Abstract
Background
Consistent use of suitable diagnostic methods is essential to evaluate the genomic diversity of E. coli strains. Advance of efficient methods to discriminate the causes of E. coli in aquatic environments is important. This study aimed to describe the strain diversity of an E. coli population retrieved from surface water.
Methods
One hundred water samples were drawn within a period of 1 year, from May 2012 to May 2013, and E. coli bacteria have been isolated from water samples. The genomic diversity analysis of 100 isolates of E. coli (one isolate per sample) has been carried out with the use of the ERIC-PCR fingerprinting method.
Results
Overall, our data indicated that complex fingerprint patterns have been obtained for totally of the isolates. Highest number of strains were in E4 (20 strains with more than 20% similarity) and lowest number of strains were in E3 (5 strains) group. In addition, there was no similarity in E1 (9 strains), E8 (10 strains) and E9 (7 strains) clusters.
Conclusion
Therefore, the occurrence of potential pathogenic E. coli and diversity of E. coli strains in surface water in Alborz province, Iran could pose a possible risk to animal health and human if not disinfected well.
Similar content being viewed by others
Background
Escherichia coli (E. coli) is a consistent dweller of the human intestinal tract, and it is the chief facultative organism in the human gastrointestinal tract [1,2,3,4]. One device for confirming being in the environment might be a variance biofilm-forming skill in some natural E. coli inhabitants. Though biofilm formation is the remaining result of multiple interacting molecular events [5,6,7,8] and is greatest usefully measured at the phenotypic level, a lack of detached genetic systems may be significant to adhesion properties, and some inhabitants level variability in their occurrence performs routine of study.
At the genotypic level, there are two phase-variable surface proteins, antigen 43 (Ag43) and type 1 fimbriae, encoded by the fim gene cluster [9, 10] and Ag43 encoding gene (agn 43) [11], respectively, which have been recommended as thoughtful in determining the adhesion properties of E. coli.
A number of high-resolution molecular fingerprinting methods have been used to reveal species and subspecies diversity [12,13,14]. PCR-based marker methods have been employed lengthily for approving genotypes of organisms at the level of species and populace. The PCR technique needs slight biological material and make available a quick technique for showing large sample sizes. PCR markers have been developed by moreover random primers or specifically designed primers from identified DNA info for instance repetitive [15].
Genomes of eukaryotes cover a large amount (50–80%) of repeated DNA sequences, simple sequence repeats (SSRs) referred to mini satellite DNA and interspersed repeated sequences together with Alu and inter simple sequence repeats (ISSRs) [16,17,18]. Moreover, repetitive portions for instance enterobacterial repetitive intergenic consensus (ERIC) [19,20,21] and repetitive extragenic palindromic (REP) [22] are as well established in prokaryotic genomes. These repeated DNA have been utilized to generate PCR primers valuable in detecting genetic variations in animals, plants, and microbes amid or inside species [23,24,25] ever since of the highly variable nature of the loci.
Advance of efficient methods to differentiate the sources of E. coli in aquatic environments is important to recover the observation of fecal contamination indicators, to improve plans to detect the sources of fecal infection, and to device suitable running performs to decrease gastrointestinal disease transmission [26].
Various DNA fingerprinting techniques exists among which, repetitive extra-genic palindromic elements-polymerase chain reaction (REP-PCR) make available great taxonomic resolution and can act as a fast detector of diversity and progress of the microbial genomes [27]. Among REP fingerprinting methods (REP/ERIC/BOX), ERIC PCR is more preferred because of simple protocol and discriminatory power similar to PFGE [28]. So, the aims of the current research were to describe the strain diversity of an E. coli populace recovered from surface water via employing a whole-genome fingerprinting method.
Methods
E. coli source and water sampling
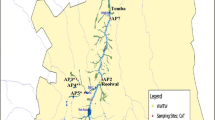
Alborz province with 2.413 million populations is located in south west of Tehran province in central region of Iran. Amirkabir dike and Karaj River are the main sources of water in this province.
This descriptive cross-sectional study was made from May 2012 to May 2013. The authors collected 100 water samples from a given sampling site in Karaj River (with geographic coordinate of 35.9404423, 51.0742861) according to standard microbiological sampling protocols (APHA 2012). They were directly placed in a lightproof insulated box inclosing ice-packs to make sure fast cooling and shipped to the laboratory. The determination of the fecal coliform has been carried out with the use of 9221-A and 9221-D. To isolate E. coli, water samples were inoculated into lauryl tryptose broth (Merck KGaA) followed by E. coli (EC) broth (Merck KGaA) at 44.5 °C and streaked onto eosin methylene blue (EMB) agar (Merck KGaA) [29]. Colonies display metal sheen were considered as presumptive E. coli isolates and were subjected to IMViC (Merck KGaA), glucuronidase, and tryptophanase (Merck KGaA) tests for last approval [29]. All recovered E. coli isolates were put in storage at −70 °C in brain heart infusion (BHI) broth comprising 15% glycerol for further use.
DNA extraction and ERIC PCR
We had evaluated one hundred isolates of E. coli which were separated from Alborz province water sources. Genomic bacterial DNA of 95 E. Coli isolates was extracted via column based technique as described previously by Tolosa (2007) [30]. ERIC-PCR was performed with the two primer sequences of ERIC1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) as described previously [31]. The 25 µl PCR mixture included 17.5 µl distilled water, 2.5 µl 10× PCR buffer, 1.25 µl MgCl2 (2.5 mM), 0.5 µl dNTP (200 µM), 1 µl from each primer ERIC1 and ERIC2, 0.4 µM), 0.25 µl Taq DNA polymerase (1.25 U) and 1 µl template DNA. PCR amplification was performed using an Eppendorf Thermal Cycler as follows: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 52 °C for 40 s, and extension at 72 °C for 5 min followed by a last extension at 72 °C for 5 min. Then, electrophoresis was performed using 1.5% agarose gel, at 70 v for 2 h and dyed with ethidium bromide. Credibility of the ERIC-PCR patterns for each E. coli isolate was proved using parallel runs on separate occasions but on the same thermo cycler. Finally, the loaded gels were visualized by Gel DOC™ XR+ (BIORAD), banding patterns and size were determined via Image Lab ™ 4.0.
Results
One hundred E. coli were isolated from 100 water sample indicating high fecal pollution of river. The genomic diversity analysis of 100 isolates of E. coli has been carried out with the use of the ERIC-PCR fingerprinting method. Complex fingerprint patterns have been found for totally of the isolates studied. A typical fingerprint for some E. coli isolates generated by ERIC PCR has been shown in Fig. 1. Then, our data were inserted in http://insilico.ehu.es/PCR site to construction of the dendrogram. The received dendrogram has grouped the 100 strains of E. coli into nine similarity groups with 75% similarity (Fig. 2).
Cluster analysis by ERIC-PCR fingerprint of 100 E. coli isolates. The bottom bar indicates the isolates number, while the right bar indicates the percentage of similarity. Cluster analysis at a coefficient of 75% similarity (red line), grouped 100 isolates into 9 clusters, designated E1–E9. Each cluster has demonstrated a characteristic number of strains as well as separate inter- and intra-group similarity relations
The percentages of E. coli strains, according in dendrogram, are summarized in Table 1. Highest number of strains were in E4 (20 strains with more than 20% similarity) and lowest number of strains were in E3 (5 strains) group. 18 strains with 75% similarity were belonging to E2 group. In addition, there was no similarity in E1 (9 strains), E8 (10 strains) and E9 (7 strains) between their strains.
Discussion
One hundred E. coli clones isolated from surface water were involved in the genomic diversity analysis. The detection of E. coli in water is an implicit indicator of fresh fecal pollution and consequently of the hazard of co-occurrence of enteric pathogens that can reason illness in susceptible populations [32] and the great ERIC diversity obtained in this study confirms the entirely different sources of water contamination, because river as a non-selective media can receive pollution from many sources.
In the current study, complex fingerprint patterns have been obtained for all E. coli isolates using ERIC PCR. Moreover, the received dendrogram has grouped the 100 strains of E. coli into nine similarity groups with 75% similarity. Our finding is in line with a study conducted to analyse the genomic diversity in E. coli strains isolated from surface water using rep-PCR fingerprinting technique, by the support of REP and ERIC primers. The result showed that, the great genomic diversity of the E. coli of the surface region was expressed as a dendrogram in the form of eight similarity groups including strains isolated from samples drawn above 1 month. The bottom-zone strains, which show a lesser degree of genomic diversity (5 similarity groups), presented different communal structures in their DNA fingerprints. In the similarity dendrogram for the bottom-zone, strains resulting in changed months of sampling were separated hooked on the similar similarity groups [33].
The percentages of E. coli strains, according in dendrogram, in our study showed highest number of strains were in E4 (20 strains with more than 20% similarity) and lowest number of strains were in E3 (5 strains) group. Eighteen strains with 75% similarity were belonging to E2 group. In addition, there was no similarity in E1 (9 strains), E8 (10 strains) and E9 (7 strains) between their strains. Another study conducted on the relative usefulness of five different rep-PCR techniques to discriminate E. coli inhabitants indicated that, cluster analysis of ERIC-PCR and ERIC2-PCR profiles of 270 E. coli revealed 23 clusters and 14 clusters, respectively. The discriminant analysis of rep-PCR genomic fingerprints of 271 E. coli isolates yielded an regular rate of correct classification (watershed-specific) of 72.6 and 55.8% for ERIC-PCR and ERIC2-PCR, respectively [26]. So, the genomic diversity in E. coli strains put on REP primers in rep-PCR generates extra complex fingerprints increasing the discriminatory power of the analysis, whereas the ERIC primer generates less complex fingerprint patterns, and is thus clearer to interpret.
Conclusion
Accessory gene fingerprinting may have significant applied suggestions for improving the specificity of techniques that are usually used to describe the strain diversity of an E. coli population from surface water. ERIC-PCR is an appropriate method to rapidly evaluate the genetic diversity of E. coli strains. To, by this assay, genomic fingerprints from E. coli isolates were distinct and displayed differences in the number of bands, intensity and fragment size.
Abbreviations
- agn 43:
-
ag43 encoding gene
- BHI:
-
brain heart infusion
- ERIC:
-
enterobacterial repetitive intergenic consensus
- E. coli :
-
Escherichia coli
- ISSRs:
-
Alu and inter simple sequence repeats
- PCR:
-
polymerase chain reaction
- REP:
-
repetitive extragenic palindromic
References
Drasar BS, Barrow PS. Aspects of microbiology 10. Intestinal microbiology. Washington, DC: American Society for Microbiology; 1985.
Hemmatinezhad B, Khamesipour F, Mohammadi M, Safarpoor Dehkordi F, Mashak Z. Microbiological Investigation of O-Serogroups, virulence factors and antimicrobial resistance properties of Shiga toxin-producing Escherichia coli isolated from Ostrich, Turkey and Quail Meats. J Food Saf. 2015;35:491–500.
Tajbakhsh E, Khamesipour F, Ranjbar R, et al. Prevalence of class 1 and 2 integrons in multi-drug resistant Escherichia coliisolated from aquaculture water in Chaharmahal Va Bakhtiari province, Iran. Ann Clin Microbiol Antimicrob. 2015;14:37.
Ranjbar R, Hosseini S, Zahraei-Salehi T, Kheiri R, Khamesipour F. Investigation on prevalence of Escherichia coli strains carrying virulence genes ipaH, estA, eaeA and bfpA isolated from different water sources. Asian Pac J Trop Dis. 2016;6(4):278–83.
Hall-Stoodley L, Stoodley P. Developmental regulation of microbial biofilms. Curr Opin Biotechnol. 2002;13:228–33.
Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, Romeo T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol. 2002;184:290–301.
Tajbakhsh E, Ahmadi P, Abedpour-Dehkordi E, Arbab-Soleimani N, Khamesipour F. Biofilm formation, antimicrobial susceptibility, serogroups and virulence genes of uropathogenic E. coli isolated from clinical samples in Iran. Antimicrob Resist. Infect Control. 2016;5:11. doi:10.1186/s13756-016-0109-4.
Torkan S, Bahadoranian MA, Khamesipour F, Anyanwu MU. Detection of virulence and antimicrobial resistance genes in Escherichia coli isolates from diarrhoiec dogs in Iran. Arch Med Vet. 2016;48(2):181–90.
Klemm P, Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987;208:439–45.
Otto K, Elwing H, Hermansson M. The role of type 1 fimbriae in adhesion of Escherichia coli to hydrophilic and hydrophobic surfaces. Coll Surf. 1999;B15:99–111.
Henderson IR, Meehan M, Owen P. Antigen 43, a phase variable bipartite outer membrane protein, determines colony morphology and auto aggregation in Escherichia coli K-12. FEMS Microbiol Lett. 1997;149:115–20.
Vaneechoutte M. DNA fingerprinting techniques for microorganisms. A proposal for classification and nomenclature. Mol Biotechnol. 1996;6:115–42.
Rademaker J, Hoste B, Louws F, Kersters K, Swings J, Vauterin L, Vauterin P, de Bruijn F. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA–DNA homology studies: Xanthomonasas a model system. Int J Syst Evol Microbiol. 2000;50:665–77.
Schloter M, Lebuhn M, Heulin T, Hartmann A. Ecology and evolution of bacterial micro diversity. FEMS Microbiol Rev. 2000;24:647–60.
Hee-Wan K, Dong-Suk P, Seung-Joo G, Moo-Young E. Fingerprinting of diverse genomes using PCR with universal rice primers generated from repetitive sequence of Korean weedy rice. Mol Cells. 2001;13(2):281–7.
Sinnett D, Deragon JM, Simard LR, Labuda D. Alumorphs–human DNA polymorphisms detected by polymerase chain reaction using Alu-specific primers. Genomics. 1990;7:331–4.
Nelson DL. Interspersed repetitive sequence polymerase chain reaction (IRS PCR) for generation of human DNA fragments from complex sources. Methods Companion Methods Enzymol. 1991;2:60–74.
Zeitkiewicz E, Rafalski A, Abuda D. Genome fingerprinting by simple sequencing repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20:176–83.
Hulton CJS, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typimurium and other enterobacteria. Mol Microbiol. 1991;5:825–34.
Ranjbar R, Ghazi FM. Antibiotic sensitivity patterns and molecular typing of Shigella sonnei strains using ERIC-PCR. Iran J Public Health. 2013;42(10):1151.
Afkhami M, Ranjbar R. Molecular typing of uropathogenic E. coli strains by ERIC-PCR method. Electron Physician. 2016;8(4):2291–6.
De Brujin FJ. Use of repetitive (repetitive extragenic pal-indromic and enterobacterial repetive intergenic consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–7.
Caetano-Anolles G, Gresshoff PM. DNA markers: protocols, applications, and overviews. New York: WILEY-VCH; 1997.
Johnson JR, O’Bryan TT. Improved repetitive element PCR fingerprinting for resolving pathogenic and nonpathogenic phylogenic groups within Escherichia coli. Clin Diagn Lab Immunol. 2000;7:265–73.
Ranjbar R, Karami A, Farshad S, Giammanco GM, Mammina C. Typing methods used in the molecular epidemiology of microbial pathogens: a how-to guide. New Microbiol. 2014;37(1):1–15.
Mohapatra BR, Mazumder A. Comparative efficacy of five different rep-PCR methods to discriminate Escherichia coli populations in aquatic environments. Water Sci Technol. 2008;58(3):537–47.
Dombek IE, Johnson LK, Zimmerley ST, Sadowsky MJ. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Appl Environ Microbiol. 2000;66(6):2572–7.
Lin C-W, Chiou C-S, Chang Y-C, Yang T-C. Comparison of pulsed-field gel electrophoresis and three rep-PCR methods for evaluating the genetic relatedness of Stenotrophomonas maltophilia isolates. Lett Appl Microbiol. 2008;47(5):393–8. doi:10.1111/j.1472-765X.2008.02443.
Association American Public Health. Standard methods for the analysis of water and 440 wastewaters. 22nd ed. Washington, DC: American Public Health Association; 2012.
Tolosa JM, Schjenken JE, Civiti TD, Clifton VL, Smith R. Column-based method to simultaneously extract DNA, RNA, and proteins from the same sample. BioTechniques. 2007;43(6):799–805.
Versalovic J, Koeuth T, Lupski R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19(24):6823–31.
Yates MV. Classical indicators in the 21st century-far and beyond the coliform. Water Environ Res. 2007;79:279–86.
Baldy-Chudzik K, Niedbach J, Stosik M. Rep-PCR fingerprinting as a tool for the analysis of genomic diversity in Escherichia coli strains isolated from an aqueous/freshwater environment. Cell Mol Biol Lett. 2003;8(3):793–8.
Authors’ contributions
RR carried out the molecular genetic studies, and participated in its design. PP carried out the biochemical study. KA and RK participated in the design of the study and performed the statistical analysis. FK conceived of the study, and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data related with this manuscript will only can be found with communication with the authors with the following link ranjbarre@gmail.com.
Ethics approval
The present study was approved by the Ethics Committee of the Saveh Science and Research Branch, Islamic Azad University. Because the samples were collected from surface water, no permission was required.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ranjbar, R., Pezeshknejad, P., Khamesipour, F. et al. Genomic fingerprints of Escherichia coli strains isolated from surface water in Alborz province, Iran. BMC Res Notes 10, 295 (2017). https://doi.org/10.1186/s13104-017-2575-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-017-2575-z