Abstract
Objective
Increased expression of the amino acid transporter solute Carrier Family 7 Member 5 (SLC7A5) has been observed in neoplastic cells during hypoxic conditions in vitro, indicating an adaptation for cell survival. To further explore this, we evaluated hypoxia-mimetic by CoCl2 as a model for hypoxia in breast cancer cell lines and the effect on SLC257A5 expression. Four different breast cancer cell lines (MCF7, T-47D, BT-474 and ZR-75-1) were exposed to 100 µM CoCl2 for 48 h. Subsequently, cell viability, gene- and protein expression analyses were performed.
Results
The gene expression of VEGF, a marker of hypoxia, was significantly elevated in all four cell lines compared to the control (p < 0.0001), indicating that CoCl2 exposure generates a hypoxic response. Moreover, CoCl2 exposure significantly upregulated SLC7A5 gene expression in T-47D (p < 0.001), BT-474 (p < 0.0001) and ZR-75-1 (p < 0.0001) cells, as compared to vehicle control. Immunofluorescence staining showed increased SLC7A5 protein expression in MCF7, T-47D and BT-474 cells compared to vehicle control. This report suggests that hypoxia-mimetic by CoCl2 can be used as a simple model for inducing hypoxia in breast cancer cell lines and in fact influence SLC7A5 gene and protein expression in vitro.
Similar content being viewed by others
Background
An increasing number of studies have associated the transmembrane amino acid transporter SLC7A5, also known as large neutral amino acid-transporter 1 (LAT1), with worse outcome in cancer [1]. As SLC7A5 relates to unfavorable prognostic factors and chemoresistance, the protein may be a potential target for treatment with inhibitors [2]. SLC7A5 plays a crucial role in transporting amino acids, such as leucine, tyrosine and tryptophan, which are required for sustaining biological functions. Upregulation of SLC7A5 provides cancer cells with advantages including increased access to necessary building blocks for protein synthesis. Additionally, SLC7A5 activates the nutrient signaling pathway Mechanistic Target of Rapamycin Kinase Complex 1 (mTORC1) leading to changes in cellular metabolism [3]. The expression of SLC7A5 has been shown to be regulated by the oncogenic c-Myc in pancreatic cancer cells in vitro [4], and may be altered as a result from adaption to hypoxic microenvironment. In a previous study, we found that SLC7A5 is positively correlated to hypoxia inducible factor (HIF) 1 in clinical breast cancer samples [5]. The upregulation of SLC7A5 appears to be predominantly linked to cell survival in estrogen receptor positive (ER+) breast cancer subtypes, supporting amino acid metabolism during nutritional stress [6, 7]. Morotti et al. has shown increased SLC7A5 mRNA levels in breast cancer cell lines during hypoxic conditions (0.1% O2) [8]. Hypoxia-mimetic agents such as CoCl2 is a suggested in vitro model [9, 10]. For this report, we aimed to evaluate cellular responses to hypoxia-mimetic by CoCl2 in ER+ breast cancer cell lines and the possible influence on SLC7A5 expression.
Methods
Cell culture conditions
The ER+ breast cancer cell lines MCF7 (HTB-22™), T-47D (HTB-133™), BT-474 (HTB-20™) and ZR-75-1 (CRL-1500™) were obtained through the American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). The cells were cultured in RPMI-1640 GlutaMAX™ medium (Gibco, Thermo Fisher Scientific, Waltham, MA, U.S.A.) supplemented with 10% fetal bovine serum (FBS), 0.01 mg/ml human recombinant insulin (Gibco) and penicillin (50 U/ml)-streptomycin (50 µg/ml) (Gibco). Cells were cultured in humidified atmosphere with 5% CO2 at 37 °C.
Cell viability assay
The in vitro effect of CoCl2 on cell viability was evaluated using the CellTiter Blue® assay (Promega, Nacka, Sweden) according to manufacturer’s instructions. Cells were seeded onto 96-well plates (104 cells/well). After 24 h, the cells were exposed to different concentrations of CoCl2 (Sigma-Aldrich, Saint Louis, MO, U.S.A.), dissolved in sterile Milli-Q H2O, varying from 0.1 to 1000 µM in triplicate for 48 h. The viability analysis was repeated once using cells from a different passage (n = 6). The effect from the CoCl2 was controlled against medium complemented with 0.1% sterile Milli-Q H2O (hereafter H2O). Fluorescence was measured using the Cytation™ 3 Cell Imaging Multi-Mode Reader (BioTek Instruments, Winooski, VT, U.S.A.) together with the Gen5 Software (BioTek).
Gene expression analysis
The cells were seeded onto six-well plates with a density of 3 × 105 cells per well. After 24 h the medium was changed to new medium with either 100 µM of CoCl2, based on viability test and previously established concentration for cell culture use [11], or 0.1% H2O as a vehicle control, and incubated further 48 h. The experiment was set in triplicate and was repeated once using cells from a different passage (n = 6). Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA (500 ng) was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit® (Applied Biosystems, Foster City, CA, USA) with a 2720 Thermal Cycler (Applied Biosystems). Gene expression analyses were run on duplicate sample cDNA (10 ng) with mixture of TaqMan gene expression assays, targeting SLC7A5 (Hs01001183_m1), MKI67 (Hs00606991_m1), MTOR (Hs00234508_m1) as well as the established hypoxia markers VEGF (Hs00900055_m1) and HIF1A (Hs00153153_m1) and TaqMan Fast Advanced Master Mix (Applied Biosystems). The mixtures and samples were set onto MicroAmp™ Optical 384-well plates and underwent 40 cycles of amplification using QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems) according to manufacturer’s protocol. Extraction Non-Template Control (ENTC), Reverse Transcriptase negative (RT-) control, Non-Template Control (NTC) as well as three internal controls were run on each plate. The quantification cycle (Cq) threshold was automatically set and the expression of the target genes was normalized against a mean of the two reference genes (TOP1, Hs00243257_m1 and CYC1 Hs00357717_m1). Duplicate samples with a standard deviation (SD) value of ≥ 0.167 were re-analyzed. Fold change gene expression (CoCl2 vs. control) was calculated using the method 2− ΔΔCq [12].
Immunofluorescence staining
The cells were cultured in 8-well Permanox® Chamber Slides™ (Lab-Tek®, Thermo Fisher) at a density of 20 000 cells/well for 24 h and then exposed to either 100 µM of CoCl2 or 0.1% H2O for another 48 h. The Image-IT Fixation/Permeabilization Kit (ThermoFisher Scientific) was used followed by 1 h incubation with a rabbit monoclonal anti-SLC7A5 antibody diluted 1:500 (#ab208776, Abcam Cambridge, UK) and then 1 h with AffiniPure anti-rabbit Cy™3-labeled secondary antibody 1:400 (#711-165-152, Jackson ImmunoResearch Europe Ltd, Cambridge, UK). Nuclei were stained with 1 µg/mL DAPI (ThermoFisher Scientific) and F-actin with ActinGreen™ 488 ReadyProbes™ Reagent (AlexaFluor™ 488 phalloidin, Invitrogen, Carlsbad, CA, U.S.A.) for 15 min. All reactions were carried out at room temperature and the cells were washed with PBS + 0.1% Tween20 (Sigma-Aldrich, Saint Louis, MO, U.S.A.) between all steps. Confocal images were acquired using a TCS SP8 confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) and the software Leica Application Suite X version 3.4. All images were collected using identical instrument settings. The level of integrated density of SLC7A5 (mean gray value * area) was measured in raw images using the open source software Fiji (ImageJ) [13]. Briefly, five circled areas were positioned in different cell regions and three areas outside of the cells (background correction) was measured. The corrected total cell fluorescence was calculated in each region with SLC7A5 expression by subtracting the area * mean gray value of background readings from the integrated density.
Statistical analyses
For differences in cell viability and corrected total cell fluorescence, Mann-Whitney U-test was used. The results are presented as median values and 95% confidence intervals (CI). Differences in fold change gene expression were tested using multiple t-test with the Holm-Sidak method for correction of multiple comparisons with results presented as adjusted p-values and graphs with mean values and standard deviations (SD). Data analysis was performed using GraphPad Prism version 7.03 (GraphPad Software, La Jolla, CA, U.S.A.). For all analyses, p-values < 0.05 were considered statistically significant. In figures, asterisks represent the following p-values; * = p < 0.05, ** = p < 0.01, *** = p < 0.001 and **** = p < 0.0001.
Results
Effect of CoCl2 on cell viability
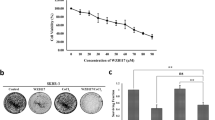
Cell viability was analyzed in order to assess if CoCl2 introduced toxic effects (Fig. 1A). MCF7 cells exposed to 100 µM CoCl2 did not show any difference in viability compared to cells exposed to 0.1% H2O, though viability decreased to 86% in T-47D cells (p < 0.05, Fig. 1B). In BT-474 cells the cell viability increased to 141% (p < 0.01) and in ZR-75-1 cells to 107% (p < 0.01, Fig. 1B).
The effect of CoCl2 on viability. (A) Cell viability of MCF7, T-47D, BT-474 and ZR-75-1 cells in % after 48 h exposure to 0.1–1000 µM CoCl2. The bars and whiskers represent mean values with 95% confidence intervals (95% CI). The fluorescence value (i.e. viability) in each biological replicate (n = 6) was tested against the mean of the controls (n = 6). The mean of the controls was set to 100%. (B) Comparison of viability at 100 µM CoCl2 in MCF7 (p = 0.82), T-47D (p = 0.015), BT-474 (p = 0.0022) and in ZR-75-1 (p = 0.0022). The bars represent median values and the whiskers represent 95% CI. * = p < 0.05, ** = p < 0.01, ns = non-significant
Effect of hypoxia-mimetic on gene expression
After 48 h of exposure to CoCl2, the hypoxia marker VEGF showed a significant increase of at least 2-fold in all cell lines (Fig. 2), with the lowest increase observed in MCF7 cells and the highest in BT474 cells. HIF1A was found to be increased only in ZR-75-1 cells (mean FC = 1.88, SD = 0.22, p < 0.01). No statistical differences in gene expression were observed for MKI67 or mTOR, although MKI67 appeared to decrease in all cell lines, disregarding the high standard deviations. SLC7A5 expression showed a significant increase in T-47D, BT-474 and ZR-75-1. However, there was no significant change in SLC7A5 expression in MCF7 cells. Compared to control cells, SLC7A5 exhibited a mean FC of 1.92 (SD = 0.26, p < 0.001) in T47D cells, 1.82 (SD = 0.071, p < 0.0001) in ZR-75-1 cells and 2.07 (SD = 0.30, p < 0.0001) in BT-474 cells.
The effect of CoCl2 on gene expressions. The relative gene expression of VEGF, HIF1A, Ki67, mTOR and SLC7A5 in MCF7 (n = 6), T-47D (n = 6), BT-474 (n = 6) and ZR-75-1 cells (n = 6). The bars represent mean values and the whiskers represent the standard deviation. Fold change gene expression is shown on the Y-axis and adjusted p-values < 0.01 are represented by **, < 0.001 by *** and < 0.0001 by ****
Effect of hypoxia-mimetic on SLC7A5 protein expression
The cell lines MCF7, T-47D and ZR-75-1 demonstrated higher basal expression of SLC7A5 than BT-474 (Fig. 3). Exposure to CoCl2 for 48 h resulted in significant increase in SLC7A5 protein expression in MCF7, T-47D and BT-474 cells. In ZR-75-1 cells, no significant difference was observed.
The effect of CoCl2 on SLC7A5 protein expression. Raw confocal images of SLC7A5 expression (gray) in MCF7 cells (upper left), T-47D cells (upper right), BT-474 cells (lower left) and ZR-75-1 cells (lower right). The white scale bars in bottom left corners of microscope images represent 50 μm. The graphs show median corrected total cell fluorescence (bars) with interquartile range (whiskers). p-values < 0.01 are represented by **. Ns = non-significant
Discussion
In this report, we evaluated hypoxia-mimetic by CoCl2 in the ER+ breast cancer cell lines MCF7, T-47D, BT-474 and ZR-75-1. The use of CoCl2 as a hypoxia model is well-established as it stabilizes HIF-1α and HIF-2α under normal oxygen levels [14]. Exposure to 100 µM CoCl2 resulted in increased gene expression of SLC7A5 in three out of four cell lines, with no significant change in MCF7 cells. Nevertheless, protein expression of SLC7A5 increased significantly in MCF7, T-47D and BT-474 cells, but not in ZR-75-1 cells. These findings indicate that SLC7A5 expression is regulated by hypoxic condition.
Cellular hypoxia occurs when oxygen levels range between 0.5 and 2% [15]. Morotti et al., explored endocrine resistance and the effect of hypoxia at 0.1% O2 in ten different breast cancer cell lines [8]. They consistently observed SLC7A5 upregulation in nine cell lines, including the four used in present report. SLC7A5 upregulation may serve as an adaptation to hypoxia, which has previously been suggested in clinical breast cancer samples [5].
Vascular endothelial growth factor (VEGF) is a recognized marker of cellular hypoxia at both translational and transcriptional level [15]. We found that all four cell lines showed significantly increased VEGF gene expression following CoCl2 exposure. The gene expression of HIF1A was only upregulated in ZR-75-1 cells. Decreased oxygen levels affect HIF-1α protein stability and subcellular localization rather than its gene expression, which could explain this result [16]. As VEGF and HIF-1α expression do not necessarily correlate, our findings are consistent with the existing literature and suggest that the CoCl2- model reliably attains a hypoxia-like condition [17, 18].
Rana et al. (2019) used CoCl2 as a hypoxic model in MCF7 and MDA-MB-231 cells and demonstrated that the proliferation increased, reaching its peak at concentrations of 150 µM for MCF7 and 25 µM for MDA-MB-231 [18]. Our results showed increased viability of BT-474 and ZR-75-1 at 100 µM CoCl2, while MCF7 and T-47D did not show the same response. All four cell lines are classified as ER+, with MCF7 and T-47D being HER2-negative (luminal A) and BT-474 and ZR-75-1 being HER2-positive (luminal B) [19, 20]. CoCl2 activates targets downstream of HER2 (PI3K/Akt) which could possibly explain the increased viability observed in the luminal B cell lines [21]. However, we were not able to show any statistical significance change in gene expression of the proliferation marker MKI67.
mTOR serves as a downstream mediator of cellular response to environmental factors such as nutrient availability and oxygen levels. It interacts with subunits to form mTOR complex 1 and 2 (mTORC1/mTORC2), with mTORC1 known for regulating cell growth, autophagy, mitochondrial respiration and amino acid metabolism [22]. Importantly, SLC7A5 imports the amino acid leucine, which promotes mTORC1 activity [23]. Although the activity of mTOR signaling typically decreases in hypoxic cells, our results did not demonstrate any changes in mTOR gene expression following CoCl2 exposure. In conclusion our study confirms increased SLC7A5 levels in breast cancer cells under hypoxia-mimetic conditions induced by CoCl2, in line with existing research. This suggests a potential of CoCl2 to upregulate SLC7A5 and exploring LAT1-inhibiting drug impacts on breast cancer cells, offering valuable insights for future studies.
Limitations
There are several limitations of using CoCl2 as a model for hypoxia in cell culture experiments. CoCl2 does not replicate all mechanisms involved in the cellular properties presented at low oxygen levels. True hypoxia triggers intricate signaling pathways leading to acute and chronic effects, molecular changes and cellular adaption. While CoCl2 does mimic certain aspects of these responses, it falls short of reproducing the full spectrum of changes associated with true hypoxia. Also, the use of CoCl2 may introduce unpredictable off-target effect. There are also limitations of extrapolating results from cell culture experiments to an in vivo setting. A true hypoxic environment characterized by low oxygen levels, coupled with the presence of stromal components surrounding tumor cells, might induce changes, such as metabolic reprogramming, that cannot be adequately observed using a chemical inducer of a hypoxia-like condition.
Data Availability
All raw data are available from the corresponding author on reasonable request.
Abbreviations
- CoCl2 :
-
Cobalt chloride
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HIF:
-
Hypoxia–inducible factor
- LAT1:
-
Large neutral amino acids transporter small subunit 1
- MKI67:
-
Marker of proliferation Ki–67
- mTOR:
-
Mechanistic target of rapamycin
- qPCR:
-
Quantitative polymerase chain reaction
- SLC7A5:
-
Solute carrier family 7 member 5
- VEGF:
-
Vascular endothelial growth factor
References
Zhang J, Xu Y, Li D, Fu L, Zhang X, Bao Y, et al. Review of the correlation of LAT1 with Diseases: mechanism and treatment. Front Chem. 2020;8:564809.
Sato M, Harada-Shoji N, Toyohara T, Soga T, Itoh M, Miyashita M, et al. L-type amino acid transporter 1 is associated with chemoresistance in Breast cancer via the promotion of amino acid metabolism. Sci Rep. 2021;11(1):589.
Cormerais Y, Giuliano S, LeFloch R, Front B, Durivault J, Tambutte E, et al. Genetic disruption of the multifunctional CD98/LAT1 Complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and Tumor Growth. Cancer Res. 2016;76(15):4481–92.
Hayashi K, Jutabha P, Endou H, Anzai N. c-Myc is crucial for the expression of LAT1 in MIA Paca-2 human Pancreatic cancer cells. Oncol Rep. 2012;28(3):862–6.
Tornroos R, Tina E, Gothlin Eremo A. SLC7A5 is linked to increased expression of genes related to proliferation and hypoxia in estrogen–receptor–positive Breast cancer. Oncol Rep. 2022;47(1).
El Ansari R, Craze ML, Miligy I, Diez-Rodriguez M, Nolan CC, Ellis IO et al. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative Breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 2018;20.
Saito Y, Li L, Coyaud E, Luna A, Sander C, Raught B, et al. LLGL2 rescues nutrient stress by promoting leucine uptake in ER(+) Breast cancer. Nature. 2019;569(7755):275–9.
Morotti M, Bridges E, Valli A, Choudhry H, Sheldon H, Wigfield S, et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in Breast cancer. Proc Natl Acad Sci USA. 2019;116(25):12452–61.
Nowak-Stepniowska A, Osuchowska PN, Fiedorowicz H, Trafny EA. Insight in Hypoxia-Mimetic Agents as Potential Tools for Mesenchymal Stem Cell Priming in Regenerative Medicine. Stem Cells Int. 2022;2022.
Lee HR, Leslie F, Azarin SM. A facile in vitro platform to study cancer cell dormancy under hypoxic microenvironments using CoCl(2). J Biol Eng. 2018;12:12.
Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Visualized Experiments: JoVE. 2011(54).
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Munoz-Sanchez J, Chanez-Cardenas ME. The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol. 2019;39(4):556–70.
Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Bio. 2020;21(5):268–83.
Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12.
Li Q, Ma R, Zhang M. CoCl2 increases the expression of hypoxic markers HIF-1alpha, VEGF and CXCR4 in Breast cancer MCF-7 cells. Oncol Lett. 2018;15(1):1119–24.
Rana NK, Singh P, Koch B. CoCl2 simulated hypoxia induce cell proliferation and alter the expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis. Biol Res. 2019;52(1):12.
Dai X, Cheng H, Bai Z, Li J. Breast Cancer cell line classification and its relevance with breast Tumor subtyping. J Cancer. 2017;8(16):3131–41.
Lin TY, Wang PW, Huang CH, Yang PM, Pan TL. Characterizing the Relapse potential in different luminal subtypes of breast cancers with functional proteomics. Int J Mol Sci. 2020;21(17).
Zhang C, Chen M, Tao Q, Chi Z. Cobalt chloride-stimulated hypoxia promotes the proliferation of cholesteatoma keratinocytes via the PI3K/Akt signaling pathway. Int J Med Sci. 2021;18(15):3403–11.
Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960–76.
Chen J, Ou Y, Luo R, Wang J, Wang D, Guan J, et al. SAR1B senses leucine levels to regulate mTORC1 signalling. Nature. 2021;596(7871):281–4.
Acknowledgements
Not applicable.
Funding
The present report was funded with the help of contributions by the Lions Cancer Research fund (Region Uppsala Örebro), Research committee, Region Örebro County and ALF grants, Region Örebro County.
Open access funding provided by Örebro University.
Author information
Authors and Affiliations
Contributions
LC and AGE contributed with the writing of the original draft, methodology, and data analysis. AGE and ET contributed with reviewing and editing, as well as with project supervision and acquisition of funding. The final manuscript has been read and approved by all contributing authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In present research there is no direct involvement of living human subjects, and no identifiable information about individuals is available. Ethical concerns related to human research participants do not apply and approval from the Swedish Ethical Review Authority is therefore not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Canhasi, L., Tina, E. & Eremo, A.G. Hypoxia-mimetic by CoCl2 increases SLC7A5 expression in breast cancer cells in vitro. BMC Res Notes 16, 366 (2023). https://doi.org/10.1186/s13104-023-06650-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06650-2