Abstract
Background
Glucagon-like peptide-1 receptor (GLP-1R) activation may improve myocardial performance in the context of ischaemia, independent of glycaemic control, in individuals with and without type 2 diabetes mellitus.
Methods
The LIONESS trial was a single-centre randomised double-blind placebo-controlled crossover study to determine whether prolonged GLP-1R activation could improve exercise haemodynamics in chronic stable angina patients. Eligibility criteria comprised angiographic evidence of obstructive coronary artery disease (CAD) and an abnormal baseline exercise tolerance test (ETT) demonstrating > 0.1 mV of planar or downsloping ST-segment depression (STD). Those randomised to active agent started with a 1-week run-in phase of 0.6 mg liraglutide daily, an established injectable GLP-1R agonist, followed by 1 week of 1.2 mg liraglutide, after which patients performed a week 2 ETT. Patients then self-administered 1.8 mg liraglutide for a week before completing a week 3 ETT. The placebo arm received visually and temporally matched daily saline injections. Participants then crossed over to a 3-week course of saline injections interspersed with a week 5 ETT and week 6 ETT and vice versa. Co-primary endpoints were rate pressure product (RPP) at 0.1 mV STD and magnitude of STD at peak exercise.
Results
Twenty-two patients (21 without diabetes) were randomised. There was no significant difference between saline versus liraglutide in the co-primary endpoints of RPP achieved at 0.1 mV STD (saline vs. liraglutide 1.2 mg p = 0.097; saline vs. liraglutide 1.8 mg p = 0.48) or the degree of STD at peak exercise (saline vs. liraglutide 1.2 mg p = 0.68; saline vs. liraglutide 1.8 mg p = 0.57). Liraglutide did not cause symptomatic hypoglycaemia, renal dysfunction, acute pancreatitis or provoke early withdrawal from the trial. Liraglutide significantly reduced weight (baseline 88.75 ± 16.5 kg vs. after liraglutide 87.78 ± 16.9 kg; p = 0.0008) and improved the lipid profile (mean total cholesterol: at baseline 3.97 ± 0.88 vs. after liraglutide 3.56 ± 0.71 mmol/L; p < 0.0001).
Conclusion
Liraglutide did not enhance exercise tolerance or haemodynamics compared with saline placebo during serial treadmill testing in patients with established obstructive CAD. It did, however, significantly reduce weight and improve the lipid profile.
Trial Registration ClinicalTrials.gov Identifier NCT02315001. Retrospectively registered on 11th December 2014.
Similar content being viewed by others
Background
Cardiovascular morbidity and mortality predominates in diabetes with at least a two-fold excess risk of developing ischaemic heart disease, stroke, peripheral arterial disease and heart failure [1]. Efforts to ameliorate this risk by intensifying glycaemic control have, for the most part, had limited success [2,3,4,5] and in the case of rosiglitazone, an association with increased adverse cardiovascular events [6]. Regulatory guidance concerning the safety of novel antidiabetic agents followed, mandating the need for large pre- and post-approval outcomes trials in type 2 diabetes mellitus (T2DM) patients already at high cardiovascular risk. Several of these trials have studied incretin-based antidiabetic therapies, namely dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1Ra) [7,8,9,10,11,12,13,14]. These trials were primarily designed to establish cardiovascular safety. They were not conducted as glycaemia lowering intensification studies to demonstrate cardiovascular risk reduction. Nevertheless, the LEADER (liraglutide) and SUSTAIN-6 (semaglutide) trials, in particular, have both demonstrated significant reductions in the primary composite endpoint of cardiovascular death, nonfatal myocardial infarction (MI) and stroke when a GLP-1Ra was added to standard therapy for T2DM versus placebo [10, 15]. Furthermore several preclinical and human studies of GLP-1Ra have shown evidence of direct cardioprotection in the failing heart or the myocardium under threat of ischaemia/reperfusion injury [16,17,18]. A definitive mechanism by which direct or indirect cardioprotection is mediated remains elusive. There are currently, however, no studies in the literature looking at the potential role of GLP-1Ra in an anti-anginal capacity. This is an important prelude to the use of these agents in the setting of acute MI, since their use could potentially deliver benefits to those who fall victim to silent MI, late presentation or early death before access to medical services. It is in this context that we set out to determine whether the putative anti-ischaemic properties of GLP-1 could translate into an anti-anginal action during sequential exercise stress testing and in so doing demonstrate whether chronic GLP-1 receptor activation could reproduce the beneficial sequelae of the warm-up angina effect without the patient first having to perform symptom-limiting, ischaemia-provoking exercise.
Methods
The Liraglutide to Improve coROnary haemodynamics during Exercise streSS (LIONESS) trial (NCT02315001) was an investigator-initiated single-centre randomised double-blind placebo-controlled crossover proof-of-principle study designed to characterise the physiological effect of chronic GLP-1R activation on validated haemodynamic and electrophysiological parameters during serial exercise treadmill testing (ETT) in patients shown to have inducible myocardial ischaemia and obstructive coronary artery disease (CAD). The hypothesis being chronic GLP-1R activation can positively augment exercise haemodynamics during serial exercise stress testing when compared with saline placebo. The LIONESS trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All participants provided written informed consent prior to randomisation.
Study population
The full inclusion and exclusion criteria can be found in Additional file 1. Participants were required to have chronic stable ischaemic heart disease (SIHD), either managed conservatively, or awaiting elective coronary revascularisation either by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery. Eligible individuals had angiographic evidence of a > 70% stenosis in a main epicardial coronary artery, with or without coronary stenoses elsewhere, a normal resting electrocardiogram (ECG) in sinus rhythm without bundle branch aberration or other conduction disturbance and a recent/baseline abnormal ETT demonstrating > 0.1 mV of planar or down-sloping ST-segment depression.
Protocol
The full protocol has been published previously [19]. In brief enrolled participants were randomised 1:1 to Group A (Treatment A then Treatment B sequence) or Group B (Treatment B then Treatment A sequence); i.e. starting with liraglutide or matched-volume saline placebo.
Participants had their heart rate-limiting drugs cautiously withdrawn prior to study commencement. Long-acting oral nitrates and nicorandil were also discontinued to avoid potential interference with haemodynamics and masking of angina burden (Additional file 1: Table S1). After trial completion individuals were recommenced on all baseline pharmacotherapy.
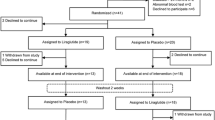
After a 1-week run-in phase of daily 0.6 mg liraglutide followed by a 1-week course of daily 1.2 mg liraglutide, patients in the active intervention arm proceeded to a Week 2 ETT. A run-in phase of once daily 0.6 mg liraglutide was incorporated to encourage tolerance to the known gastrointestinal side effects of GLP-1Ra’s and therefore minimise premature patient withdrawal. Patients were then up-titrated to high-dose 1.8 mg liraglutide for another week before performing a Week 3 ETT. Those patients randomised to the placebo arm were given visually and temporally matched-volume saline subcutaneous injections for the first two weeks before the Week 2 ETT and then another week of saline injections before the Week 3 ETT. Patients then crossed over to complete the 6-week protocol so that those first randomised to the active agent arm switched to the placebo arm and vice versa (Fig. 1). This was a proof of principle trial with no planned follow up beyond the 6-week protocol.
Liraglutide (Victoza®, NovoNordisk, Bagsvaerd, Denmark) is a guideline-approved drug that shares 97% structural homology with native GLP-1. Liraglutide is an appropriate surrogate for studying chronic GLP-1R activation in humans. Furthermore it has a half-life of approximately 10–14 h after subcutaneous injection, which allows for use as a once-daily preparation. This permitted a crossover trial design, helped to maximise patient compliance and precluded the risk of fluid overload in individuals with established CAD. The small risk of hypoglycaemia associated with liraglutide lent further support to it being used in an all comers setting.
Endpoints
Co-primary endpoints
-
Change in rate pressure product (RPP) at 0.1 mV ST-segment depression (STD)
-
Change in magnitude of STD at peak exercise during sequential ETTs
The RPP is the product of heart rate and systolic blood pressure expressed as beats/minute mmHg. It is a recognised indicator of myocardial oxygen consumption (MVO2) and a validated marker of cardiac function and overall physical fitness [20]. The RPP can be used to gauge haemodynamic response to exercise and can be used to titrate exercise intensity to remain below the angina threshold in those with chronic stable angina.
Secondary inducible ischaemia endpoints
-
Change in time to 0.1 mV STD
-
Change in time to maximum STD
Secondary exercise capacity endpoints
-
Change in total exercise time
-
Change in recovery time to 0.05 mV STD
Secondary safety endpoints
-
Evidence of hypoglycaemia
-
Evidence of renal dysfunction
-
Evidence of acute pancreatitis
All exercise tests were analysed by two independent investigators blinded to treatment allocation and sequence (see Additional file 1). All exercise tests were analysed and the results documented before the trial was un-blinded. A stepwise increase in liraglutide dosage was factored into the trial protocol with the aim of observing a dose–response effect on haemodynamics during serial exercise testing. The ETTs performed after each week during the liraglutide treatment period were analysed independently and compared with the corresponding ETT performed on saline placebo. Therefore the week 2 ETT-1 was compared with the week 5 ETT-3 and the week 3 ETT-2 with the week 6 ETT-4 (Fig. 1).
Statistical analysis
Previous studies have shown that the time taken to 0.1 mV STD (368 ± 34 → 418 ± 36 s) and the rate pressure product at 0.1 mV STD (20,500 ± 755 → 21,907 ± 764 mmHg/min) are significantly increased during the second of two serial exercise tests separated by 15 min in chronic CAD patients with known left anterior descending artery stenoses [21, 22]. This warm-up angina effect is thought to augment the innate resistance of the myocardium to an ischaemic insult [23, 24]. We postulated that the administration of liraglutide would mimic the beneficial cardioprotective effects of warm-up angina. A sample size of 26 patients randomised in a 1:1 fashion to each treatment arm (taking into consideration a > 10% drop-out rate) followed by crossover would have 90% power to detect a difference between the means of approximately 15% (2-sided α = 0.05).
The LIONESS trial is effectively a two-period two-treatment crossover trial with a basic AB/BA design. The 2-week transition period from the Week 3 ETT to the Week 5 ETT was deemed sufficient to allow adequate washout of the active drug and therefore minimise the impact of a potential carryover effect. The D’Agostino-Pearson test for normality was performed on each of the sample population parameters under investigation followed by analysis of the two sequence groups A-B and B-A. A rule out test for a carryover effect was performed using a parametric unpaired t-test for a normal distribution or a non-parametric Mann Whitney test for a non-normal distribution. If the test yielded a statistically significant result, this would indicate a significant carryover effect from one treatment sequence to the next. In such cases the first period results before crossover were analysed in isolation [25].
In the absence of a carryover effect outcome after Treatment A is compared directly with outcome after Treatment B (i.e. we ignore whether Treatment A is given in Period 1 or Period 2, given that a significant carryover effect would have been ruled out). A parametric paired t-test or a non-parametric Wilcoxon matched-pairs signed rank test was adopted for this analysis, guided by the baseline test for normality. A one-way ANOVA was used for multiple comparisons. Linear regression would be used thereafter to study the correlation between outcome parameters and baseline patient characteristics if a significant treatment effect were to be discovered. The trial was analysed on a per protocol basis. A p value < 0.05 was considered statistically significant. All analyses were conducted using GraphPad Prism (San Diego, CA, USA).
Results
From December 2013 to October 2014, 105 chronic SIHD patients were screened. Of these, 26 individuals fulfilled the inclusion criteria and consented to participate. Two patients subsequently withdrew consent prior to randomisation and a further 2 withdrew during the trial protocol to proceed to elective CABG surgery. Therefore 22 patients completed the 6-week protocol which met the target sample size. Baseline demographics are shown in Table 1. The mean age (standard deviation—SD) of the per protocol cohort was 62.5 (8.6) years and the mean (SD) body mass index was 29.8 (4.0). Subjects were predominantly male (n = 20/22; 90.9%) and white British (n = 20/22; 90.9%). Most were awaiting elective CABG surgery (n = 20) and the remainder were being managed conservatively (n = 5) or awaiting elective PCI (n = 1). All but two had angiographic multivessel obstructive CAD.
No patient withdrew from the trial prematurely due to adverse events. Furthermore there were no specific adverse events associated with the cautious withdrawal of beta-blockers in the 20 out of 22 individuals that completed the trial protocol. Once the final patient had completed their 6-week protocol, and all exercise tests had been analysed, subsequent un-blinding revealed the treatment sequences (Fig. 1).
Percentage target heart rate (THR) achieved
Mean percentage of THR achieved across all ETTs performed during the trial was approximately 81 ± 10%. Percentage THR achieved by all trial participants at baseline and after each week of treatment (irrespective of treatment period) can be seen in Additional file 1: Table S2. Using a one-way ANOVA with multiple comparisons, there was no significant difference in percentage of THR achieved by all trial participants during all four of the serial ETTs as per protocol. There was, however, a significant difference between the percentage of THR achieved at baseline in comparison to rates achieved during the trial protocol (p = 0.011) (Fig. 2).
Primary endpoints
Change in RPP at 0.1 mV STD
There was no carryover effect during the transition from ETT-1 (matched placebo or liraglutide 1.2 mg) to ETT-3 (1.2 mg liraglutide or matched placebo) (Mann Whitney test p = 0.18) (Additional file 1: Table S3). The test for normality revealed a non-parametric distribution. When the difference between the RPP at 0.1 mV STD after the first ETT on placebo was compared with the RPP at 0.1 mV STD after liraglutide 1.2 mg (i.e. we ignore whether placebo or liraglutide 1.2 mg is given in Period 1 or Period 2), there was no significant treatment effect (Wilcoxon matched-pairs signed rank test p = 0.097) (Fig. 3a).
Primary endpoints. a Rate pressure product at 0.1 mV ST-segment depression ETT-1 placebo vs. ETT-3 1.2 mg liraglutide. b Rate pressure product at 0.1 mV ST-segment depression ETT-2 placebo vs. ETT-4 1.8 mg liraglutide. c ST-segment depression at peak exercise ETT-1 placebo vs. ETT-3 1.2 mg liraglutide. d ST-segment depression at peak exercise ETT-2 placebo vs. ETT-4 1.8 mg liraglutide
There was no carryover effect during the transition from ETT-2 (matched placebo or liraglutide 1.8 mg) to ETT-4 (1.8 mg liraglutide or matched placebo) (Mann Whitney test p = 0.381) (Additional file 1: Table S4). The test for normality revealed a non-parametric distribution. When the difference between the RPP at 0.1 mV STD after the second ETT on placebo was compared directly with the RPP at 0.1 mV STD after liraglutide 1.8 mg (irrespective of trial period), no significant treatment effect was discovered (Wilcoxon matched-pairs signed rank test p = 0.483) (Fig. 3b).
Change in degree of STD at peak exercise
There was no carryover effect during the transition from ETT-1 to ETT-3 (Mann Whitney test p = 0.76) (Additional file 1: Table S5). No significant treatment effect was seen after comparing the STD at peak exercise between the first ETT on placebo with the ETT on liraglutide 1.2 mg irrespective of trial period (Wilcoxon matched-pairs signed rank test p = 0.684) (Fig. 3c).
There was no carryover effect from ETT-2 to ETT-4 (Mann Whitney test p = 0.69) (Additional file 1: Table 6). No significant treatment effect was found after comparing the STD at peak exercise between the second ETT on placebo with the ETT on liraglutide 1.8 mg irrespective of trial period (Wilcoxon matched-pairs signed rank test p = 0.571) (Fig. 3d).
Secondary inducible ischaemia endpoints
Change in time to 0.1 mV STD
Of the 22 patients who completed the protocol, 16 patients achieved STD ≥ 0.1 mV during serial exercise stress testing and were subsequently analysed. There was no carryover effect from ETT-1 to ETT-3 (Mann Whitney test p = 0.28). No significant treatment effect was observed between ETT-1 on placebo and ETT-3 on liraglutide 1.2 mg irrespective of trial period (Wilcoxon matched-pairs signed rank test p = 0.782) (Fig. 4a) (Additional file 1: Table S7).
Secondary inducible ischaemia endpoints. a Time to 0.1 mV ST-segment depression ETT-1 placebo vs. ETT-3 1.2 mg liraglutide. b Time to 0.1 mV ST-segment depression ETT-2 placebo vs. ETT-4 1.8 mg liraglutide. c Time to maximum ST-segment depression ETT-1 placebo vs. ETT-3 1.2 mg liraglutide. d Time to maximum ST-segment depression ETT-2 placebo vs. ETT-4 1.8 mg liraglutide
There was no carryover effect from ETT-2 to ETT-4 (Mann Whitney test p = 0.279). No significant treatment effect was seen between ETT-2 on placebo ETT-4 on liraglutide 1.8 mg irrespective of trial period (Wilcoxon matched-pairs signed rank test p = 0.472) (Fig. 4b) (Additional file 1: Table S8).
Change in time to maximum STD
There was no carryover effect from ETT-1 to ETT-3 (Mann Whitney test p = 0.261). A paired comparison of the time to maximum STD between ETT-1 on placebo with ETT-3 on liraglutide 1.2 mg irrespective of trial period demonstrated no significant treatment effect (Wilcoxon matched-pairs signed rank test p = 0.111) (Fig. 4c) (Additional file 1: Table S9).
There was no carryover effect from ETT2 to ETT4 (Mann Whitney test p = 0.381). A paired comparison of the time to maximum STD between ETT-2 on placebo ETT-4 on liraglutide 1.8 mg irrespective of trial period, showed no significant treatment effect (Wilcoxon matched-pairs signed rank test p = 0.4826) (Fig. 4d) (Additional file 1: Table S10).
Secondary exercise capacity endpoints
Change in total exercise time
There was no carryover effect from ETT-1 to ETT-3 (Mann Whitney test p = 0.72). No significant treatment effect was found between ETT-1 on placebo and ETT-3 on liraglutide 1.2 mg irrespective of trial period (Wilcoxon matched-pairs signed rank test p = 0.06) (Fig. 5a) (Additional file 1: Table S11).
Secondary exercise capacity endpoints. a Total exercise time ETT-1 placebo vs. ETT-3 1.2 mg liraglutide. b Total exercise time ETT-2 placebo vs. ETT-4 1.8 mg liraglutide. c Recovery time to 0.05 mV ST-segment depression ETT-1 placebo vs. ETT-3 1.2 mg liraglutide. d Recovery time to 0.05 mV ST-segment depression ETT-2 placebo vs. ETT-4 1.8 mg liraglutide
There was no carryover effect from ETT-2 to ETT-4 (Mann Whitney test p = 0.46). No significant treatment effect was found between ETT-2 on placebo and ETT-4 on liraglutide 1.8 mg irrespective of trial period (Wilcoxon matched-pairs signed rank test p = 0.99) (Fig. 5b) (Additional file 1: Table S12).
Change in recovery time to 0.05 mV STD
There was no carryover effect from ETT-1 to ETT-3 (Mann Whitney test p = 0.54). A paired comparison of the recovery time to 0.05 mV STD between ETT-1 on placebo with ETT-3 on liraglutide 1.2 mg, irrespective of trial period, confirmed no significant treatment effect (Wilcoxon matched-pairs signed rank test p = 0.17) (Fig. 5c) (Additional file 1: Table S13).
There was no carryover effect from ETT-2 to ETT-4 (Mann Whitney test p = 0.314). A paired comparison of the recovery time to 0.05 mV STD directly between ETT-2 on placebo with ETT-4 on Liraglutide 1.8 mg, irrespective of trial period, also confirmed no significant treatment effect (Wilcoxon matched-pairs signed rank test p = 0.265) (Fig. 5d) (Additional file 1: Table S14).
Secondary safety endpoints
Hypoglycaemia
Recruits were asked to take twice daily home blood glucose measurements (HBGM) throughout the course of the trial. From a possible 1848 measurements (i.e. 44 per day multiplied by 42 days), 1818 recordings were available for analysis: a response rate of 98.4%. There were no symptomatic episodes of hypoglycaemia recorded for any participant throughout the course of the trial [26]. Overall average blood glucose levels were significantly lower in the morning (Wilcoxon matched-pairs signed rank test p < 0.0001) and the afternoon (paired t-test p < 0.0001) during the liraglutide treatment phase versus placebo (Additional file 1: Fig. S1).
Random plasma glucose (RPG) was also checked at baseline and at each study visit. A repeated measures one-way ANOVA demonstrated a significant difference (p = 0.001) between mean RPG at baseline (5.8 ± 1.5 mmol/L) versus mean RPG after liraglutide (5.2 ± 0.7 mmol/L) versus mean RPG after placebo (5.7 ± 0.9 mmol/L). There was a significant difference when mean RPG after liraglutide was compared with either baseline or placebo separately. There was no significant difference between mean RPG at baseline versus mean RPG after placebo (Additional file 1: Fig. S2).
Renal function
A repeated measures one-way ANOVA demonstrated no significant difference in the mean serum creatinine at baseline (91.6 ± 24.6 μmol/L) versus after the placebo trial period (89.6 ± 24.4 μmol/L) versus after the liraglutide trial period (88.8 ± 21.0 μmol/L) (p = 0.46). Likewise, there was no significant difference in the estimated glomerular filtration rate at baseline (76.2 ± 21.1 mL/min/1.73 m2) versus after placebo (77.7 ± 18.8 mL/min/1.73 m2) versus after liraglutide (77.7 ± 18.6 mL/min/1.73 m2) (p = 0.451) (Additional file 1: Fig. S3).
Acute pancreatitis
There were no documented cases of acute pancreatitis during the trial. A repeated measures one-way ANOVA of serum amylase at baseline (68.1 ± 27.4 IU) versus mean amylase during placebo (75.1 ± 34.4 IU) versus mean amylase during liraglutide (77.6 ± 41.5 IU) showed no significant difference between the treatment periods (p = 0.06) (Additional file 1: Fig. S4).
Assessment of chronic GLP-1 receptor activation
GLP-1Ra’s in general, and liraglutide in particular, have been shown to mediate indirect benefits on cardiovascular risk such as weight loss, improvements in lipid profile, and net reductions in blood pressure (BP) [27,28,29,30,31,32,33,34,35]. These parameters were readily available for measurement during the course of the 6-week protocol and were designed to act as surrogate markers of patient compliance with the blinded study drugs.
Weight
A repeated measures one-way ANOVA comparing weight at baseline (88.75 ± 16.5 kg) versus mean weight after placebo (88.11 ± 16.27 kg) versus mean weight after liraglutide (87.78 ± 16.86 kg) confirmed a significant difference (p = 0.0008) between mean weight at baseline versus mean weight after liraglutide. There was no difference between mean weight at baseline versus mean weight after placebo or mean weight after placebo versus mean weight after liraglutide (Additional file 1: Fig. S5).
Blood pressure
A repeated measures one-way ANOVA of mean systolic BP at baseline (134.4 ± 18.3 mmHg) versus mean systolic BP after placebo (139.2 ± 17.7 mmHg) versus mean systolic BP after liraglutide (137.1 ± 16.5 mmHg) showed no significant differences (p = 0.23) (Additional file 1: Fig. S6A).
A repeated measures one-way ANOVA of mean diastolic BP at baseline (77.8 ± 9.8 mmHg) versus mean diastolic BP after placebo (80.1 ± 12.8 mmHg) versus mean diastolic BP after liraglutide (81.6 ± 13.6 mmHg) also revealed no significant differences (p = 0.80) (Additional file 1: Fig. S6B).
Lipid profile
Repeated measures one-way ANOVA of total cholesterol (TC) measurements throughout the trial demonstrated a significant difference (p < 0.0001). There was a significant difference seen between baseline TC (3.97 ± 0.88 mmol/L) versus TC after liraglutide (3.56 ± 0.71 mmol/L) and a significant difference between TC after placebo (4.14 ± 0.91 mmol/L) versus TC after liraglutide (Fig. 6a).
Repeated measures one-way ANOVA of low-density lipoprotein cholesterol (LDL-C) levels throughout the trial also revealed a significant difference between LDL-C after saline placebo (2.14 ± 0.84 mmol/L) versus LDL-C after liraglutide (1.77 ± 0.67 mmol/L) only (p = 0.003) (Fig. 6b).
Repeated measures one-way ANOVA of HDL-C measurements throughout the trial demonstrated no significant differences (p = 0.36) (Fig. 6c).
Assessment of symptoms
Angina
Trial participants were given a patient diary and asked to note down any episodes of angina occurring throughout the study duration. Diary entries were recorded at every trial visit. The frequency of angina episodes were, however, relatively low (Additional file 1: Table S15). There was also no significant difference in frequency of angina on placebo when compared with liraglutide (Wilcoxon matched-pairs signed rank test p = 0.14) (Additional file 1: Fig. S7A).
Gastrointestinal adverse effects
Gastrointestinal (GI) upset is fairly common when commencing GLP-1Ra therapy. As with angina frequency, we asked all trial recruits to note down any episodes of nausea, vomiting, abdominal discomfort or diarrhoea in their diaries. Overall there was a relatively low incidence of GI symptoms (Additional file 1: Table S16). As expected, however, there was a significantly higher incidence of GI symptoms after liraglutide therapy compared with placebo (Wilcoxon matched-pairs signed rank test p = 0.008) (Additional file 1: Fig. S7B). These GI symptoms did not lead to the premature withdrawal of any patient from the trial.
Discussion
To the best of our knowledge, we have shown for the first time in the literature that chronic GLP-1R activation, mediated by liraglutide, does not act in an anti-anginal capacity in a cohort of SIHD patients with obstructive CAD. Liraglutide did not enhance any parameters of haemodynamic performance measured during serial exercise testing. Liraglutide did not significantly augment indices of cardiac function such as the rate pressure product and did not attenuate the magnitude of ST-segment depression seen at peak exercise. There was no dose–response effect seen, such that neither the standard dose of 1.2 mg, or a higher dose of 1.8 mg, were able to provoke a significant anti-anginal or anti-ischaemic effect compared to saline placebo. Furthermore, liraglutide did not significantly reduce the number of angina episodes during daily activity when compared with placebo, although the overall incidence of angina in the cohort was relatively low. The validity of these findings are further reinforced by the relative parity noted in the percentage of THR achieved across all four of the serial ETTs. This would suggest there was no training effect acting as a potential confounder during the 6-week trial protocol. Recent evidence has emerged linking liraglutide to increased heart rate, reduced heart rate variability and a detrimental effect on sympathovagal balance [36,37,38]. In the present trial this putative haemodynamic effect was not observed.
We used a crossover design for the LIONESS trial so each patient served as their own control. This helps to avoid problems associated with the comparability of study and control groups and confounding variables such as age and gender since intra-individual rather than inter-individual differences were studied (Table 1). A comparison of treatments on the same subject is also expected to be more precise which means a smaller sample size could be utilised to achieve adequate statistical power. We were able to achieve our target sample size.
Moreover, crossover can be advantageous with respect to the power of the statistical test carried out to confirm the existence of a treatment effect. To exploit these advantages it is imperative to factor in a washout phase that is sufficiently long enough to rule out a carryover effect. The 2-week interval between ETTs from the first treatment period to the next is well over the accepted mark of ≥ 5 half-lives of the active agent under investigation. Indeed a carryover effect was not detected for any of the haemodynamic primary and secondary endpoints measured in the trial, which confirmed a suitable washout period had been incorporated.
We did, however, demonstrate the safety of using liraglutide in a predominantly non-diabetes population, reflected by the absence of symptomatic hypoglycaemia throughout the study. GLP-1Ra are known to mediate beneficial effects on lipid profile and stimulate weight loss [12, 39,40,41]. This positive modulation of cardiovascular risk was replicated in the trial with significant reductions in weight, total cholesterol and LDL-cholesterol recorded after the liraglutide phase of treatment. Modification of the lipid profile could be due to biological variation or a play of chance as opposed to a direct (or indirect) GLP-1-mediated effect. However, 21 out of 22 patients continued taking statins throughout the 6-week trial (Additional file 1: Table S1). This may reinforce the plausibility of the significant changes seen in the lipid profile.
We did not see any significant effects on BP. Again the short duration of active agent uptake may have been a factor here along with the temporary cessation of beta-blocker therapy in 20 participants across the 6 weeks [40, 42]. This may have negated any net GLP-1-mediated modulation of BP. Ultimately, we should be clear that the trial was not powered (nor designed) to detect differences in weight, lipid profile, or BP.
The GLP-1Ra’s have been studied in several large cardiovascular outcomes trials in patients with type 2 diabetes and elevated cardiovascular risk to demonstrate their safety. Trials of liraglutide (LEADER), exenatide (EXSCEL), lixisenatide (ELIXA), semaglutide (SUSTAIN-6), and albiglutide (HARMONY OUTCOMES) have all confirmed cardiovascular safety but interestingly, liraglutide, semaglutide and albiglutide have also significantly reduced the number of cardiovascular events [9, 10, 12,13,14]. Furthermore, in a post hoc analysis of the LEADER trial, liraglutide was shown to consistently reduce major cardiovascular events in patients with established multi-vessel and single vessel CAD [43]. A recent systematic review and meta-analysis of cardiovascular outcome trials, also confirmed the observation that treatment with GLP-1Ra’s in T2DM patients improves cardiovascular, mortality and kidney outcomes [44]. The mechanisms by which these beneficial cardiovascular effects are mediated remain elusive but are thought to be driven by their non-glycaemic effects [40]. The LIONESS trial would suggest the modulation of cardiovascular risk secondary to chronic GLP-1R activation is unlikely to be via an anti-anginal or anti-ischaemic effect, particularly in the context of SIHD. In acute coronary syndromes, however, small studies have shown liraglutide can improve myocardial salvage and ameliorate infarct size after ST-elevation myocardial infarction (STEMI) and left ventricular function following STEMI and non-STEMI [45,46,47]. Similar effects have been noted for exenatide in STEMI [17, 18].
Limitations
The duration of liraglutide therapy may be a limiting factor. A more sustained period of active agent may have stimulated greater improvements in exercise haemodynamics. Adequate compliance with study agents was strongly encouraged and the number of empty syringes were recorded at every trial visit. Significant differences in HBGM, weight, lipid profile and gastrointestinal side effects would suggest participants were compliant with their study agents and biologically meaningful GLP-1R activation was achieved. Moreover extending the trial beyond 6 weeks would have been logistically difficult, may have allowed a training effect to emerge, and could have resulted in more recruits withdrawing from the trial prematurely to proceed to elective coronary revascularisation.
The diagnostic value of exercise tolerance testing as a modality to detect reversible myocardial ischaemia in the context of current guideline-mandated algorithms, might also be questioned. We accept exercise stress testing has relatively poor sensitivity (mean 67%) and specificity (mean 72%) depending on which published report you read. In the context of the LIONESS trial, however, this was acceptable given that all patients were required to have established CAD confirmed by coronary angiography. The determination of the functional significance of every coronary stenosis deemed obstructive angiographically was not a prerequisite for trial inclusion, but this potential flaw was countered by the need for evidence of inducible ischaemia from a baseline ETT for every trial participant.
Pharmacological agents used to replicate exercise stress are more likely to achieve ≥ 85% of THR during stress echocardiography, stress perfusion cardiac magnetic resonance imaging and nuclear myocardial perfusion scanning. These imaging modalities study left ventricular function and regional wall motion (so can therefore better localise the distribution of ischaemia) but not the exercise haemodynamics via specific electrophysiological parameters we were investigating. Moreover exercise is preferred over pharmacological testing because exercise can induce higher physiological stress and better correlation between symptoms and physical work capacity than that achieved by pharmacological testing. Moreover, recruits achieved on average > 81% of their THR during the trial which validates the ability of serial exercise testing to induce reproducible myocardial ischaemia.
Conclusions
In the LIONESS trial, chronic GLP-1R activation mediated by liraglutide, did not improve exercise haemodynamics or augment established indices of cardiac function in an all comer population of SIHD patients with known obstructive CAD and inducible myocardial ischaemia. Liraglutide did not cause symptomatic hypoglycaemia (in a predominantly non-diabetes trial cohort) and significantly reduced weight and stimulated net improvements in lipid profile, in line with the known effects of injectable GLP-1R agonists.
Availability of data and materials
All data generated or analysed during this study are included in this published article and associated additional files.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- DPP-4:
-
Dipeptidyl peptidase-4
- GLP-1Ra:
-
Glucagon-like peptide-1 receptor agonist
- MI:
-
Myocardial infarction
- ETT:
-
Exercise treadmill test
- CAD:
-
Coronary artery disease
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass graft
- ECG:
-
Electrocardiogram
- BP:
-
Blood pressure
- HBGM:
-
Home blood glucose measurements
- STD:
-
ST-segment depression
- RPP:
-
Rate pressure product
References
Sarwar N, Gao P, Seshasai SRK, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
The Action to Control Cardiovascular Rise in Diabetes Study Group, Group TA to CCR in DS. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–59.
The ADVANCE Collaborative Group, Chalmers J, MacMahon S, Patel A, Cooper M. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year Follow-Up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–89.
Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–8.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–42.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247–57.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–22.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29.
Zinman B, Wanner C, Lachin JM, Fitchett DH, Bluhmki E, Hantel S, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. N Engl J Med. 2015;373:311–22.
Noyan-Ashraf M, Momen M, Ban K, Al-Muktafi S, Zhou Y, Riazi A, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83.
Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–519.
Lønborg J, Kelbæk H, Vejlstrup N, Bøtker HE, Kim WY, Holmvang L, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012;5:288–95.
Myat A, Arri S, Bhatt DL, Gersh BJ, Redwood SR, Marber MS. Design and rationale for the randomised, double-blinded, placebo-controlled Liraglutide to Improve corONary haemodynamics during Exercise streSS (LIONESS) crossover study. Cardiovasc Diabetol. 2015;14:27.
Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–56.
Edwards RJ, Redwood SR, Lambiase PD, Tomset E, Rakhit RD, Marber MS. Antiarrhythmic and anti-ischaemic effects of angina in patients with and without coronary collaterals. Heart. 2002;88:604–10.
Edwards RJ, Redwood SR, Lambiase PD, Marber MS. The effect of an angiotensin-converting enzyme inhibitor and a K+(ATP) channel opener on warm up angina. Eur Heart J. 2005;26:598–606.
Williams RP, Manou-Stathopoulou V, Redwood SR, Marber MS. “Warm-up Angina”: harnessing the benefits of exercise and myocardial ischaemia. Heart. 2014;100:106–14.
Saha M, Redwood SR, Marber MS. Exercise training with ischaemia: is warming up the key? Eur Heart J. 2007;28:1543–4.
Wellek S, Blettner M. Vom richtigen Umgang mit dem Crossover-Design in klinischen Studien: Teil 18 der Serie zur Bewertung wissenschaftlicher Publikationen. Dtsch Arztebl Int. 2012;109:276–81.
Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36:1384–95.
Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–16.
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–50.
Horton E, Silberman C, Davis K. Changes in Cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759–65.
Marre M, Shaw J, Brändle M, Bebakar WMW, Kamaruddin N a, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268–78.
Zinman B, Gerich J, Buse J, Lewin A, Schwartz S, Raskin P. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224–30.
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55.
Sivertsen J, Rosenmeier J, Holst JJ, Vilsbøll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9:209–22.
Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–24.
Rosenstock J, Klaff L, Schwartz S. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes. 2010;33:6–8.
Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77.
Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, et al. Effects of liraglutide on heart rate and heart rate variability: A randomized, double-blind, placebo-controlled crossover study. Diabetes Care. 2017;40:117–24.
Lorenz M, Lawson F, Owens D, Raccah D, Roy-Duval C, Lehmann A, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16:1–10.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Nauck MA, Meier JJ, Cavender MA, El Aziz MA, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–70.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11–22.
von Scholten BJ, Lajer M, Goetze JP, Persson F, Rossing P. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabet Med. 2015;32:343–52.
Verma S, Bhatt DL, Bain SC, Buse JB, Mann JFE, Marso SP, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease results of the LEADER trial. Circulation. 2018;137:2179–83.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85.
Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2015;170:845–54.
Chen WR, Shen XQ, Zhang Y, Chen YD, Hu SY, Qian G, et al. Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction. Endocrine. 2016;52:516–26.
Chen WR, Chen YD, Tian F, Yang N, Cheng LQ, Hu SY, et al. Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2016;9:1–9.
Acknowledgements
Not applicable.
Funding
The trial was supported by the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The trial was also supported by the British Heart Foundation via a Clinical Research Training Fellowship awarded to AM (Grant Number FS/11/70/28917).
Author information
Authors and Affiliations
Contributions
AM and MSM conceived the study and participated in its design, coordination, registration and acquisition of ethical approval. AM and SA were responsible for patient screening, recruitment and examination of patients during the study. MSM was the principal investigator. SRR was the co-principal investigator and contributed to the analysis and interpretation of data. DLB and BJG also contributed to the analysis and interpretation of data. AM wrote the original draft of the manuscript. The remaining authors then revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in compliance with the principles of the Declaration of Helsinki (2008), the principles of Good Clinical Practice and in accordance with all applicable requirements laid down by the Co-Sponsors of the study, namely: King’s College London and Guy’s and St Thomas’ NHS Foundation Trust. The trial protocol received a favourable ethical opinion by the National Research Ethics Service (NRES) Committee London—Westminster (Research Ethics Committee reference 11/LO/1564) of the United Kingdom. The trial was adopted by the National Institute for Health Research Clinical Research Network (NIHR CRN) portfolio (ID 11112) and has been registered on its publicly accessible database since May 2012. Annual progress and safety reports and a final report at conclusion of the study were submitted to the Co-Sponsors, the Research Ethics Committee and the NIHR CRN. The Medicines and Healthcare products Regulatory Agency (MHRA) approved the study as a Non-Clinical Trial of an Investigational Medicinal Product.
Consent for publication
Not applicable.
Competing interests
AM, SA, BJG, SRR and MSM declare that they have no competing interests.
DLB discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Myat, A., Redwood, S.R., Arri, S. et al. Liraglutide to Improve corONary haemodynamics during Exercise streSS (LIONESS): a double-blind randomised placebo-controlled crossover trial. Diabetol Metab Syndr 13, 17 (2021). https://doi.org/10.1186/s13098-021-00635-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-021-00635-6