Abstract
Background/aims
Hypoxia microenvironment plays a crucial role during tumor progression and it tends to exhibit poor prognosis and make resistant to various conventional therapies. HIF-1α acts as an important transcriptional regulator directly or indirectly associated with genes involved in cell proliferation, angiogenesis, apoptosis and energy metabolism during tumor progression in hypoxic microenvironment. This study was aimed to investigate the expression pattern of the hypoxia associated genes and their association during breast cancer progression under hypoxic microenvironment in breast cancer cells.
Methods
Cell proliferation in MCF-7 and MDA-MB-231 cell lines treated with different concentration of CoCl2 was analyzed by MTT assay. Flow cytometry was performed to check cell cycle distribution, whereas cell morphology was examined by phase contrast microscopy in both the cells during hypoxia induction. Expression of hypoxia associated genes HIF-1α, VEGF, p53 and BAX were determined by semiquantitative RT-PCR and real-time PCR. Western blotting was performed to detect the expression at protein level.
Results
Our study revealed that cell proliferation in CoCl2 treated breast cancer cells were concentration dependent and varies with different cell types, further increase in CoCl2 concentration leads to apoptotic cell death. Further, accumulation of p53 protein in response to hypoxia as compare to normoxia showed that induction of p53 in breast cancer cells is HIF-1α dependent. HIF-1α dependent BAX expression during hypoxia revealed that after certain extent of hypoxia induction, over expression of BAX conquers the effect of anti-apoptotic proteins and ultimately leads to apoptosis in breast cancer cells.
Conclusion
In conclusion our results clearly indicate that CoCl2 simulated hypoxia induce the accumulation of HIF-1α protein and alter the expression of hypoxia associated genes involved in angiogenesis and apoptosis.
Similar content being viewed by others
Background
Breast cancer is the most commonly diagnosed cancer in women. About one out of eight women develop breast cancer throughout life [1]. Early detection through screening programs and new therapeutic strategies have improved the chances to survive; however, many women still die because of metastasis. Prognosis and survival rates for breast cancer vary according to cancer type, stage, treatment, and geographical location of the patient. Survival rates in western world are quite high as compare to developing countries and more than 8 out of 10 women diagnosed with breast cancer survive for at least 5 years in England (84%). Whereas in India incidence of breast cancer is rapidly rising but the survival rate is not even more than 60% [2]. Hypoxia can be defined as the reduction of oxygen or increase in consumption of oxygen relative to the supply in cells, tissue or organs. It is well known that hypoxia is associated with poor prognosis [3], increased angiogenesis [4], tumor growth and resistance to several therapies [5]. Although hypoxia is toxic to both cancer cells and normal cells, cancer cells undergo genetic and adaptive changes that allow them to survive and even proliferate in a hypoxic environment [6, 7]. Multiple studies suggest that hypoxia inducible factor alpha (HIF-1α) get stabilized during hypoxic condition and regulates various genes involved in angiogenesis or apoptosis. It was reported that HIF-1α, VEGF (vascular endothelial growth factor) and p53 play an important role in radiation resistance of tumor cells therefore they can be the potential therapeutic targets to eradicate cancer [8,9,10]. Hypoxia has been described as p53 inducer and as we know p53 plays important role in various pathways of cell cycle delay, apoptosis and cells survival in hypoxic microenvironment [11]. Due to increase in expression of anti-apoptotic proteins cancer cells became resistant to chemotherapy and radiotherapy. Whereas reports suggest that BAX gene surmount the effect of anti-apoptotic proteins and over expression of BAX gene can lead to apoptosis in cancer cells [12,13,14]. However molecular mechanism responsible for the hypoxic survival of breast cancer cells are not well characterised therefore the direct interaction among HIF-1α, p53 and BAX may affect hypoxia induced apoptosis.
Therefore, the present study was undertaken to established a relation between CoCl2 simulated cell proliferation and apoptosis in breast cancer cells under hypoxic condition and to investigate the expression pattern of these factors and their association during breast cancer progression under hypoxic microenvironment.
Materials and methods
Cell culture
Two human breast cancer cell lines (MCF-7 and MDA-MB-231) were grown in Dulbecco Modified Eagle Medium (DMEM; Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen, Carlsbad, CA, USA), 100 units/mL penicillin and 100 µg/mL streptomycin (Cellclone; Genetix Biotech Asia Pvt. Ltd.) in a CO2 incubator (Heal Force HF 90 Shanghai China) with humidified air containing 5% CO2 at 37 °C. 1 × 106 cells from each cell lines (MCF-7 and MDA-MB-231) were seeded in T-25 culture flask (Eppendorf, Hamburg, Germany) and left for 24 h in CO2 incubator.
Preparation of CoCl2 stock solution and hypoxia treatment
Stock solution 25 mM of cobalt chloride (CoCl2) was prepared in sterile distilled water and further diluted in medium in order to obtain the final desired concentrations. MCF-7 as well as MDA-MB-231 cells were cultured in DMEM supplemented with 10% FBS and 100 units/mL penicillin and 100 µg/mL streptomycin in standard CO2 incubator. After 24 h of incubation, the breast cancer cell lines were treated with different concentration of CoCl2.
MTT cell proliferation assay
Briefly, 4000 cells from each cell line (MCF-7 and MDA-MB-231) were seeded treated in 96-well plate with increasing concentration of CoCl2 (0–250 µM for MCF-7 and 0–200 µM for MDA-MB-231) to induce hypoxia. The cells were then incubated for 72 h in a CO2 incubator with humidified atmosphere at 37 °C and 5% CO2. The media was replaced and 10 µL of MTT (Himedia, India) from the stock (5 mg/mL in PBS) was added into each well of 96-well plate and incubated for 2 h at 37 °C. MTT was removed and 100 µL DMSO was added to dissolve the formazan crystal and left for 30 min at 37 °C. Absorbance was detected in ELISA plate reader at 570 nm.
Cell cycle analysis by flow cytometry
Distribution of cells in cell cycle was analysed by flow cytometry. In brief, MCF-7 and MDA-MB-231 cells were seeded in a 6-well cell culture plate and treated with different concentration of CoCl2 to induce hypoxia (50, 100, 150 and 200 µM for MCF-7; 10, 20, 25 and 50 µM for MDA-MB-231 cells). The cell harvested followed by fixation in 70% chilled ethanol. Staining of the cells was carried out with PI-RNase solution (1 mg/mL PI, 0.1% V/V Triton X-100 and 10 mg/mL RNase) and analysed in FACScan using Cell Quest software (Becton–Dickinson).
Study of cell morphology during hypoxia induction
Cells were incubated at a density of 4000 cells per well onto a 96-well cell culture plate for 72 h in a standard CO2 incubator and images were taken in inverted phase contrast microscope after every 24 h following treatment with different concentration of CoCl2 to observe the morphology.
RT-PCR
Following treatment of CoCl2 in MCF-7 and MDA-MB-231, RNA were isolated using TRI reagent (Sigma-Aldrich) according to manufacturer’s instruction. Consequently c-DNA was synthesised with 1 µg RNA by RevertAid H Minus First Strand c-DNA synthesis kit (Thermo Fisher Scientific) according to the manufacturer protocol. Further to check differential expression pattern of HIF-1α, VEGF, p53 and BAX; amplification of these genes were performed in a DNA Thermal Cycler (Applied Biosystem) with gene specific primers obtained from Eurofins Genomics India Pvt. Ltd. (Additional file 1: Table S1), while reaction procedure during PCR amplification has been used as follows: β-actin: 28 cycles at 95 °C for 4 m, 95 °C for 45 s, 50 °C for 45 s, 72 °C for 45 s; HIF-1α: 28 cycles at 95 °C for 4 m, 95 °C for 45 s, 51 °C for 45 s, 72 °C for 45 s; VEGF: 35 cycles at 95 °C for 4 m, 95 °C for 45 s, 57.3 °C for 45 s, 72 °C for 45 s; p53: 32 cycle at 95 °C for 4 m, 95 °C for 45 s, 56.5 °C for 45 s, 72 °C for 45 s; BAX: 32 cycles at 95 °C for 4 m, 95 °C for 45 s, 54.5 °C for 45 s, 72 °C for 45 s; final extension at 72 °C for 10 min. In order to evaluated the differential expression pattern of HIF-1α, VEGF, p53 and BAX with increasing concentration of CoCl2 relative densitometry values were calculated after normalisation with β-actin.
Western blot analysis
MCF-7 and MDA-MB-231 were treated with selected concentration of CoCl2 (50 µM, 100 µM, 150 µM for MCF-7 and 10 µM, 25 µM, 50 µM and 100 µM for MDA-MB-231) and incubated under CO2 incubator for 24 h. Total proteins were extracted by RIPA buffer (Bio-Chemax, Axiva Sichem Biotech, India) and protein concentration were determined by standard bicinchoninic acid (BCA; Bio vision USA) method [15]. 40 µg of protein was loaded into each well and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to polyvinylidene fluoride (PVDF; Merck Millipore, USA). Consequently, primary antibody for each protein like anti-β-actin (Mouse monoclonal antibody obtained from ambion by life technologies; Cat. No. AM4302), anti-p53 (Mouse monoclonal antibody from Puregene by Genetix Biotech Asia Pvt. Ltd; Cat. No. GX-8701M), anti-HIF-1α (Rabbit monoclonal antibody by Invitrogen Cat. No. 700505) and anti-VEGF (Rabbit polyclonal antibody from Puregene by Genetix Biotech Asia Pvt. Ltd) was added in blocking solution individually with standardized dilution (1:500 to 1:1000) and incubated for 16 h at 4 °C. The membrane was washed three times in TBST and then incubated for 2 h with secondary antibody (for β-actin and p53 Rabbit anti-mouse IgG-ALP from GeNei Cat. No. 621100980011730, while for HIF-1α and VEGF Goat anti-rabbit IgG-ALP has been used from GeNei Cat. No. 621100180011730) (secondary antibodies have been used with 1:16,000 dilution). Subsequently membrane was washed thrice in TBST and exposed to the NBT/BCIP (AMRESCO). The expression of protein was detected and captured in E-Gel Imager (Thermo Fisher, USA).
Real-time PCR (qPCR)
qPCR has been performed by using following protocol: denaturation program (95 °C for 10 min), amplification and quantification program repeated 40 times (95 °C for 15 s, 60 °C for 1 min, 95 °C for 30 s with a single fluorescence measurement), melting curve program (60–95 °C with a heating rate of 0.1 °C per second and a continuous fluorescence measurement) and finally a cooling step to 4 °C.
Statistical analysis
All values obtained were represented as mean ± standard error (SE) of at least three independent experiments. Statistical analysis were performed with SPSS version 16.0 (SPSS, Chicago, IL, USA). Differences between control (normoxia, N) and CoCl2 treated groups (hypoxia) were evaluated by One-way ANOVA followed by Tukey post hoc test. The P value less than 0.05 was considered as statistically significant.
Results
MTT assay
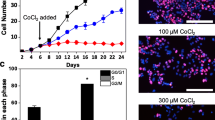
Both the cell lines showed significant proliferation in CoCl2 treated cells as compare to untreated cells, but the concentration of CoCl2 that showed maximum proliferation differ in two cell lines. MCF-7 cell line showed maximum proliferation (P < 0.05) when treated with 150 µM of CoCl2, whereas MDA-MB-231 promotes significant increased in proliferation (P < 0.05) at 25 µM concentration. However, it worth to mention that further increased in the CoCl2 concentration gradually decrease their proliferation rates in both the cell line which showed their inhibitory effect after certain concentration.
Cell cycle distribution analysis by FACS
CoCl2 treated cells showed significant increase in cell population particularly at G2/M phase in both the cell lines after 72 h of treatment as compare to untreated cells. The increase in G2/M phase was dose dependent of maximum increased was observed when CoCl2 was given 150 µM in MCF-7 cell and 25 µM in case of MDA-MB-231 cells. Further increase in concentration of CoCl2 leads to enhancement of Sub G1 cell population also in both the cell lines. Our results also depicts that during hypoxia induction by CoCl2 in MDA-MB-231cells as the concentration increases up to 25 µM cell population in S phase also increases significantly in dose dependent manner (Figs. 1, 2).
Cell proliferation in MCF-7 cells after 72 hrs treatment with different concentration of CoCl2 was determined by MTT assay and represented in bar diagram (a), whereas b illustrates graphical represenation of flow cytometry data for cell cycle distribution of MCF-7 cells exposed to CoCl2 in concentration dependent manner obtained from histogram analysis through FACScan (Becton Dickinson). *Denotes significant difference (P <0.05) as compared to control
Cell proliferation in MDA-MB-231 cells after 72 hrs treatment with different concentration of CoCl2 was determined by MTT assay and represented in bar diagram (a), whereas b illustrates graphical represenation of flow cytometry data for cell cycle distribution of MCF-7 cells exposed to CoCl2 in concentration dependent manner obtained from histogram analysis through FACScan (Becton Dickinson). *Denotes significant difference (P <0.05) as compared to control
Effects of CoCl2 induced hypoxia on cells morphology
The effect of CoCl2 in cell proliferation has also been observed in a dose dependent manner under an inverted light microscope for both the cell lines (MCF-7 and MDA-MB-231) treated with different concentration of CoCl2. It was observed that there was an increased in the cell population in a dose dependent manner up to a certain concentration (50–150 µM in MCF-7 and 10–25 µM in MDA-MB-231) whereas further increase in concentration of CoCl2 exhibit cell shrinkage and cytoplasmic condensation which ultimately leads to cell death [16, 17]. Cell death at higher concentration of CoCl2 in beyond its proliferative dose (150 µM for MCF-7; 25 µM for MDA-MB-231) clearly indicates that the toxicity of compound (Fig. 3a, b).
Cell proliferation with increase in concentration of CoCl2 (MCF-7 50–200 µM; MDA-MB-231 10–200 µM) in MCF-7 (a) and MDA-MB-231 (b) cell lines were analysed under phase contrast microscopy in a time dependent manner. Maximum cell proliferation has been observed at 150 µM and 25 µM in MCF-7 and MDA-MB-231 respectively. Cell morphology was also analysed at ×10 objective in inverted light microscope (EVOS FL cell imaging system, Life Technologies, USA) where cytoplasmic condensation and cell shrinkage were observed at higher CoCl2 concentration i.e., above 150 µM in case of MCF-7 (a) and above 25 µM in MDA-MB-231 (b) which ultimately leads to cell death
Differential expression of hypoxia associated genes
PCR results show a band of expected size for each gene (395 bp for β-actin; HIF-1α-252 bp; VEGF-189 bp; p53-173 bp; BAX-256 bp), which reflects the differential expression of hypoxia associated genes in both cells before and after CoCl2 treatment. Analysis of the observed bands clearly indicate the degree of expression of HIF-1α and VEGF increases in a dose dependent manner in both the cell lines (50–150 µM in MCF-7; 10–25 µM in MDA-MB-231) which strongly support the hypoxia induction by CoCl2 [18,19,20]. Increase in p53 expression in a dose dependent manner was also observed in breast cancer cells treated with CoCl2 which depicts that hypoxia induces p53 expression from its basal level as compared to cells grown under normoxia [11]. It was also observed that expression of pro-apoptotic genes like BAX [12] increases in a dose dependent manner and a significant increase in expression of BAX has been observed at 25 µM. Further increase in CoCl2 concentration enhance the extent of hypoxia which leads apoptotic cell death.
Western blot for analysis of expression of HIF-1α, VEGF and p53 protein
Further, in order to investigate CoCl2 simulated hypoxia induction in both the cell lines (MCF-7 and MDA-MB-231) expression of HIF-1α and VEGF protein were detected by western blot where β-actin is used as internal control. As hypoxia accumulates p53 protein [21] therefore expression level of p53 protein was also investigated in one of these two cell lines (MDA-MB-231) to confirmed the role of p53 in cell survival during hypoxic stress. A band of an expected size (β-actin— ~ 42 kDa; HIF-1α— ~ 92 kDa; VEGF— ~ 45 kDa; p53— ~ 53 kDa) for each protein were obtained with their specific primary antibodies. Interestingly, a dose dependent (In MCF-7: 50–150 µM; MDA-MB-231: 10–25 µM) increase in HIF-1α and VEGF protein expression has been observed in both the cell lines which strongly supports the hypoxia induction by CoCl2. In contrast to this p53 protein expression was also increased in a dose dependent manner as hypoxia has been considered as p53 inducer in cancer cells.
Real-time PCR
Validation of hypoxia induction has been done by investigating the mRNA level of HIF-1α and VEGF gene through qPCR. In addition to this mRNA level of p53 as well as BAX gene were also validated by real-time PCR in MDA-MB-231. Data from our study demonstrate a significant increase in expression level of HIF-1α (> twofold) and VEGF (> 20-fold in MCF-7 cells; > twofold in MDA-MB-231) in both the cell lines at different CoCl2 concentration (150 µM in MCF-7; 25 µM in MDA-MB-231) [22]. Dose dependent enhancement of HIF-1α and VEGF mRNA levels during qPCR validate the expression pattern observed at transcript as well as protein level. In addition to this dose dependent enhancement in expression of p53 as well as BAX mRNA has also been observed in MDA-MB-231. It was also found that the maximum fold change for both the genes (p53- > threefold; BAX- > threefold) were obtained at similar concentration (25 µM) which eventually validate the RT-PCR and western blot data (Figs. 4, 5, 6).
Differential expression pattern of HIF-1α and hypoxia associated genes involved in angiogenesis and apoptosis like VEGF, p53 and BAX in breast cancer cells, where graphical data represents the relative density value after densitometry analysis of band of PCR amplified product. a Depicts the expression pattern of β-actin, HIF-1α and VEGF in MCF-7 cells treated with different concentration of CoCl2 as compare to control by RT-PCR, similarly b shows expression of β-actin, HIF-1α, VEGF, p53 and BAX genes in MDA-MB-231 cells treated with increasing concentration of CoCl2, where it was clearly observed that expression of these genes increases significantly in a dose dependent manner. Error bar represents mean ± SEM of minimum three independent experiments and * Denotes significant difference (P < 0.05) as compared to control (N)
Western blot analysis of β -actin, HIF-1α, VEGF, p53 and BAX protein in breast cancer cells grown under hypoxia (treated with CoCl2) as well as in normoxia (without treatment). a Differential expression pattern of HIF-1α and VEGF protein isolated from MCF-7 cells. b Shows the dose dependent enhancement in expression of hypoxia associated proteins HIF-1α, VEGF, p53 and BAX in MDA-MB-231 cells grown under hypoxic condition, P < 0.05
Relative fold change in expression of HIF-1α and VEGF in MCF-7 cells treated with CoCl2 in a dose dependent manner has been analysed in qPCR data a as compare to cells grown under normoxia, where β-actin used as a endogenous control. While b represents the qPCR data of HIF-1α, VEGF, p53 and BAX gene in MDA-MB-231 cells, P < 0.05
Discussion
In the present study we first investigated whether CoCl2, a well known hypoxia mimetic agent [23], mediated hypoxia induction in breast cancer cells at variable concentration. Then we investigated expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis during breast cancer progression under hypoxic microenvironment. Our study demonstrated that significant increase in cell proliferation up to certain concentration in both the cell lines clearly indicates the role of hypoxia in cell proliferation while the difference in concentration of CoCl2 at which maximum proliferation has been observed in both cells (150 µM in MCF-7; and 25 µM in MDA-MB-231) demonstrate that hypoxia induction by hypoxia mimetic agent differ with different cell types [24]. In addition to this, our study also revealed that increase in extent of hypoxia due to further increase in CoCl2 concentration beyond a certain level which differs for different cell types plays a crucial role in apoptotic cell death. It was reported that hypoxia show signs of dual roles in cell proliferation as well as apoptotic cell death [25]. Therefore to induce hypoxia in breast cancer cells, MCF-7 and MDA-MB-231 cells were treated with different concentration of CoCl2 for 72 h. Further, to investigate the effect of CoCl2 in cell proliferation, cell cycle analysis has been performed, a large proportion of cell population in G2/M phase were observed in both the CoCl2 treated cell lines as compared to untreated cells. However, further increase in CoCl2 concentration triggers apoptotic cell death and population of cells in G0/G1 phase increases significantly in both cells which may due to increase in extent of hypoxia by further increase in CoCl2 concentration that drags cells towards apoptosis [26]. Morphological study also confirms the cell proliferation that occurs in a dose dependent manner up to certain concentration in both the cell lines (MCF-7, 50–150 µM; MDA-MB-231, 10–25 µM) and further increase in CoCl2 concentration disturbs the cellular morphology which ultimately leads to cell death [27]. It was reported that CoCl2 artificially induce the hypoxia by blocking the degradation of HIF-1α [18, 19] and when HIF-1α gets activated it further stimulates transcription of hypoxia associated genes like VEGF which promotes angiogenesis [20]. In order to evaluate the hypoxia induction we first examined the expression of HIF-1α and its associated gene like VEGF as the hypoxia is well known inducer of angiogenesis which is necessary for tumor progression [28]. It was observed that CoCl2 induced HIF-1α accumulation and consequently up-regulated the expression of VEGF in dose dependent manner in both the cell lines. Significant increase in the level HIF-1α and VGEF confirms that CoCl2 artificially induce hypoxia by blocking the degradation HIF-1α which normally occurs in the presence of adequate oxygen or normoxia [22] Several reports suggest that CoCl2 and hypoxia regulate the expression of similar group of genes like HIF-1α, VEGF and p53 [29]. Hypoxia is well known to induce p53 expression and modulate the p53 pathway, in a HIF-1α dependent or independent manner [9]. It was also reported that accumulation of p53 protein induce apoptosis and the presence of HIF-1α and p53 seems to be important for hypoxia induced cell death [30]. Our present study indicate that there was significant increase in expression of p53 in a dose dependent manner in both the cells which confer the HIF-1α dependent induction of p53 at transcript as well as protein level, further validated by qPCR. It was reported that hypoxia induces the expression of p53 which play a major role in cells survival during hypoxic condition [11]. In contrast, there were some reports which suggest that over expression of BAX gene leads to apoptosis [12]. It was also reported that over expression of pro apoptotic gene like BAX induce apoptosis in hypoxia [31]. Various study suggest that during cancer progression amount of anti-apoptotic proteins get increased in cancer cells which makes them resistant to apoptosis during conventional therapy [32, 33]. In our study we found that there was significant increase in expression of BAX in breast cancer cells up to a certain concentration of CoCl2, where further increase in CoCl2 concentration creates high extent of hypoxia which cause the over expression of proapototic gene which ultimately induces apoptosis in breast cancer cells treated with CoCl2. From our present study we speculated that hypoxia alters the p53 dependent pathway in HIF-1α dependent manner thereby targeting the genes involved in p53 pathways which alters the expression of pro apoptotic genes. Our data demonstrate that hypoxic environment promotes the proliferation of breast cancer cells by altering the expression of genes involved in angiogenesis and apoptosis.
Conclusion
In summary, we primarily aimed to established a relation between CoCl2 simulated cell proliferation and apoptosis in breast cancer cells under hypoxic condition. Our study revealed that CoCl2 mediated hypoxia induction and cell proliferation in breast cancer cells is concentration dependent and differ with different cell types. In order to evaluate the effect of CoCl2 in cell proliferation MTT assay followed by flow cytometry has been performed simultaneously, where a large proportion of cell population in G2/M phase were observed in both the cells at their respective concentration (150 μM in MCF-7; 25 μM in MDA-MB-231). However, further increase in CoCl2 concentration triggers apoptotic cell death and population of cells in G0/G1 phase increases significantly. It was observed that CoCl2 induced HIF-1α accumulation and consequently up-regulated the expression of VEGF up to certain concentration, which confers the hypoxia induction at their respective concentration in both the cells. Consequently increase in expression of p53 and BAX in a dose dependent manner up to certain concentration where hypoxia induced significantly confers the HIF-1α dependent induction of p53 which ultimately alters the expression of pro apoptotic gene. Here we conclude that further increase in CoCl2 concentration creates high extent of hypoxia which cause the over expression of proapototic gene which ultimately induces apoptosis in breast cancer cells.
References
Breast Cancer Research U.S. Cancer statistics report for the U.S. 2016. https://www.breastcancer.org/symptoms/understand_bc/risk/understanding. Accessed 23 June 2016.
Agarwal G, Ramakant P. Breast cancer care in India: the current scenario and the challenges for the future. Breast Care. 2008;3:505–14.
Giatromanolaki A, Sivridis E, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Koukourakis MI. Hypoxia-inducible factors 1α and 2α are associated with VEGF expression and angiogenesis in gallbladder carcinomas. J Surg Oncol. 2006;94:242–7.
Maxwell PH, Dachs GU, Nicholls LG, Nicholls AL, Harris I, Stratford O, Hankison CW, Ratciliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94:8104–9.
Shannon AM, Bouchier-Hayes DJ, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307.
Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worral L, Gillies RJ. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97:646–53.
Adamaki M, Georgountzou A, Moschovi M. Cancer and the cellular response to hypoxia. Pediatr Ther. 2012;S1. https://doi.org/10.4172/2161-0665.s1-002.
Mohamed KM, Le A, Duong H, Zhang Y, Wu Q, Messadi DV. Correlation between VEGF and HIF-1α expression in human oral squamous cell carcinoma. Exp Mol Pathol. 2004;76:143–52.
Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophy Res Commun. 2005;331:718–25.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32.
Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, Murphy M, Derr J, Taya Y, Lowe SW, Kastan M, Giaccia A. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol. 2001;21:1297–310.
Ghanbariasad A, Bandehpour M, Kazemi B. Hypoxia response elements can cause the overexpression of the BAX mRNA under hypoxic condition. Iran J Cancer Prev. 2016;9:e4554.
Huang J, Gao J, Lv X, Li G, Hao D, Yao X, Zhou L, Liu D, Wang R. Target gene therapy of glioma: overexpression of BAX gene under the control of both tissue-specific promoter and hypoxia-inducible element. Acta Biochim Biophys Sin. 2010;42:274–80.
Pirocanac EC, Nassirpour R, Yang M, Wang J, Nardin SR, Gu J, Fang B, Moossa AR, Hoffman RM, Bouvet M. Bax-induction gene therapy of pancreatic cancer. J Surg Res. 2002;106:346–51.
Sharma G, Rana NK, Singh P, Dubey P, Pandey DS, Koch B. p53 dependent apoptosis and cell cycle delay induced by heteroleptic complexes in human cervical cancer cells. Biomed Pharmacother. 2017;88:218–31.
Mahey S, Kumar R, Arora R, Mahajan J, Arora S, Bhardwaj R, Thukral AK. Effect of cobalt(II) chloride hexahydrate on some human cancer cell lines. Springer Plus. 2016;5:930. https://doi.org/10.1186/s40064-016-2405-0.
Hong B, Zhou C, Du X, Chen S, Deng X, Duoerkun S, et al. Application of hypoxia to establish epithelial–mesenchymal transition models in renal cell carcinoma cell lines. J Cell Sci Ther. 2016;7. https://doi.org/10.4172/2157-7013.1000242.
Huang Y, Du KM, Xue ZH, Yan H, Li D, Liu W, Chen Z, Zhao Q, Tong JH, Zhu YS, Chen GQ. Cobalt chloride and low oxygen tension trigger differentiation of acute myeloid leukemic cells: possible mediation of hypoxia-inducible factor-1α. Leukemia. 2003;17:2065–73.
Zhang YB, Wang X, Meister EA, Gong KR, Yan SC, Lu GW, Shao G. The effects of CoCl2 on HIF-1α protein under experimental conditions of autoprogressive hypoxia using mouse models. Int J Mol Sci. 2014;15:10999–1012.
Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–33.
Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ Jr, Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–77.
Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89.
Fu OY, Hou MF, Yang SC, Lee WY. Cobalt chloride-induced hypoxia modulates the invasive potential and matrix metalloproteinases of a primary and metastatic breast cancer cells. Anticancer Res. 2009;29:3131–8.
Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2:e164. https://doi.org/10.1038/cddis.2011.48.
Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92.
Bae S, Jeong HJ, Cha HJ, Kim K, Choi YM, An IS, Koh HJ, Lim DJ, Lee SJ, An S. The hypoxia-mimetic agent cobalt chloride induces cell cycle arrest and alters gene expression in U266 multiple myeloma cells. Int J Mol Med. 2012;30:1180–6.
Orozco-Ibarra M, Muñoz-Sánchez J, Zavala-Medina ME, Pineda B, Magaña-Maldonado R, Vázquez-Contreras E, Maldonado PD, Pedraza-Chaverri J, Chánez-Cárdenas ME. Aged garlic extract and S-allylcysteine prevent apoptotic cell death in a chemical hypoxia model. Biol Res. 2016;49:7. https://doi.org/10.1186/s40659-016-0067-6.
Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncol. 2004;9:10–7.
Fu Z, Chen D, Cheng H, Wang F. Hypoxia-inducible factor-1α protects cervical carcinoma cells from apoptosis induced by radiation via modulation of vascular endothelial growth factor and p53 under hypoxia. Med Sci Monit. 2015;21:318–25.
Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–9.
Lowe SL, Rubinchik S, Honda T, McDonnell TJ, Dong JY, Norris JS. Prostate specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther. 2001;8:1363–71.
Ozawa T, Hu JL, Hu LJ, Kong EL, Bollen AW, Lamborn KR, Deen DF. Functionality of hypoxia-induced BAX expression in a human glioblastoma xenograft model. Cancer Gene Ther. 2005;12:449–55.
Honda T, Kagawa S, Spurgers KB, Gjertsen BT, Roth JA, Fang B. A recombinant adenovirus expressing wild-type Bax induces apoptosis in prostate cancer cells independently of their Bcl-2 status and androgen sensitivity. Cancer Biol Ther. 2002;1:163–7.
Authors’ contributions
The study was designed by NKR and BK. Cell proliferation assay, morphological study and flow cytometry experiments were performed and analysed by NKR. NKR and PS performed the mammalian cell culture as well as molecular studies. The data was acquired and analysed by NKR. The manuscript was written and drafted by NKR and BK and reviewed by all authors. All authors read and approved the final manuscript.
Acknowledgements
The authors kindly acknowledge Interdisciplinary School of Life Sciences (ISLS), Institute of Science, Banaras Hindu University for providing the flow cytometry and qPCR (quantitative realtime PCR) facility. We are highly thankful to the Department of Zoology (CAS), Institute of Science, Banaras Hindu University, Varanasi for extending laboratory facilities.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data and materials are available under the permission of authors.
Consent for publication
All the authors agree with publication.
Ethics approval and consent to participate
The authors have no ethical conflicts to disclose.
Funding
The present study was financially supported by SERB-DST, New Delhi, India (SB/EMEQ-263/2014).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Table S1 illustrates designed primer sequences used for RT-PCR, where mRNA sequnce of all five genes were obtained from the Gene database (www.ncbi.nlm.nih.gov), and primers were designed by a tool for finding specific primers, Prime3Plus (http://www.bioinformatics.nl/cgibin/primer3plus/primer3plus.cgi).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rana, N.K., Singh, P. & Koch, B. CoCl2 simulated hypoxia induce cell proliferation and alter the expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis. Biol Res 52, 12 (2019). https://doi.org/10.1186/s40659-019-0221-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-019-0221-z