristics, lifestyle factors, neurological and physical examination, and a full neuropsychological battery. Brain MRI (mandatory), lumbar puncture (LP) (optional), and 18F-fluorodeoxyglucose positon emission tomography (FDG-PET) (optional) were performed at baseline and every 2 years. Amyloid-PET scans were obtained as part of the MEMENTO-Amyging ancillary study.
Social and lifestyle variables
We selected a set of social and lifestyle factors available for the Memento participants and hypothesized to be cognitively stimulating and associated with cognitive functions during early to midlife and latelife [8].
Early to midlife social factors included:
-
- Education level defined in 4 categories: no diploma or primary school level, validated short secondary school level, validated long secondary school level, and some college or higher
-
- Occupations gathered by complexity level: lower (blue-collar workers: i.e. …), intermediate (white-collar workers: i.e. …), and higher complexity (executive positions: i.e. …)
-
- Monthly household income recorded in 8 categories ranging from 400–800€ to 6000€+
Latelife lifestyle factors included:
-
- Physical activity assessed with the International Physical Activity Questionnaire (IPAQ) [27], defined as low, moderate, and vigorous
-
- Leisure activity in 4 categories being quartiles (< 6; 6–7; 8–9; >9) according to the number of physical, cognitive, and social activities carried out at least once a week from a list of 15 (producing a score from 0 to 15)
-
- Social network index (SNI), adapted from The Berkman-Syme SNI, combining information on marital status, sociability (number of close relatives/friends), and membership in community organizations [28]. SNI ranged from 0 to 3, with higher scores corresponding to increasing social connectedness.
ADRD biomarkers
ADRD biomarkers were measured through either neuroimaging (MRI, FDG-PET, and amyloid-PET) or CSF.
All neuroimaging acquisitions and analyses were coordinated by the Center for Acquisition and Treatment of Images (CATI; cati-neuroimaging.com), a platform dedicated to the management of multicentre neuroimaging [29]. Scans were harmonized across centre, centralized, quality checked, and postprocessed to obtain standardized measurements.
MRI measures
Brain MRI was mandatory, and 86% of participants had a 3.0 Tesla MRI scan (vs 1.5 Tesla otherwise) at baseline. Neurodegeneration and white matter lesion measures were computed using automated procedures. Whole-brain and grey/white volumetry was performed using the method “segment” in SPM12 software. Hippocampal volumetry was performed with SACHA software [30, 31]. Cortical thickness was computed with the FreeSurfer software for each region of interest (ROI) of the Desikan-Killiany Atlas,[32, 33] and the AD cortical signature was estimated (including entorhinal, inferior temporal, middle temporal, inferior parietal, fusiform, and precuneus) [34]. White matter hyperintensity volume (WMHV) was estimated using WHASA software [35] complemented by a visual assessment of deep and periventricular lesions done centrally by two trained raters using the Fazekas scale [36].
FDG-PET
FDG-PET scans were acquired in 57% of participants 30 min after injection of 2 MBq/kg of 2-deoxy-2-18F-fluoro-d-glucose. All acquisitions consisted of 3 × 5-min frames. Images were then reconstructed using an iterative algorithm, and last, frames were realigned, averaged, and quality-checked. Mean FDG-PET uptake for a set of disease-specific ROIs (posterior cingulate cortex, inferior parietal lobule, precuneus and inferior temporal gyrus) inferred from the ADNI database was estimated [37]. Further details on the FDG-PET procedure are available in Additional file 1.
Amyloid-PET
Amyloid PET examinations (MEMENTO-Amyging substudy) were performed in 28% of participants using either Florbetapir (18F) or Flutemetamol (18F) radioligands. Florbetapir scans (3 × 5 min) were acquired 50 min after injection of 370 (± 10%) MBq. Flutemetamol scans (4 × 5 min) were acquired 90 min after injection of 185 (± 10%) MBq. Images were then reconstructed using an iterative algorithm. Frames were realigned, averaged, and quality-checked. Standard uptake value ratios (SUVR) for target areas such as the medial frontal cortex, temporal cortex, parietal cortex, posterior cingulate cortex, anterior cingulate cortex, and precuneus were calculated with respect to the entire cerebellum [38]. Further details are available in Additional file 1.
Cerebrospinal fluid sampling AD markers
LP was performed in 18% of participants at baseline. Each CSF sample was transferred to a local biobank within 4 h after collection and was centrifuged at 1000 × g at 4 °C for 10 min. CSF samples were aliquoted in polypropylene tubes (16 tubes of 250 μl) and stored at − 80 °C. All tubes were further shipped for storage in a centralized biobank (LAG-CRB, Pasteur Institut Lille, BB-0033–00,071). Measurements from CSF of amyloid-β 42 peptide (Aβ42), Aβ40, total tau, and phosphorylated tau (p-tau) were realized using the standardized commercially available INNOTEST sandwich enzyme-linked immunosorbent assay (Fujirebio, Ghent, Belgium).
Cognitive testing
At baseline, participants were administered a neuropsychological test battery that included the four following cognitive tests: (1) the Free and Cued Selective Reminding Test (FCSRT) [39], measuring verbal episodic memory. In this associative memory test, an individual has to learn 16 words by groups of four with each corresponding cue provided verbally by the tester (e.g. “fish” is the cue for the word “herring”). Here, we used the sum of the three free recalls: (2) the Verbal Fluency (VF) test [40], which consists in producing as many words (animals) as possible within 2 min, assessing lexical access and semantic memory; (3) the Trail Making Test B (TMT-B) [41], measuring attention and executive functioning by recording the time in seconds to complete the task; and (4) the Rey–Osterrieth Complex Figure test [42], assessing visuospatial and visuoconstructive abilities by reproducing complex drawing first by copying and then from memory assessed at 3 min. For the FCSRT, verbal fluency, and the Rey figure test, a higher score indicates better performance, whereas for the TMT-B test, a higher score (in seconds) indicates worse performance.
Statistical analysis
Participants’ characteristics at baseline were described and compared according to sex using analysis of variance (means) and χ2 tests to assess differences in means and proportions, respectively. A description of ADRD biomarker distribution across ages was also performed.
Social and lifestyle latent factors
The first step of our modelling approach consisted in the creation of two latent global indicators constituted of early to midlife social and latelife lifestyle factors. We used Item Response Theory (IRT) models, and more specifically the Graded Response Model (GRM), a class of latent variable models that links ordered polytomous manifest variables (i.e. response) to their underlying single latent trait of interest. Each individual’s response to an item of the trait is considered as a manifestation of this trait. The latent trait value of each participant can be thought as its “ability” at the time of data collection. Ability scores for each participant can be predicted from the GRM parameters and the participant’s responses. In this work, two different measures from two separate IRT models were established based on an a priori life course hypothesis. The first targeted early to midlife social factors, including education level, occupation complexity, and salary, while the second targeted latelife lifestyle factors, comprising physical activity, leisure activities, and social network at study entry. GRM fits were assessed using two-way margins and were deemed satisfactory. We then extracted and standardized predicted scores that ranged from − 2.33 to 1.87 and − 1.71 to 2.14 for early to midlife social and latelife lifestyle indicators, respectively, with higher values representing more stimulating levels of social and lifestyle factors. Additional information regarding the two latent indicators is reported in Additional file 2.
To assess further the robustness of the two latent indicator-related results, we performed the following two sensitivity analyses: first, as correlations between social network and other components were low, we repeated our original analysis excluding social network from the latelife lifestyle latent indicator. Second, we re-ran our main model after excluding the two physical items of the leisure activity questionnaire from the latelife lifestyle indicator.
Mediation analysis
A pathway analysis was conducted to explore relationships between the social and lifestyle indicators, ADRD biomarkers, and cognitive functions using structural equation models (SEM). The two latent indicators established during the first step were used as exposures of interest. We hypothesized three different pathways of actions of social and lifestyle indicators on cognition: SVD, AD-specific pathology, and neurodegeneration.
-
- SVD was represented by a latent variable constituted of WMH volumes (standardized on total intracranial volume (TIV) and log-transformed) and Fazekas scales of paraventricular and deep WMH (see Additional file 7: Fig. S1 for latent variable details).
-
- AD pathology was represented by a latent variable including CSF Ab42/Ab40 ratio, CSF p-tau (log transformed), and mean global SUVr amyloid-PET (log transformed and standardized by radioligand).
-
- Neurodegeneration was represented by a latent variable, comprising hippocampal volume (standardized on TIV and log-transformed), cortical thickness, brain parenchymal fraction, and SUVr FDG-PET. Latent variable indicators were reverse coded so that a higher score represented greater neurodegeneration.
The outcome of interest, cognitive functioning, was represented by a latent variable constituted by the following four cognitive tests: FCSRT, Animal Fluency, Rey figure test, and TMT-B (the latter was log-transformed and inversed so that higher scores correspond to higher performances). Higher values of the latent variable represent higher cognitive performances.
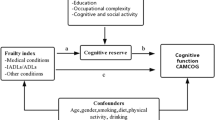
We built a SEM to test whether the association between latent life course social and lifestyle indicators and cognitive performances was mediated by AD pathology, SVD, and neurodegeneration biomarkers (Fig. 1). All potential paths were adjusted for potential confounders: age at baseline, sex, and APOE-e4 status. We used maximum likelihood estimators and full information maximum likelihood (FIML) to handle missing data under the missing at random hypothesis. Coefficients for direct and indirect effects are presented and standard errors for indirect effect parameters were computed using the Delta method. Standardized coefficients as well as 95% confidence intervals (CI) for endogenous variables (i.e. SVD, AD pathology, neurodegeneration, and cognition) and latent social and lifestyle indicators are reported. Only coefficients on associations going to a particular outcome are comparable.
Structural equation model representing the pathways involved in the association between social and lifestyle indicators and cognition. SI, social indicator; LI, lifestyle indicator; SVD, small vessel disease; AD, Alzheimer’s disease; CI, confidence interval. Each pathway was adjusted for age, sex, and APOE status, yet they were not represented here for clarity. Solid arrows represent associations. Dashed bidirectional arrows represent correlations. Bolded arrows represent significant associations. Estimates presented in the graph are standardized. P values: * < 0.05, ** < 0.01, *** < 0.001. Social and lifestyle latent indicators were obtained through a graded response model, and circles represent latent variables from SEM. Variables composition: early to midlife SI: education, occupational complexity, and salary; latelife LI: physical activity, leisure activities, and social network; SVD: white matter hyperintensity volume, paraventricular white matter lesions, and deep white matter lesions; AD pathology: CSF Aβ42/Aβ40 ratio, CSF phosphorylated Tau, and SUVr amyloid-PET; neurodegeneration: hippocampal volume, cortical thickness, SUVr FDG-PET, and brain parenchymal fraction; cognition: Verbal Fluency, Free and Cued Selective Reminding test, Trail making test B, and Rey figure test
Analyses were conducted in R (version 3.6.0), using ltm (version 1.1–1) [43] and lavaan (version 0.6–4) [44] packages.
Results
A description of the sample characteristics is presented in Table 1. Participants’ mean age at baseline was 70.9 years old and 61.9% were women. Twenty-eight percent were APOE-ɛ4 carriers and 59.2% had a CDR score of 0.5. Women had on average lower educational level, occupational complexity, income, and social network index score than men. As expected, markers of SVD, AD pathology, neurodegeneration, and cognition worsened with age (Additional file 3: Table S1).
Structural equation model
The final SEM model fit was acceptable with a root mean square error of approximation (RMSEA) of 0.049 (95% CI = 0.046–0.052), a standardized root mean square residual (SRMR) of 0.047, and a Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI) of 0.942 and 0.922, respectively. Figure 1 shows the full SEM with direct estimates. Factor loadings between indicators and latent common variables, as well as explained variances are presented in Additional file 4: Table S2. Residual correlations of the model remained low (Additional file 8: Fig. S2).
Estimates of mediation effects between social and lifestyle indicators and ADRD markers are presented in Table 2. A higher level of early to midlife latent social indicator was only directly associated with better cognitive function (βdirect = 0.364 (0.322; 0.405)). Indeed, the early to midlife social indicator was not associated with SVD and AD pathology nor neurodegeneration markers. A higher level of latelife latent lifestyle indicator was associated with better cognitive function, both directly (βdirect = 0.076 (0.033; 0.118)) and indirectly (βindirect = 0.077 (0.046; 0.108)). This association was mediated by a direct effect of the latelife lifestyle indicator on lower neurodegeneration (βdirect = − 0.116 (− 0.153; − 0.079); βindirect = 0.066 (0.042; − 0.090)). However, the latelife lifestyle indicator was not associated with AD pathology nor with SVD at the significance level. Total association between latent indicators and cognition was more than twice stronger for the early to midlife social indicator than for the latelife lifestyle indicator (βtotal equal to respectively 0.390 (0.351; 0.429) vs 0.153 (0.112; 0.193)). Higher AD pathology and neurodegeneration, but not SVD, were directly associated with lower cognitive function (βdirect_AD = − 0.264 (− 0.362; − 0.165) and βdirect_N = − 0.574 (− 0.665; − 0.483)). AD pathology, SVD, and neurodegeneration were all three positively correlated (Table 2). Sensitivity analysis excluding social network from the latelife lifestyle indicator yielded similar results compared to the primary analysis (Additional file 5: Table S3). In addition, analysis excluding physical leisure activity items showed results similar to the main analysis, with slightly higher effect sizes for the direct effect between the latelife lifestyle indicator and SVD and AD pathology, but not significant (Additional file 4: Table S4).
Associations between covariates and ADRD markers are presented in Table 3. Women had lower neurodegeneration (βdirect = − 0.603 (− 0.677; − 0.529)) and higher cognitive function (βtotal = 0.186 (0.100; 0.273)). Increasing age was directly associated with worse SVD, AD pathology, and neurodegeneration. It was associated with worse cognition in total (βtotal = − 0.042 (− 0.047; − 0.038)). APOE-e4 carrier status was directly associated with higher AD pathology (βdirect = 0.835 (0.703; 0.967)) and slightly with SVD (βdirect = 0.085 (− 0.004; 0.174)). It was indirectly associated with lower cognitive function through worse AD pathology (βindirect = − 0.338 (− 0.438; − 0.237)).
Discussion
In this cross-sectional analysis of data from a clinic-based study, we aimed at understanding the role of different ADRD biomarkers in the association between factors known to enhance resilience over the life course and cognitive function at older ages. Our results suggest that social and lifestyle factors favour cognitive performances, directly for both early and latelife factors, and also indirectly for latelife lifestyle factors, through lower neurodegeneration. Moreover, our work highlights the importance of investigating different factors over the life course.
More stimulating social and lifestyle factors have often been associated with improved cognitive performances and lower dementia risk [8]. However, it remains unclear how higher levels of such factors may lead to improved cognitive performances at older ages. Studies identifying underlying mechanisms through multiple markers of brain pathology are thus required to better understand the influence of these factors on cognitive and brain ageing, which could ultimately inform on innovative strategies for dementia prevention. In this work, we hypothesized that social (earlylife) and lifestyle (latelife) factors may improve cognitive function either directly through potential compensation strategies or indirectly by lowering brain pathology (through cerebrovascular lesion, AD pathology, or neurodegeneration).
First, regarding the potential direct effect of social and lifestyle factors on cognitive function, it has previously been evidenced that factors such as education level, physical activities, or cognitively enhancing activities are involved in resilience against ADRD, i.e. the ability of the brain to cope against adversity and maintain “normal” cognitive functions. For instance, these factors are hypothesized to influence cognitive reserve, which allows individuals to maintain cognitive functions in the presence of brain alterations [45]. In this work, both early to midlife latent social indicator and latelife lifestyle indicator were directly associated with higher cognitive performances, independently of AD or SVD pathologies, and neurodegeneration. Yet, the effect size for the association of the latelife lifestyle indicator with cognitive functions was 4.8 times lower than for the early to midlife social indicator. These results are in agreement with the hypothesis that intellectual stimulations throughout life (mostly through education, occupation, and SES) help in maintaining cognitive performances despite brain pathology, by enhancing compensation strategies and brain network efficiency, capacity, or flexibility [46].
Then, regarding the indirect impact of social and lifestyle factors on cognition through brain pathology, we did not observe a significant association between SVD and more stimulating latelife lifestyle, contrary to previous reports in favour of a protective effect of lifestyle factors on cerebrovascular pathology [9, 14, 47, 48]. Moreover, although some studies found an association between markers of cognitive reserve and AD pathology [10,11,12, 14, 16, 49, 50], our results are in agreement with evidence showing no associations between social and lifestyle factors measured at different times over the lifecourse and AD pathology [9, 17, 18, 20, 25]. Our results did not evidence any mediated pathways between the early to midlife social indicator and cognitive function. On the other side, the latelife lifestyle indicator showed an indirect effect on cognition, mostly through lower neurodegeneration, in line with previous reports linking physical activity or leisure activities with hippocampal volume or other brain volume markers [47, 51,52,53].
Overall, our results suggest that social and lifestyle factors may influence cognition through different mechanisms. Our findings do not support a contribution of social and lifestyle factors to resistance against AD and cerebrovascular pathology given the absence of associations between the latent indicators and AD pathology or SVD markers. The divergent results across studies could be explained by variations in pathology levels and participants’ clinical status, where participants with more advanced pathology may be exhibiting lower variability, potentially explaining the lack of associations in studies with MCI patients. Moreover, variability in definitions or timing of social and lifestyle factors makes comparisons across studies difficult. Latent factors allow capturing the comprehensive effect of multiple resilience-enhancing factors over the lifespan. Our a priori hypothesis, which appears to be a strength and novelty of this work, was that differentiating the time window of exposure to social and lifestyle factors that can influence resilience may be of importance when assessing their association with cognitive decline or dementia risk [8]. In addition, if early to midlife factors are most of the time not influenced by the latelife outcome, associations between latelife factors, such as physical, cognitive, or social activities, and latelife outcomes may be the results of reverse causality.
Limitations
This work has some limitations. First, given data are cross-sectional, causal inference must be discussed with caution, as reverse causality cannot be excluded and temporal ordering between ADRD biomarkers cannot be determined. In addition, our study sample consists of persons presenting with cognitive complaints or MCI in memory clinics. Results thus apply to a population of individuals at risk for ADRD and may not be generalizable to the general population. However, comprehensive neuroimaging and CSF markers being less widely available in population-based cohorts, our study provides valuable insights regarding the different mechanisms involved in cognitive ageing. Moreover, social and lifestyle factors were self-reported at baseline; thus, measurement bias cannot be excluded, especially for latelife lifestyle factors. Other factors (such as midlife lifestyle factors, childhood cognitive enrichment, or diet) may contribute to resilience over the life course but were not recorded. Finally, it could be argued that amyloid-PET and lumbar punctures were realized in smaller and potentially selected samples. However, to ensure the validity of our findings, we applied a Full Information Maximum Likelihood approach handling missing data under the missing at random hypothesis. Given the large number of information considered in the analysis, the missing at random assumption is plausible.
Despite these limitations, this study has important strengths and contributes to the sparse literature on mechanisms underlying the influences of social and lifestyle factors on cognition. The Memento study provides an adequate setting to investigate the mediating role of ADRD biomarkers, due to the availability of various neuroimaging and CSF markers. In addition, this study enabled us to assess different social and lifestyle factors, in line with the hypothesis that resilience is built over multiple experiences across the lifespan. Finally, structural equation models are a powerful tool to model complex relationships with brain and cognitive health as latent constructs and robustly estimate direct and indirect effects.
Conclusions
In conclusion, this work suggests that more stimulating levels of social and lifestyle factors may be associated with better cognitive function through different mechanisms over the life course. Our results support the hypothesis that investing in education and earlylife cognitive-enhancing activities may have an impact on cognitive health later in life. Complementary findings based on longitudinal evaluation of pathological markers and cognitive function could help to build new comprehensive strategies for dementia prevention.
Availability of data and materials
Memento data access request is available via the Dementia Platform UK Data Access appliance form (https://portal.dementiasplatform.uk/Apply) or via the Memento Secretariat (sophie.lamarque@u-bordeaux.fr).
References
Alzheimer’s Disease International. World Alzheimer Report: the global impact of dementia. London; 2015. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf.
Yates LA, Ziser S, Spector A, Orrell M. Cognitive leisure activities and future risk of cognitive impairment and dementia: systematic review and meta-analysis. Int Psychogeriatr. 2016;28(11):1791–806.
Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS ONE. 2012;7(6):e38268.
Members ECC, Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(Pt 8):2210–6.
Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: a systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510.
Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age-related cognitive decline: a review of recent research. Psychogeriatrics. 2015;15(2):154–62.
Penninkilampi R, Casey AN, Singh MF, Brodaty H. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2018;66(4):1619–33.
Fratiglioni L, Marseglia A, Dekhtyar S. Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference? Lancet Neurol. 2020;19(6):533–43.
Pettigrew C, Soldan A, Zhu Y, Cai Q, Wang M-C, Moghekar A, et al. Cognitive reserve and rate of change in Alzheimer’s and cerebrovascular disease biomarkers among cognitively normal individuals. Neurobiol Aging. 2020;88:33–41.
Almeida RP, Schultz SA, Austin BP, Boots EA, Dowling NM, Gleason CE, et al. Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol. 2015;72(6):699–706.
Arenaza-Urquijo EM, Bejanin A, Gonneaud J, Wirth M, La Joie R, Mutlu J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. 2017;59:72–9.
Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, et al. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol. 2012;69(5):623–9.
Schreiber S, Vogel J, Schwimmer HD, Marks SM, Schreiber F, Jagust W. Impact of lifestyle dimensions on brain pathology and cognition. Neurobiol Aging. 2016;40:164–72.
Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging. 2014;35(8):1873–82.
Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J Neurosci. 2014;34(25):8612–7.
Lo RY, Jagust WJ, Alzheimer’s Disease Neuroimaging I. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord. 2013;27(4):343–50.
Soldan A, Pettigrew C, Li S, Wang MC, Moghekar A, Selnes OA, et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol Aging. 2013;34(12):2827–34.
Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72(5):730–8.
Soldan A, Pettigrew C, Lu Y, Wang MC, Selnes O, Albert M, et al. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum Brain Mapp. 2015;36(7):2826–41.
Gidicsin CM, Maye JE, Locascio JJ, Pepin LC, Philiossaint M, Becker JA, et al. Cognitive activity relates to cognitive performance but not to Alzheimer disease biomarkers. Neurology. 2015;85(1):48–55.
Ko K, Byun MS, Yi D, Lee JH, Kim CH, Lee DY. Early-life cognitive activity is related to reduced neurodegeneration in Alzheimer signature regions in late life. Front Aging Neurosci. 2018;10:70.
Pettigrew C, Soldan A, Zhu Y, Wang MC, Brown T, Miller M, et al. Cognitive reserve and cortical thickness in preclinical Alzheimer’s disease. Brain Imaging Behav. 2017;11(2):357–67.
Shpanskaya KS, Choudhury KR, Hostage C Jr, Murphy KR, Petrella JR, Doraiswamy PM, et al. Educational attainment and hippocampal atrophy in the Alzheimer’s disease neuroimaging initiative cohort. J Neuroradiol. 2014;41(5):350–7.
Cook IA, Leuchter AF, Morgan ML, Dunkin JJ, Witte E, David S, et al. Longitudinal progression of subclinical structural brain disease in normal aging. Am J Geriatr Psychiatry. 2004;12(2):190–200.
Vemuri P, Lesnick TG, Knopman DS, Przybelski SA, Reid RI, Mielke MM, et al. Amyloid, vascular, and resilience pathways associated with cognitive aging. Ann Neurol. 2019;86(6):866–77.
Dufouil C, Dubois B, Vellas B, Pasquier F, Blanc F, Hugon J, et al. Cognitive and imaging markers in non-demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther. 2017;9(1):67.
Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–62.
Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204.
Operto G, Chupin M, Batrancourt B, Habert MO, Colliot O, Benali H, et al. CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14(3):253–64.
Chupin M, Hammers A, Liu RS, Colliot O, Burdett J, Bardinet E, et al. Automatic segmentation of the hippocampus and the amygdala driven by hybrid constraints: method and validation. Neuroimage. 2009;46(3):749–61.
Chupin M, Mukuna-Bantumbakulu AR, Hasboun D, Bardinet E, Baillet S, Kinkingnehun S, et al. Anatomically constrained region deformation for the automated segmentation of the hippocampus and the amygdala: method and validation on controls and patients with Alzheimer’s disease. Neuroimage. 2007;34(3):996–1019.
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80.
Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016;11:802–12.
Samaille T, Fillon L, Cuingnet R, Jouvent E, Chabriat H, Dormont D, et al. Contrast-based fully automatic segmentation of white matter hyperintensities: method and validation. PLoS ONE. 2012;7(11):e48953.
Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13(Suppl 2):31–6.
Toussaint PJ, Perlbarg V, Bellec P, Desarnaud S, Lacomblez L, Doyon J, et al. Resting state FDG-PET functional connectivity as an early biomarker of Alzheimer’s disease using conjoint univariate and independent component analyses. Neuroimage. 2012;63(2):936–46.
Habert M-O, Bertin H, Labit M, Diallo M, Marie S, Martineau K, et al. Evaluation of amyloid status in a cohort of elderly individuals with memory complaints: validation of the method of quantification and determination of positivity thresholds. Ann Nucl Med. 2018;32(2):75–86.
Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–3.
Thurstone LL. Psychophysical analysis. By L.L. Thurstone, 1927. Am J Psychol. 1987;100(3–4):587–609.
Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14.
Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test Arch Neurol. 1978;35(6):364–7.
Rizopoulos D. ltm: an R package for latent variable modeling and item response analysis. J Stat Softw. 2006;17(1):1–25.
Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(1):1–36.
Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305-11. https://doi.org/10.1016/j.jalz.2018.07.219. Epub 2020 Jan 6.
Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci (Regul Ed). 2013;17(10):502–9.
Casaletto KB, Rentería MA, Pa J, Tom SE, Harrati A, Armstrong NM, et al. Late-life physical and cognitive activities independently contribute to brain and cognitive resilience. J Alzheimers Dis. 2020;74(1):363–76.
Valenzuela MJ, Matthews FE, Brayne C, Ince P, Halliday G, Kril JJ, et al. Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol Psychiatry. 2012;71(9):783–91.
Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83(19):1753–60.
Oveisgharan S, Wilson RS, Yu L, Schneider JA, Bennett DA. Association of early-life cognitive enrichment with Alzheimer disease pathological changes and cognitive decline. JAMA Neurology. 2020;77(10):1217–24.
Valenzuela MJ, Sachdev P, Wen W, Chen X, Brodaty H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS ONE. 2008;3(7):e2598.
Neth BJ, Graff-Radford J, Mielke MM, Przybelski SA, Lesnick TG, Schwarz CG, et al. Relationship between risk factors and brain reserve in late middle age: implications for cognitive aging. Frontiers in Aging Neuroscience. 2019;11:355.
Arenaza-Urquijo EM, de Flores R, Gonneaud J, Wirth M, Ourry V, Callewaert W, et al. Distinct effects of late adulthood cognitive and physical activities on gray matter volume. Brain Imaging Behav. 2017;11(2):346–56.
Acknowledgements
The full list of names of the Memento study group is presented in Additional file 9.
Funding
The MEMENTO cohort is funded by the Fondation Plan Alzheimer (Alzheimer Plan 2008–2012) and the French Ministry of Research (MESRI, DGRI) through the Plan Maladies Neurodégénératives (2014–2019). This work was also supported by CIC 1401‐EC, Bordeaux University Hospital (CHU Bordeaux, sponsor of the cohort), Inserm, and the University of Bordeaux. The MEMENTO cohort has received funding support from AVID, GE Healthcare, and FUJIREBIO through private–public partnerships. The Insight-PreAD substudy was promoted by INSERM in collaboration with the Institut du Cerveau et de la Moelle Epinière, Institut Hospitalo-Universitaire, and Pfizer and has received support within the “Investissement d’Avenir” (ANR-10-AIHU-06) programme. This work was undertaken using resources on the Dementias Platform UK (DPUK) Data Portal; the Medical Research Council supports DPUK through grant MR/L023784/2. Sponsor and funders were not involved in the study conduct, analysis, or interpretation of data nor in the writing of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
LG designed and conceptualized the study, analysed and interpreted the data, and drafted the manuscript for intellectual content. CPL interpreted the data and revised the manuscript for intellectual content. JFM, MOH, ML, and MC designed and conceptualized the study and revised the manuscript for intellectual content. BD, CP, OH, AG, MC, CA, RD, TJ, CB, AJ, IRL, and JP had a major role in the acquisition of the data and revised the manuscript for intellectual content. GC and CD designed and conceptualized the study, interpreted the data, and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent. The MEMENTO cohort protocol has been approved by the local ethics committee (“Comité de Protection des Personnes Sud-Ouest et Outre Mer III”; approval number 2010-A01394-35) and was registered in ClinicalTrials.gov (Identifier: NCT01926249).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
PET measurement additional information
Additional file 2:
Early to midlife ‘social factors’ and latelife ‘lifestyle factors’ latent measures additional information
Additional file 3:
Table S1. Description of ADRD biomarkers measures by age group, the Memento study
Additional file 4:
Table S2. Estimates of the latent variables’ measurement equations from the structural equation model.
Additional file 5:
Table S3. Estimates of the direct and indirect effects of social and lifestyle indicators on ADRD biomarkers and cognitive performance using structural equation models, excluding social network from the lifestyle indicator.
Additional file 6:
Table S4. Estimates of the direct and indirect effects of social and lifestyle indicators on ADRD biomarkers and cognitive performance using structural equation models, excluding physical leisure activities items.
Additional file 7:
Fig. S1. Detailed latent variables composition.
Additional file 8:
Fig. S2. Residual correlations of the structural equation model.
Additional file 9:
Memento Cohort Study Group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Grasset, L., Proust-Lima, C., Mangin, JF. et al. Explaining the association between social and lifestyle factors and cognitive functions: a pathway analysis in the Memento cohort. Alz Res Therapy 14, 68 (2022). https://doi.org/10.1186/s13195-022-01013-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-022-01013-8