Abstract
Objective
Our aim is to validate the process steps implemented by the French CATI platform to assess amyloid status, obtained from 18F-Florbetapir PET scans, in a cohort of 318 cognitively normal subjects participating in the INSIGHT-preAD study. Our objective was to develop a method with partial volume effect correction (PVEC) on untransformed PET images, using an automated pipeline (“RACHEL”) adapted to large series of patients and including quality checks of results.
Methods
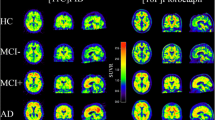
We compared RACHEL using different options (with and without PVEC, different sets of regions of interest), to two other methods validated in the literature, referred as the “AVID” and “CAEN” methods. A standard uptake value ratio (SUVR) was obtained with the different methods for participants to another French study, IMAP, including 26 normal elderly controls (NEC), 11 patients with mild cognitive impairment (MCI) and 16 patients with Alzheimer’s disease (AD). We determined two cutoffs for RACHEL method by linear correlation with the other methods and applied them to the INSIGHT-preAD subjects.
Results
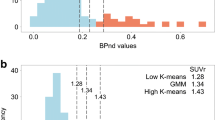
RACHEL including PVEC and a combination of the whole cerebellum and the pons as a reference region allowed the best discrimination between NEC and AD participants. A strong linear correlation was found between RACHEL and the other two methods and yielded the two cutoffs of 0.79 and 0.88. According to the more conservative threshold, 19.8% of the INSIGHT-preAD subjects would be considered amyloid positive, and 27.7% according to the more liberal threshold.
Conclusions
With our method, we clearly discriminated between NEC with negative amyloid status and patients with clinical AD. Using a linear correlation with other validated cutoffs, we could infer our own positivity thresholds and apply them to an independent population. This method might be useful to the community, especially when the optimal cutoff could not be obtained from a population of healthy young adults or from correlation with post-mortem results.






Similar content being viewed by others
References
Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–16.
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323.
Operto G, Chupin M, Batrancourt B, Habert MO, Colliot O, Benali H, et al. CATI: A large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14(3):253–64.
Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (Florbetapir [corrected] F 18). J Nucl Med. 2010;51(6):913–20.
Besson FL, La Joie R, Doeuvre L, Gaubert M, Mézenge F, Egret S, et al. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J Neurosci. 2015;35(29):10402–11.
La Joie R, Landeau B, Perrotin A, Bejanin A, Egret S, Pélerin A, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer’s and semantic dementia-targeted networks. Neuron. 2014;81(6):1417–28.
Habert MO, Marie S, Bertin H, Reynal M, Martini JB, Diallo M, et al. Optimization of brain PET imaging for a multicentre trial: the French CATI experience. EJNMMI Phys. 2016;3(1):6.
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of Florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305(3):275–83.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with Florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669–78.
Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, et al. Performance characteristics of amyloid PET with Florbetapir F 18 in patients with Alzheimer’s disease and cognitively normal subjects. J Nucl Med. 2012;53(3):378–84.
Chételat G, La Joie R, Villain N, Perrotin A, de La Sayette V, Eustache F, Vandenberghe R. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2013;2:356–65.
La Joie R, Perrotin A, Barré L, Hommet C, Mézenge F, Ibazizene M, et al. Region-specific hierarchy between atrophy, hypometabolism, and β-amyloid (aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012;32(46):16265–73.
Thomas BA, Erlandsson K, Modat M, Thurfjell L, Vandenberghe R, Ourselin S, Hutton BF. The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2011;38(6):1104–19.
Brendel M, Högenauer M, Delker A, Sauerbeck J, Bartenstein P, Seibyl J, et al. Improved longitudinal [(18)F]-AV45 amyloid PET by white matter reference and VOI-based partial volume effect correction. Neuroimage. 2015;108:450–9.
Chen K, Roontiva A, Thiyyagura P, Lee W, Liu X, Ayutyanont N, et al. Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med. 2015;56(4):560–6.
Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, et al. Amyloid PET imaging in Alzheimer’s disease: A comparison of three radiotracers. Eur J Nucl Med Mol Imaging. 2014;41(7):1398–407.
Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, Sherwin P. Automated quantification of 18f-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014;55(10):1623–8.
Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319–29.
Villemagne VL, Mulligan RS, Pejoska S, Ong K, Jones G, O’Keefe G, et al. Comparison of 11c-pib and 18f-florbetaben for aβ imaging in ageing and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39(6):983–9.
Jagust WJ. Amyloid imaging: Liberal or conservative? Let the data decide. Arch Neurol. 2011;68(11):1377–8.
Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh compound-b positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138(Pt 7):2020–33.
Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, Jagust WJ. Not quite PIB-positive, not quite PIB-negative: Slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59(2):1152–60.
Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(Pt 11):2837–44.
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–17.
Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–83.
Acknowledgements
We sincerely thank Alain Giron for helping with the statistical analyses and Anne Bertrand for her input on the manuscript. INSIGHT-AD study group Audrain C, Bakardjian H, Benali H, Bertin, H, Boukadida L, Cacciamani F, Causse-Lemercier V, Cavedo E, Chiesa P, Colliot O, Dos Santos A, Dubois B, Durrleman S, Epelbaum S, Gagliardi G, Genthon R, Habert M-O, Hampel H, Jungalee N, Kas A, Lehericy S, Lamari F, Letondor C, Levy M, Lista S, Mochel F, Nyasse F, Poisson C, Potier MC, Revillon M, Rojkova K, Roy P, Santos-Andrade K, Santos A, Simon V, Sole M, Tandetnik C, Thiebaud De Schotten M.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Research involving human participants
A national ethics committee approved the INSIGHT study (ANSM 130134B-31), and all subjects gave written informed consent before their inclusion in the study. The IMAP study was approved by a regional ethics committee (Comité de Protection des Personnes Nord-Ouest III) and all participants gave written informed consent to the study prior to the investigation.
Funding
The INSIGHT-preAD study is supported by the IHU-A-ICM, Investissement d’Avenir from the French Ministry of Health, the French Foundation Plan-Alzheimer, Pfizer and AVID/Lilly companies. The French Foundation Plan-Alzheimer supports the CATI. Harald Hampel is supported by the AXA Research Fund, the Fondation Université Pierre et Marie Curie and the “Fondation pour la Recherche sur Alzheimer”, Paris, France. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06 (Agence Nationale de la Recherche-10-IA Institut Hospitalo-Universitaire-6).
Additional information
Members of INSIGHT-AD study group are listed in acknowledgements.
Rights and permissions
About this article
Cite this article
Habert, MO., Bertin, H., Labit, M. et al. Evaluation of amyloid status in a cohort of elderly individuals with memory complaints: validation of the method of quantification and determination of positivity thresholds. Ann Nucl Med 32, 75–86 (2018). https://doi.org/10.1007/s12149-017-1221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-017-1221-0