Abstract
Background
Parasitic nematodes, including large roundworms colloquially known as ascarids, affect the health and well-being of livestock animals worldwide. The equine ascarids, Parascaris spp., are important parasites of juvenile horses and the first ascarids to develop widespread anthelmintic resistance. The microbiota has been shown to be an important factor in the fitness of many organisms, including parasitic nematodes, where endosymbiotic Wolbachia have been exploited for treatment of filariasis in humans.
Methods
This study used short-read 16S rRNA sequences and Illumina sequencing to characterize and compare microbiota of whole worm small intestinal stages and microbiota of male and female intestines and gonads. Diversity metrics including alpha and beta diversity, and the differential abundance analyses DESeq2, ANCOM-BC, corncob, and metagenomeSeq were used for comparisons.
Results
Alpha and beta diversity of whole worm microbiota did not differ significantly between groups, but Simpson alpha diversity was significantly different between female intestine (FI) and male gonad (MG) (P= 0.0018), and Shannon alpha diversity was significantly different between female and male gonads (P = 0.0130), FI and horse jejunum (HJ) (P = 0.0383), and FI and MG (P= 0.0001). Beta diversity (Fig. 2B) was significantly different between female and male gonads (P = 0.0006), male intestine (MI) and FG (P = 0.0093), and MG and FI (P = 0.0041). When comparing organs, Veillonella was differentially abundant for DESeq2 and ANCOM-BC (p < 0.0001), corncob (P = 0.0008), and metagenomeSeq (P = 0.0118), and Sarcina was differentially abundant across four methods (P < 0.0001). Finally, the microbiota of all individual Parascaris spp. specimens were compared to establish shared microbiota between groups.
Conclusions
Overall, this study provided important information regarding the Parascaris spp. microbiota and provides a first step towards determining whether the microbiota may be a viable target for future parasite control options.
Graphical abstract

Similar content being viewed by others
Background
The order Ascaridida consists of nematode parasites colloquially known as roundworms that infect a variety of host species and include important parasites of poultry, fish, dogs, cats, cattle, weasels, bears, pigs, horses, and humans. Ascarids can cause a wide array of clinical signs that can result in severe consequences, including death, which causes large economic losses in the agriculture industry [1,2,3,4,5]. The equine ascarids, Parascaris spp., are considered by veterinary parasitologists to be the most pathogenic parasites affecting juvenile horses worldwide [6, 7]. These parasites can cause coughing, lethargy, poor appetite, diarrhea, nasal discharge [6, 7], liver and lung lesions [8, 9], and impaction colic that may require surgical intervention [7, 10,11,12].
At present, Parascaris spp. are the only ascarid parasites that have evolved widespread anthelmintic resistance. Anthelmintic resistance has been reported in all three drug classes available for the treatment of Parascaris spp. in horses—macrocyclic lactones [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], tetrahydropyrimidines [13, 31,32,33, 39, 40], and benzimidazoles [13, 33, 42]—in multiple countries and continents. Anthelmintic resistance is of major concern in veterinary parasitology. In livestock parasitology, anthelmintic resistance is widespread in many important parasite species [43, 44] and has been for decades. It is also a rising concern in companion animal parasitology [45]. It is thought that frequent anthelmintic treatment intervals have contributed to the development of resistance and thus necessitate treatment efficacy monitoring programs [46], and some parasitologists believe that medical parasitology needs to learn from veterinary parasitology by reducing treatment frequency, identifying causes of resistance, and monitoring for decreased treatment efficacy [46,47,48,49,50]. Little has changed, however, in public health approaches to parasite control [50], and with the lack of new drug development, it is imperative to explore alternative options for parasite control as anthelmintic resistance becomes a major concern in other ascarid species, and before current drugs completely stop working against Parascaris spp.
Over the past couple of decades, the microbiota has been associated with a plethora of health outcomes including but not limited to Crohn’s disease [51], cancer [52], depression [53], equine oral health [54], and bovine respiratory disease [55]. In recent years, microbiota manipulation has emerged as a method to achieve a variety of results, including improved health outcomes in medicine, and is being explored in veterinary science [56,57,58], coral reefs [59, 60], bioremediation [61], and agriculture [62]. One particularly powerful example of the practical application of microbiota manipulation is that of endosymbionts in the genus Wolbachia which are found in filarial nematodes. Wolbachia are important for female worm development [63], larval and microfilarial development, molting, survival [64,65,66], and embryogenesis [67, 68]. Antibiotics in combination with anthelmintics [69, 70] were initially used as a treatment for filarial nematodes in humans, and two candidate drugs were recently developed and are in clinical trials for the treatment and prevention of filariasis [71, 72].
Despite the successful use of the microbiota for control of filarial nematodes, parasite microbiota as a whole remain understudied. Four microbiota studies have been completed for Haemonchus contortus, the most important nematode parasite affecting small ruminants, and have explored the microbiota of life stages [73, 74] and sexes [73, 75], the effect of antibiotics on parasite longevity [76], and the identification of intracellular bacteria via microscopy [74]. Two microbiome studies have been completed for Trichuris spp., an important parasite of humans and pigs [77, 78]. Microbiota studies have also been completed for Schistocephalus solidus [79], Coitocaecum parvum [80], and Philophthalmus attenuatus [81], all of which are flatworms infecting fish. The microbiota has also been associated with various factors in nematodes affecting plants, including virulence [82], life stages [83], development [84], and host symbiosis [85, 86], in Bursaphelenchus spp., endosymbiosis in Xiphinema americanum [87], Radopholus similis [88], and Heterodera glycines [87], and acquisition of parasitism genes in Meloidogyne spp. [90, 91].
In 2019, a paper was published highlighting 100 questions that need to be addressed in livestock helminthology research, voted on from 385 questions submitted by veterinary parasitology researchers in an effort to help close knowledge gaps and direct research efforts; the nematode microbiome was number 73 on that list [92]. Subsequently, a few papers have been published calling for more research and highlighting potential challenges, reinforcing the notion that helminth microbiota research is an important up-and-coming area of study [93,94,95,96]. This study aimed to, for the first time, characterize the bacterial population within Parascaris spp. by determining a shared microbiota and comparing microbiota diversity metrics for the whole worm at different life stages. Additionally, Parascaris spp. provided a unique opportunity to delve further into the parasite microbiome by studying individual organs such as the gonads and intestine due to its large size, which makes it easy to dissect, and allows for comparisons within the worm itself, which has not previously been done for helminth microbiome studies.
Methods
Parasites
All parasites were collected between August 2019 and November 2020 from 5-month-old foals euthanized as part of a routine research program under the University of Kentucky Institutional Animal Care and Use Committee (IACUC) protocol 2012-1046. Foals were not weaned prior to euthanasia and lived outdoors on pasture within the same herd 24/7, with free access to hay and daily grain. Intestinal content samples from the jejunum were also collected at necropsy. Parasites were placed into phosphate-buffered saline (PBS) immediately after removal from the small intestine, rinsed with water, and then placed into sterile PBS for further processing. Jejunal samples were snap-frozen in liquid nitrogen and stored in an −80 °C freezer. For the whole worm microbiota, three adult male, three adult female, and three immature worms were collected from each of three foals. Parasites were serially washed in 70% ethanol and sterile water three times before being snap-frozen in liquid nitrogen and stored in an −80 °C freezer until DNA extraction. Once thawed, a section from the center of adult parasites, determined by folding the worm in half and taking an approximately 2.5 cm section, was used for DNA extraction. Whole immature parasites were used for DNA extraction due to their smaller size. For the organ microbiota, a total of 46 adult Parascaris spp. (24 male and 22 female) were collected from three foals. The parasite surface was washed with 70% ethanol, and the worm was dissected using individually packaged sterile scalpels. All other tools were sterilized with 70% ethanol. Gonads and intestines were dissected from each individual parasite and placed into sterile 15 ml tubes, snap-frozen in liquid nitrogen, and stored in an −80 °C freezer.
DNA extraction
DNA extraction was completed using the Zymo Quick-DNA Fecal/Soil Microbe Kit (Zymo Research Corporation, Irvine, CA, USA) with the following modifications. First, samples were placed in a 2 ml MP Biomedicals (Santa Ana, CA, USA) Lysing Matrix A tube with 750 µl of BashingBead™ buffer and then placed in a Bead Ruptor 12 (Omni International, Inc., Kennesaw, GA, USA) for two 90-s rounds on high. Final elution was performed using 75 µl of 10 mM Tris–HCl buffer (pH 8.5; bioWORLD, Dublin, OH, USA). DNA quantification was performed at the University of Kentucky Genomics Core Laboratory using the Qubit 2.0 (Thermo Fisher Scientific, Waltham, MA, USA).
Next-generation sequencing (NGS) library preparation and sequencing
Library preparation for gonad, intestine, and whole worm samples was completed using the Illumina 16S metagenomic sequencing protocol (Illumina, San Diego, CA, USA, 2013). Female gonad samples were additionally prepared using the Swift Amplicon™ 16S+ITS Panel (Integrated DNA Technologies, Coralville, IA, USA) following the manufacturer’s instructions. Quantification was performed on an Agilent Technologies Stratagene Mx3000P (Santa Clara, CA, USA) using the Collibri™ Library Quantification Kit (Invitrogen, Waltham, MA, USA), following the manufacturer’s instructions, and quality analysis was performed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Library pooling was completed following the respective protocols, and sequencing was performed with the Illumina MiSeq™ (San Diego, CA, USA) using the MiSeq™ reagent kit v3 2 × 300 (Illumina) at the University of Kentucky Genomics Core Laboratory.
Negative controls
Three negative reagent controls were maintained throughout the process, from DNA extraction to sequencing. All amplicon sequence variants (ASVs) found in these negative reagent controls were subsequently removed from all downstream analysis.
Sequence processing
Raw paired 16S amplicon sequence data was converted into and retrieved as fastq files from the Illumina BaseSpace (https://basespace.illumina.com) interface using an Apple Mac Pro (Apple, Inc., Cupertino, CA, USA) running macOS High Sierra 10.13.6. Unless otherwise noted, default settings were used. Fastq quality was assessed, and adapter sequences and low-quality reads were removed using dada2 (version 1.22.0) [97]. A conservative minimum read length of 250 nucleotides was imposed for all reads. R (4.1.2) [98], BiocManager (1.30.16) [99] and Bioconductor libraries BiocStyle (2.22.0) [100], phyloseq (1.38.0) [101], DECIPHER (2.22.0) [102], phangorn (2.8.1) [103], decontam (1.140) [104], and ggplot2 (3.3.5) [105], as well as standard R libraries gridExtra (2.3) [106] and knitr (1.37) [107], were used in amplicon sequence analysis. The decontam package removed common contaminants in the data using control sample and DNA quantification data. The plotQualityProfile function provided by the dada2 R package was used to visualize a summary of the distribution of quality scores for a selection of forward and reverse reads and to assess quality thresholds. The function filterAndTrim was used to filter paired reads. In order to reduce computation time by reducing redundant comparisons, the derepFastq function was used to dereplicate amplicon sequences contained within the filtered data, producing a series of “unique sequences” with corresponding “abundance” estimates. Error rates were estimated using the learnErrors function and plotted using the plotErrors function to assess whether error rates were reasonably well estimated. Samples were clustered and denoised using the dada function, reducing sample error and inferring membership composition of the samples. Paired reads were merged and tabularized, and chimeric sequences were removed using the mergePairs, makeSequenceTable, and removeBimeraDenovo functions. Phyloseq and the SILVA non-redundant rRNA sequence library (v132) [108, 109] were used to analyze microbiota data and assign taxonomic rankings. All ASVs found in control samples, identified as Eukaryota, or without a named phylum were removed from downstream analysis.
Statistical analysis
Prior to conducing diversity analyses, all abundance counts were converted to relative abundance by aggregating ASV data to genus and then dividing genus abundance counts by total reads for a particular sample. Diversity analysis was conducted on relative abundance data using the vegan (2.5–7) [110] package for R. Alpha diversity was calculated using both the Shannon and Simpson diversity indexes. Alpha diversity and relative abundance of individual genera were tested at the genus level for normality using the Shapiro–Wilk test. All normal distributions were then tested for statistical significance using analysis of variance (ANOVA) with Tukey adjustment, and all nonparametric distributions were tested using the Kruskal–Wallis and Dunn tests with Bonferroni correction. Beta diversity was calculated using the Bray–Curtis dissimilarity and was visualized using principal coordinate analysis. Statistical significance was tested for beta diversity using a beta-diversion calculation, which was then tested for significance using ANOVA with Tukey correction using the hagis (3.1.3) [111] package in R. Shared microbiota were determined using a value of > 0.5% relative abundance in > 20% of the samples. Analyses were completed using the phyloseq, knitr, microbiome (1.16.0) [112], dplyr (1.0.8) [113], lsmeans (2.30–0) [114], FSA (0.9.3) [115], and ape (5.6–2) [116] packages in R. Statistical analyses were conducted with α = 0.05.
Differential abundance analysis
Differential abundance analysis was completed using four different methods that were then compared to determine commonalities in differentially abundant taxa. This analysis was completed in R using the tidyverse (1.3.1) [117], phyloseq, edgeR (3.36.0) [118], DEFormats (1.22.0) [119], DESeq2 (1.34.0) [120], apeglm (1.16.0) [121], corncob (0.2.0) [122], ANCOMBC (1.4.0) [123], eulerr (6.1.1) [124], and metagenomeSeq (1.36.0) [125] libraries. The four differential abundance analysis methods used were ANCOM-BC, DESeq2, corncob, and metagenomeSeq, and results were compared with a Venn diagram using eulerr. Four methods were chosen and compared in order to encompass different statistical methods.
Results
Sequencing results
The Illumina MiSeq™ sequencing run included whole worm, gonad, and intestinal specimens in addition to three reagent control for a total of 129 samples and yielded 11.24 gigabases (Gb) with 87.57% passing filter. After processing through the decontam run, 7,785,001 reads remained with a mean of 60,349 reads per sample (range: 45–308,386) and a total of 4635 ASVs. There were 77 ASVs found in negative controls, which were removed from all samples for subsequent analysis. The taxonomic assignment rate after negative controls were removed was as follows: 98.95% kingdom; 80.30% phylum; 79.88% class; 79.25% order; 75.93% family; and 64.98% genus.
Whole worm: After final sequence processing, a total of 132,375 reads remained for 31 whole worm microbiota samples, with a mean of 4270 reads per sample (range: 225–22,940). Prior to downstream analysis, all samples with less than 1000 reads per sample were removed [80, 81], along with the single sample of isolated eggs, leaving 26 samples (3 horse, 7 male, 8 female, 8 immature) for diversity and differential abundance analysis.
Organs: After final sequence processing, a total of 292,667 reads remained for 95 intestinal and gonad samples, with a mean of 3080 reads per sample (range: 0–11,148). Prior to downstream analysis, all samples with less than 200 reads were removed in order to maintain sample sizes between groups, leaving 83 samples (3 horse jejunum [HJ], 20 male gonad [MG], 23 male intestine [MI], 15 female gonad [FG], 22 female intestine [FI]) for diversity and differential abundance analysis.
Whole worm microbiota of small intestinal stages of Parascaris spp.
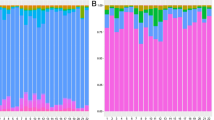
Overall, a total of 22 phyla, 118 families, and 232 genera were identified in the whole worm microbiota samples (Additional File 1: Table S1). The mean relative abundance of the five most abundant phyla are presented in Fig. 1A. There were no significant differences between groups for either alpha or beta diversity (Fig. 1B, C).
Diversity metrics, phylum relative abundance (RA), and shared genera for whole worm microbiota. A Mean RA of the five most abundant phyla, where error bars represent 95% confidence intervals. B Principal coordinate analysis plot based on Bray–Curtis dissimilarities. C Alpha diversity box plot showing both Shannon and Simpson alpha diversity, where • denotes outliers. D Venn diagram showing number of shared genera between groups. F female, M male, I immature, H horse
When comparing samples for shared genera, 28 were common among the four groups, and each group had at least 20 unique genera (Fig. 1D). A list of taxa found only within worms or found within worms with a numerically higher relative abundance compared to the horse is presented in Table 1. Only one, Pelomonas, had significantly different relative abundance based upon sex, with males having a significantly higher relative abundance than immatures (P = 0.0449), where the genus was not detected.
Only ANCOM-BC and DESeq2 returned any differentially abundant taxa for the whole worm samples, and none of them were shared. The DESeq2 results indicated that Enterococcus was differentially abundant across samples (P = 0.0058) and ANCOM-BC indicated that P: Proteobacteria (P = 0.0003) and Sphingomonas (P = 0.0003) were differentially abundant between female parasite and HJ samples.
Adult Parascaris spp. gonad and intestinal microbiota
Overall, a total of 22 phyla, 145 families, and 294 genera were identified in samples from this study (Additional File 2: Table S2). The mean relative abundance of the five most abundant phyla are presented in Fig. 2A. Alpha diversity was significantly different based upon both sex and organ (Fig. 2C). Simpson alpha diversity was significantly different between FI and MG (P= 0.0018). Shannon alpha diversity was significantly different between FG and MG (P = 0.0130), FI and HJ (P = 0.0383), and FI and MG (P = 0.0001) at the genus level. Beta diversity (Fig. 2B) was significantly different between MG and FG (P = 0.0006), MI and FG (P = 0.0093), and MG and FI (P = 0.0041). While not statistically significant based upon the alpha value set for this study, beta diversity tended to differ between MI and FI (P = 0.05602) and MG and HJ (P = 0.05776).
Diversity metrics, phylum relative abundance (RA), and shared genera for whole worm microbiota. A Mean RA of the five most abundant phyla, where error bars represent 95% confidence intervals. B Principal coordinate analysis plot based on Bray–Curtis dissimilarities. C Alpha diversity box plot showing both Shannon and Simpson alpha diversity, where • denotes outliers, and the same letters indicate significant differences. D Venn diagram showing number of shared genera between groups. FG female gonad, FI female intestine, HJ horse jejunum, MG male gonad, MI male intestine
Comparison of all genera within groups indicated 23 shared genera across all groups and at least eight unique genera for each group (Fig. 2D). A summary of taxa found only within worms or found within worms with a higher relative abundance compared to the horse is presented in Table 2.
All genera had significant differences when Kruskal–Wallis tests were performed. A full table of P-values resulting from Dunn’s testing can be found in Table 3. All four differential abundance analysis methods returned statistically significant results for the organ data, and two were shared across all methods. Sarcina was differentially abundant across all four methods (P< 0.0001) and Veillonella was differentially abundant for DESeq2 and ANCOM-BC (P < 0.0001), corncob (P= 0.0008), and metagenomeSeq (P = 0.0118).
Parascaris spp. shared microbiota
Heat plots generated to visualize the shared microbiota for the whole worm study are shown in Fig. 3. Comparison of these shared microbiota indicated that at least two taxa were unique to each group. Heat plots generated to visualize the shared microbiota for each group in the organ study are shown in Fig. 4. Comparison of these microbiota indicated unique taxa for MG and MI, and a total of four shared taxa across all groups.
Combined shared microbiota results from both whole worm and organ microbiota studies included 68 worms and identified 11 shared genera between male, female, and immature whole worm microbiota and combined male and female organ microbiota. The 11 genera were Acinetobacter, Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium (ANPR), Clostridium sensu stricto 1, Gemella, Janthinobacterium, Lactobacillus, Reyranella, Sarcina, Sphingomonas, Streptococcus, and Veillonella. The prevalence and relative abundance data for each of these genera are presented in Table 4.
Discussion
This study described the microbiota of a population of Parascaris spp. in a University of Kentucky parasitology research herd and compared microbiota of small intestinal life stages as well as different organs in adult parasites. This is the first study to describe the microbiota of an equine-specific parasite, an ascarid parasite, and individual nematode parasite organs. Mounting evidence indicates an important role in overall fitness of the parasite microbiota [63,64,65,66,67,68, 82, 84,85,86], and that a common core microbiota is maintained throughout the life-cycle and between sexes [73, 80]. The common core microbiota consists of taxa that are shared by all, or most, members of a given group [126, 127]. The prevalence and abundance of a given microbiota member are not necessarily tied to function, and rare taxa can be essential for host survival [128, 129]. The common core, however, does allow for an understanding of host-microbiota, phylogenetic, and population-level microbiota composition [130]. While the thresholds for shared microbiota were low for this study, at 20% prevalence, core microbiomes have been determined with such a low prevalence [80]; however, a more conservative approach was taken in this study, and the taxa in common are referred to as shared rather than core or common core microbiota.
Among the 11 shared genera identified across groups in this study, Sarcina and Veillonella were identified as differentially abundant in the organ microbiota study. All genera were identified in the horse jejunal microbiota, and all except Reyranella have previously been identified in equine gastrointestinal microbiota [131,132,133,134,135,136,137]. Reyranella, however, has previously been identified in other microbiota such as human neonatal and vaginal samples [138], fish [139,140,141] and shrimp [139]. Reyranella are gram-negative, non-motile, microaerophilic rod-shaped bacteria that have weak urase activity, oxidize CO to CO2, and are generally found in water and soil samples [142]. Reyranella is a relatively new genus, with the type species R. massiliensis first being described in 2011, and there are at present five named species within the genus [142]. Reyranella, along with Acinetobacter and Sphingomonas, has also been previously identified as a reagent contaminant [143, 144]; however, that is unlikely to be the case in this study, because three negative reagent controls were used and all ASVs found in the controls were removed from analysis, and the decontam [104] pipeline was also used, which takes into account sample concentration to remove contaminant sequences.
Comparisons between whole worm and organ results suggest that whole organism analysis of microbiota for helminths may mask nuances in microbiota structure within an organism. The organ study identified 27 more families and 62 more genera than were found in the whole worm study, including those that were unique to different sexes and organs. It also highlighted differences in relative abundance, such as the higher relative abundance of Aminobacter, F: Mycoplasmataceae, Ralstonia, and Reyranella in the FG and of Sphingomonas and Janthinobacterium in both MG and FG. These types of nuances that were not evident in the whole worm study are important because the microbiota can play an important role in parasite reproduction, as previously mentioned. Future studies establishing relative abundance trends in the global Parascaris spp. population, metabolomic and metaproteomic studies, and in vitro studies will be essential for determining the part that these genera play in the overall reproductive success of the parasite.
Out of the few helminth parasite microbiota studies completed to date, H. contortus and Trichuris spp. are the only other parasites of veterinary importance to have had their microbiota analyzed. Four microbiota studies have been completed for H. contortus [73,74,75,76] and two for Trichuris spp. [77, 78]. Three of those used a 16S rRNA hypervariable region and Illumina NGS to analyze the microbiota of different life stages as well as male and female H. contortus [73, 75] and a small number of Trichuris spp. [77]. Comparing microbiota studies with different methods is not always ideal because many factors such as the DNA extraction method [145, 146], PCR primers [147, 148], library preparation [149], and database [150, 151] can affect the results. One H. contortus [75] used a different DNA extraction method from that described herein but used the same database for taxonomic assignment and the same library preparation protocol, and thus the results can be compared with a good level of confidence. Another Trichuris spp. study used the same 16S rRNA hypervariable region and library preparation method as described herein, but different DNA extraction and taxonomic database, and so the results must be compared with more caution.
In one H. contortus study [75], male specimens ha higher alpha diversity than females, but both sexes clustered together for beta diversity. This is similar to results found in the present study, where there were no discernible clusters specifically for male or female worms for beta diversity. Alpha diversity was similar for the whole worm study; however, male Parascaris spp. gonad and intestine both had higher alpha diversity than female organs when parsed out. Out of the most prevalent genera in the H. contortus study, only Acinetobacter was shared with the Parascaris spp. shared microbiota, and out of the unique genera found in either male or female H. contortus, only Corynebacterium 1 and Prevotella were shared between the two parasite species. They were found in the MG, FI, and MI, and in the MG, respectively, both with prevalence ≤ 25.0%.
The Trichuris spp. study mainly contained samples of parasite intestine and a total of only seven specimens, three males and four females [77]. The genera Acinetobacter and Sphingomonas were the only two Parascaris spp. shared microbiota that were also found in Trichuris spp. [77]. Shannon alpha diversity did not show any differences between the samples; however, the sample size for this study was small, and no comparisons were made between sexes or the two species—T. trichiura and T. suis—examined in this study [77].
The five most abundant phyla for both whole worm and organ microbiota studies in Parascaris spp. were Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Tenericutes (Figs. 1A, 2A). Firmicutes was the most prevalent phylum for all groups in both Parascaris spp. studies; however, this differs from results found in the H. contortus and Trichuris spp. studies, where Proteobacteria was the most prevalent phylum [73, 75, 77]. In another Trichuris spp. study, the phylum Bacteroidetes was the most prevalent [78]. In three H. contortus studies, a limited number of phyla were detected overall [73, 74, 76], whereas 23 were identified in another [75], which is similar in number to the 22 phyla identified in this study. A total of 36 phyla were identified in Trichuris spp. [77], over 50% more than found in Parascaris spp. Overall, there were some similarities between the Parascaris spp., Trichuris spp., and H. contortus microbiota despite some differences in methodology. It is important to note, however, that there are substantial biological differences among all three parasite types. Parascaris spp. reside in the small intestine, feed on intestinal content, have migratory stages throughout the host [7], and are a clade III nematode [152]; H. contortus resides in the abomasum, feeds upon blood, does not have migratory stages in the host [153], and belongs to nematode clade V [152]; and Trichuris spp. reside in the caecum, burrow into the mucosal epithelium, do not have migratory stages in the host [154], and belong to nematode clade I [152]. These biological differences could have a substantial effect on microbiota composition due to differences in hosts, genetics [155, 156], diet [157], and environmental exposure throughout the life-cycle [158, 159].
Further studies assessing microbiota function and localization will be necessary to determine whether some of these bacterial genera are important for parasite fitness, what role they play in parasite biology, and whether they are passed down via vertical transmission or acquired from the environment. This study was limited by small sample sizes, both in number of worms and number of foals represented. A total number of six foals were represented in the entirety of the study, which was of course limited by the number of foals with a parasite burden at the time of necropsy. Jejunum content from these foals, however, does represent every population from which worms were collected, and while the number of reads (range 4023–10,431) may at first appear low, the number of ASVs (769) and genera (136) is similar to findings in previous studies. The jejunum tends to have lower bacterial diversity than other gastrointestinal compartments, and previous studies have identified 293 OTUs in the gastrointestinal tract [160], 209 OTUs in the small intestine [161], and 500 unique sequences and 135 OTUs in the jejunum [162]. Additionally, the number of parasites, particularly for the whole worm study where fewer than 10 individuals were available for each group, is another limitation, and results may change with larger sample sizes. However, when comparing results from the whole worm and organ studies, dissecting Parascaris spp. may provide more nuance and insight into the parasite microbiome and thus may be a better way forward when studying the microbiome of this particular organism.
While is it not possible to discern any functional implications of microbiota from the present research, some of the members may be worth further investigation. Reyranella, for example, has been previously identified in the human vaginal microbiota and was also identified in this study at a higher prevalence and relative abundance in the FG compared to the other investigated sites, suggesting a possible function in the female reproductive tract. Additionally, the two differentially abundant genera, Sarcina and Veillonella, are also worth deeper investigation as a differentiating factor between different organ compartments of Parascaris spp. Future research will provide further insight into the possibility of using microbiota manipulation for the control of Parascaris spp. and development of new anthelmintic treatments.
Conclusions
This study is the first to characterize the microbiota of an ascarid parasite, a parasite affecting horses, and the first parasitic nematode microbiota to include analysis of separate organs. A group of shared microbiota for the study population was determined to consist of 11 bacterial genera, two of which—Sarcina and Veillonella—were differentially abundant in organs. The gonad and intestine of female and male specimens were found to have differences for both alpha and beta diversity, suggesting that there are potentially important nuances in organ compartment microbiota versus whole-organism microbiota studies for parasitic nematodes. Ultimately, more research is needed with larger sample sizes and diverse populations to parse out differences in microbiota of Parascaris spp., and functional studies are needed to determine what role the parasite microbiota plays in host fitness.
Availability of data and materials
Sequencing data were submitted to the National Center for Biomedical Information (NCBI) Sequence Read Archive under accession number PRJNA851371; BioSample accession numbers SAMN29223031–SAMN29223159.
Abbreviations
- FG:
-
Female gonad
- FI:
-
Female intestine
- HJ:
-
Horse jejunum
- MG:
-
Male gonad
- MI:
-
Male intestine
References
Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32.
Chelladurai JJ, Bader C, Snobl T, Magstadt D, Cooper V, Brewer MT. Toxocara vitulorum infection in a cohort of beef calves in Iowa. Vet Parasitol. 2015;214:96–9.
Avery RH, Wall LA, Verhoeve VI, Gipson KS, Malone JB. Molecular confirmation of Ascaris suum: further investigation into the zoonotic origin of infection in an 8-year-old boy with Loeffler syndrome. Vector Borne Zoonotic Dis. 2018;18:638–40.
Sharma N, Hunt PW, Hine BC, Ruhnke I. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem. Poul Sci. 2019;98:6517–26.
Gakosso LGC, Baadi F, Abakka FZ, Basraoui D. The visceral larva migrans caused by Toxocara canis: a case report. Pan Afr Med J. 2020;36:150.
Reinemeyer CR. Diagnosis and control of anthelmintic-resistant Parascaris equorum. Parasit Vectors. 2009;2:S8.
Nielsen MK. Evidence-based considerations for control of Parascaris spp. infections in horses. Equine Vet Ed. 2016;28:224–31.
Nicholls JM, Clayton HM, Pirie HM, Duncan JL. A pathological study of the lungs of foals infected experimentally with Parascaris equorum. J Comp Pathol. 1978;88:261–74.
Brown PJ, Clayton HM. Hepatic pathology of experimental Parascaris equorum infection in worm-free foals. J Comp Path. 1979;89:115–23.
Southwood LL, Ragle CA, Snyder S, Hendrickson DA. Surgical treatment of ascarid impactions in horses and foals. Proc Am Assoc Equine Practnrs. 1996;42:258–61.
Cribb NC, Cote NM, Boure LP, Peregrine AS. Acute small intestinal obstruction associated with Parascaris equorum infection in young horses: 25 cases (1985–2004). N Z Vet J. 2006;54:338–43.
Tatz AJ, Segev G, Steinman A, Berlin D, Milgram J, Kelmer G. Surgical treatment for acute small intestinal obstruction caused by Parascaris equorum infection in 15 horses (2002–2011). Equine Vet J Suppl. 2012;43:111–4.
Alanazi AD, Mukbel RM, Alyousif MS, AlShehri ZS, Alanazi IO, Al-Mohammed HI. A field study on the anthelmintic resistance of Parascaris spp. in Arab foals in the Riyadh region Saudi Arabia. Vet Q. 2017;37:200–5.
Cirak VY, Kar S, Girişgin O. A survey on anthelmintic resistance in Strongyles to ivermectin and pyrantel and macrocyclic lactone-resistance in Parascaris equorum. Turkiye Paraziol Derg. 2010;34:35–9.
Schougaard H, Nielsen MK. Apparent ivermectin resistance of Parascaris equorum in Danish foals. Vet Rec. 2007;160:439–40.
Lassen B, Peltola SM. Anthelmintic resistance of intestinal nematodes to ivermectin and pyrantel in Estonian horses. J Helminthol. 2014;89:760–3.
Näreaho A, Vainio K, Oksanen A. Impaired efficacy of ivermectin against Parascaris equorum, and both ivermectin and pyrantel against strongyle infections in trotter foals in Finland. Vet Parasitol. 2011;182:372–7.
Laugier C, Sevin C, Ménard S, Maillard K. Prevalence of Parascaris equorum infection in foals on French stud farms and first report of ivermectin-resistant P. equorum populations in France. Vet Parasitol. 2012;188:185–9.
Geurden T, Betsch JM, Maillard K, Vanimisetti B, D’Espois M, et al. Determination of anthelmintic efficacy against equine cyathostomins and Parascaris equorum in France. Equine Vet Ed. 2013;25:304–7.
von Samson-Himmelstjerna G, Fritzen B, Demeler J, Schurmann S, Rohn K, Schnieder T, et al. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet Parasitol. 2007;144:74–80.
Martin F, Halvarsson P, Delhomme N, Höglund J, Tydén E. Exploring the β-tubulin gene family in a benzimidazole-resistant Parascaris univalens population. Int J Parasitol Drugs Drug Resist. 2021;17:84–91.
Veronesi F, Moretta I, Moretti A, Fioretti DP, Genchi C. Field effectiveness of pyrantel and failure of Parascaris equorum egg count reduction following ivermectin treatment in Italian horse farms. Vet Parasitol. 2009;161:138–41.
Veronesi F, Fioretti DP, Genchi C. Are macrocyclic lactones useful drugs for the treatment of Parascaris equorum infections in foals? Vet Parasitol. 2010;172:164–7.
Lindgren K, Ljungvall Ö, Nilsson O, Ljungström BL, Lindahl C, et al. Parascaris equorum in foals and in their environment on a Swedish stud farm, with notes on treatment failure of ivermectin. Vet Parasitol. 2008;151:337–43.
Lind EO, Christensson D. Anthelmintic efficacy on Parascaris equorum in foals on Swedish studs. Acta Vet Scand. 2009;51:45.
Boersema JH, Eysker M, Nas JWM. Apparent resistance of Parascaris equorum to macrocylic lactones. Vet Rec. 2002;150:279–81.
Stoneham S, Coles G. Ivermectin resistance in Parascaris equorum. Vet Rec. 2006;158:572.
Relf VE, Lester HE, Morgan ER, Hodgkinson JE, Matthews JB. Anthelmintic efficacy on UK thoroughbred stud farms. Int J Parasitol. 2014;44:507–14.
Hearn FPD, Peregrine AS. Identification of foals infected with Parascaris equorum apparently resistant to ivermectin. J Am Vet Med Assoc. 2003;223:482–5.
Slocombe JOD, de Gannes RVG, Lake MC. Macrocyclic lactone resistant Parascaris equorum on stud farms in Canada and effectiveness of fenbendazole and pyrantel pamoate. Vet Parasitol. 2007;145:371–6.
Craig TM, Diamond PL, Ferwerda NS, Thompson JA. Evidence of ivermectin resistance by Parascaris equorum on a Texas horse farm. J Equine Vet Sci. 2007;27:67–71.
Lyons ET, Tolliver SC, Ionita M, Collins SS. Evaluation of parasiticidal activity of fenbendazole, ivermectin, oxibendazole, and pyrantel pamoate in horse foals with emphasis on ascarids (Parascaris equorum) in field studies on five farms in Central Kentucky in 2007. Parasitol Res. 2008;103:287–91.
Armstrong SK, Woodgate RG, Gough S, Heller J, Sangster NC, et al. The efficacy of ivermectin, pyrantel and fenbendazole against Parascaris equorum infection in foals on farms in Australia. Vet Parasitol. 2014;205:575–80.
Beasley A, Coleman G, Kotze AC. Suspected ivermectin resistance in a south-east Queensland Parascaris equorum population. Aust Vet J. 2015;93:305–7.
Wilkes EJA, McConaghy FF, Thompson RL, Dawson K, Sangster NC, Hughes KJ. Efficacy of a morantel-abamectin combination for the treatment of resistant ascarids in foals. Aust Vet J. 2017;95:85–8.
Bishop RM, Scott I, Gee EK, Rogers CW, Pomroy WE, Mayhew IG. Sub-optimal efficacy of ivermectin against Parascaris equorum in foals on three Thoroughbred stud farms in the Manawatu region of New Zealand. N Z Vet J. 2014;62:91–5.
Cooper LG, Caffe G, Cerutti J, Nielsen MK, Anziani OS. Reduced efficacy of ivermectin and moxidectin against Parascaris spp. in foals from Argentina. Vet Parasitol Reg Stud Rep. 2020;20:100388.
Molento MB, Antunes J, Bentes RN, Coles GC. Anthelmintic resistant nematodes in Brazilian horses. Vet Rec. 2008;162:384–5.
Hautala K, Näreaho A, Kauppinen O, Nielsen MK, Sukura A, Rajala-Schultz PJ. Risk factors for equine intestinal parasite infections and reduced efficacy of pyrantel embonate against Parascaris sp. Vet Parasitol. 2019;273:52–9.
Martin F, Höglund J, Bergström TF, Lindsjö OK, Tydén E. Resistance to pyrantel embonate and efficacy of fenbendazole in Parascaris univalens on Swedish stud farms. Vet Parasitol. 2018;264:69–73.
Lyons ET, Tolliver SC, Kuzmina TA, Collins SS. Further evaluation in field tests of the activity of three anthelmintics (fenbendazole, oxibendazole, and pyrantel pamoate) against the ascarid Parascaris equorum in horse foals on eight farms in Central Kentucky (2009–2010). Parasitol Res. 2011;109:1193–7.
Martin F, Eyda M, Höglund J, Tydén E. Constitutive and differential expression of transport protein genes in Parascaris univalens larvae and adult tissues after in vitro exposure to anthelmintic drugs. Vet Parasitol. 2021;298:109535.
Sutherland IA, Leathwick DM. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–81.
Kaplan RM, Vidyashankar AN. An inconvenient truth: Global worming and anthelmintic resistance. Vet Parasitol. 2012;186:70–8.
Jimenez Castro PD, Venkatesan A, Redman E, Chen R, Malatesta A, Huff H, et al. Multiple drug resistance in hookworms infecting greyhound dogs in the USA. Int J Parasitol Drugs Drug Resist. 2021;17:107–17.
von Samson-Himmelstjerna G, Thompson RCA, Krücken J, Grant W, Bowman DD, Schnyder M, et al. Spread of anthelmintic resistance in intestinal helminths of dogs and cats is currently less pronounced than in ruminants and horses - Yet it is of major concern. Int J Parasitol Drugs Drug Resist. 2021;17:36–45.
Geerts S, Coles GC, Gryseels B. Anthelmintic resistance in human helminths: Learning from the problems with worm control in livestock. Parasitol Today. 1997;13:149–51.
Beech RN, Skuce P, Bartley DJ, Martin RJ, Prichard RK, Gilleard JS. Anthelmintic resistance: markers for resistance, or susceptibility? Parasitology. 2010;138:160–74.
Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, et al. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist. 2011;1:14–27.
Tinkler SH. Preventive chemotherapy and anthelmintic resistance of soil-transmitted helminths—Can we learn nothing from veterinary medicine? One Health. 2020;9:100106.
Ni J, Shen TCD, Chen EZ, Bittinger K, Bailey A, Roggiani M, et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017;9:6888.
Helmink BA, Wadud Khan MA, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–88.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–96.
Kennedy R, Lappin DF, Dixon PM, Buijs MJ, Zaura E, Crielaard W, et al. The microbiome associated with equine periodontitis and oral health. Vet Res. 2016;47:49.
Lima SF, Teixeira AGV, Higgins CH, Lima FS, Bicalho RC. The upper respiratory tract microbiome and its potential role in bovine respiratory disease and otitis media. Sci Rep. 2016;6:29050.
Clemmons BA, Voy BH, Myer PR. Altering the Gut Microbiome of Cattle: considerations of host-microbiome interactions for persistent microbiome manipulation. Microb Ecol. 2019;77:523–36.
Song SJ, Woodhams DC, Martino C, Allaband C, Mu A, Javorschi-Miller-Montgomery S, et al. Engineering the microbiome for animal health and conservation. Exp Biol Med. 2019;244:494–504.
Peixoto RS, Harkins DM, Nelson KE. Advances in microbiome research for animal health. Annu Rev Anim Biosci. 2021;9:289–311.
Rosado PM, Leite DCA, Duarte GAS, Chaloub RM, Jospin G, Nunes da Rocha U, et al. Marine probiotics: increasing coral resistance to bleaching through microbiome manipulation. ISME J. 2019;13:921–36.
Santoro EP, Borges RM, Espinoza JL, Freire M, Messias CSMA, Villela HDM, et al. Coral microbiome manipulation elicits metabolic and genetic restructuring to mitigate heat stress and evade mortality. Sci Adv. 2021;7:3088.
Correa-García S, Pande P, Séguin A, St-Arnaud M, Yergeau E. Rhizoremediation of petroleum hydrocarbons: a model system for plant microbiome manipulation. Microb Biotechnol. 2018;11:819–32.
Deng X, Zhang N, Shen Z, Zhu C, Liu H, Xu Z, et al. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum. NPJ Biofilms Microbiomes. 2021;7:33.
Hoerauf A, Volkmann L, Nissen-Paehle K, Schmetz C, Autenrieth I, Büttner DW, et al. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Top Med Int Health. 2000;5:275–9.
Casiraghi M, McCall JW, Simoncini K, Kramer LH, Sacchi L, Genchi C, et al. Tetracycline treatment and sex-ratio distortion: a role for Wolbachia in the moulting of filarial nematodes? Int J Parasitol. 2002;32:1457–68.
Arumugam S, Pfarr KM, Hoerauf A. Infection of the intermediate mite host with Wolbachia-depleted Litomosoides sigmodontis microfilariae: impaired L1 to L3 development and subsequent sex-ratio distortion in adult worms. Int J Parasitol. 2008;38:981–7.
Mand S, Pfarr K, Sahoo PK, Satapathy AK, Specht S, Klarmann U, et al. Macrofilaricidal activity and amelioration of lymphatic pathology in bancroftian filariasis after 3 weeks of doxycycline followed by single-dose diethcarbamazine. Am J Trop Med Hyg. 2009;81:702–11.
Hoerauf A, Mand S, Volkmann L, Büttner M, Marfo-Debrekyei Y, Taylor M, et al. Doxycycline in the treatment of human onchocerciasis: kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 2003;5:261–73.
Foray V, Pérez-Jiménez MM, Fattouh N, Landmann F. Wolbachia control stem cell behavior and stimulate germline proliferation in filarial nematodes. Dev Cell. 2018;45:198–211.
Bazzocchi C, Mortarino M, Grandi G, Kramer LH, Genchi C, Bandi C, et al. Combined ivermectin and doxycycline treatment has microfilaricidal and adulticidal activity against Dirofilaria immitis in experimentally infected dogs. Int J Parasitol. 2008;38:1401–10.
Luck AN, Evans CC, Riggs MD, Foster JM, Moorhead AR, Slatko BE, et al. Concurrent transcriptional profiling of Dirofilaria immitis and its Wolbachia endosymbiont throughout the nematode life cycle reveals coordinated gene expression. BMC Genomics. 2014;15:1041.
Taylor MJ, von Geldern TW, Ford L, Hübner MP, Marsh K, Johnston KL, et al. Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and ochocerciasis. Sci Transl Med. 2019;11:2086.
Hong WD, Benayoud F, Nixon GL, Ford L, Johnston KL, Clare RH, et al. AWZ1066S, a highly specific anti-Wolbachia drug candidate for a short-course treatment of filariasis. Proc Natl Acad Sci USA. 2019;116:1414–9.
El-Ashram S, Suo X. Exploring the microbial community (microflora) associated with ovine Haemonchus contortus (macroflora) field strains. Sci Rep. 2017;7:70.
Sinnathamby G, Henderson G, Umair S, Janssen P, Bland R, Simpson H. The bacterial community associated with the sheep gastrointestinal nematode parasite Haemonchus contortus. PLoS ONE. 2018;13:e0192164.
Mafuna T, Soma P, Tsotetsi-Khambule AM, Hefer CA, Muchadeyi FC, Thekisoe OMM, et al. Bacterial profiling of Haemonchus contortus gut microbiome infecting Dohne Merino sheep in South Africa. Sci Rep. 2021;11:5905.
Bouchet C, Deng Q, Umair S. Bacteria associated with the parasitic nematode Haemonchus contortus and its control using antibiotics. Parasitologia. 2022;2:63–70.
García-Sánchez AM, Miller AZ, Caldeira AT, Cutillas C. Bacterial communities from Trichuris spp. A contribution to deciphering the role of parasitic nematodes as vector of pathogens. Acta Trop. 2022;226:106277.
White EC, Houlden A, Bancroft AJ, Hayes KS, Goldrick M, Grencis RK, et al. Manipulation of host and parasite microbiota: Survival strategies during chronic nematode infection. Sci Adv. 2018;4:7399.
Hahn M, Piecyk A, Jorge F, Cerrato R, Kalbe M, Dheilly NM. Host phenotype and microbiome vary with infection status, parasite genotype, and parasite microbiome composition. Mol Ecol. 2022;31:1577–94.
Jorge F, Dheilly NM, Poulin R. Persistence of a core microbiome through the ontogeny of a multi-host parasite. Front Microbiol. 2020;11:954.
Jorge F, Dheilly NM, Froissard C, Wainwright E, Poulin R. Consistency of bacterial communities in a parasitic worm: variation throughout the life cycle and across geographic space. Microb Ecol. 2022;83:724–38.
Xiang Y, Wu XQ, Zhou AD. Bacterial diversity and community structure in the pine wood nematode Buesaphelenchus xylophilus and B mucronatus with different virulence by high-throughput sequencing of the 16S rDNA. PLoS ONE. 2015;10:0137386.
Wu XQ, Xue Q, Xiang Y, Ding XL, Xu XL, Ye JR. Community and functional diversity of bacteria associated with propagative and dispersal forms of Bursaphelenchus xylophilus. Nematology. 2016;18:1185–98.
Tian XJ, Wu XQ, Xiang Y, Fang X, Ye JR. The effect of endobacteria on the development and virulence of the pine wood nematode Brusaphelenchus xylophilus. Nematology. 2015;17:581–9.
Cheng XY, Tian XL, Wang YS, Lin RM, Mao ZC, Chen N, et al. Metagenomic analysis of the pinewood nematode microbiome reveals a symbiotic relationship critical for xenobiotics degradation. Sci Rep. 2013;3:1869.
Wang X, Yu Y, Ge J, Xie B, Zhu S, Cheng X. Effects of α-pinene on the pinewood nematode (Bursaphelenchus xylophilus) and its symbiotic bacteria. PLoS ONE. 2019;14:e0221099.
Vandekerckhove TTM, Willems A, Gillis M, Cooman A. Occurrence of novel verrucomicrobial species, endosymbiotic and associated with parthenogenesis in Xiphinema americanum-group species (Nematoda, Longidoridae). Int J Syst Evol Microbiol. 2000;50:2197–205.
Haegeman A, Vanholme B, Jacob J, Vandekerckhove TTM, Claeys M, Borgonie G, et al. An endosymbiotic bacterium in a plant-parasitic nematode: member of a new Wolbachia supergroup. Int J Parasitol. 2009;39:1045–54.
Noel GR, Atibalentja N. ‘Candidatus Paenicardinium endonii’, an endosymbiont of the plant-parasitic nematode Heterdera gylcines (Nemata: Tylenchida), affiliated to the phylum Bacteroidetes. Int J Syst Evol Microbiol. 2006;56:1697–702.
Bird DM, Opperman CH, Davies KG. Interactions between bacteria and plant-parasitic nematodes: now and then. Int J Parasitol. 2003;33:1269–76.
Scholl EH, Thorne JL, McCarther JP, Bird DM. Horizontally transferred genes in plant-parasitic nematodes: a high-throughput genomic approach. Genome Biol. 2003;4:R39.
Morgan ER, Aziz NA, Blanchard A, Charlier J, Charvet C, Claerebout E, et al. 100 questions in livestock helminthology research. Trends Parasitol. 2019;35:52–71.
Dheilly NM, Bolnick D, Bordenstein S, Brindley PJ, Figuères C, Holmes EC, et al. Parasite microbiome project: systematic investigation of microbiome dynamics within and across parasite-host interactions. mSystems. 2017;2:00050–17.
Dheilly NM, Martínez JM, Rosario K, Brindley PJ, Fichorova RN, Kaye JZ, et al. Parasite microbiome project: grand challenges. PLoS Pathog. 2019;15:e1008028.
Jenkins TP, Brindley PJ, Gasser RB, Cantacessi C. Helminth microbiomes —a hidden treasure trove? Trends Parasitol. 2019;35:13–22.
Formenti F, Cortés A, Brindley PJ, Cantacessi C, Rinaldi G. A bug’s life: delving into the challenges of helminth microbiome studies. PLoS Negl Trop Dis. 2020;14:e0008446.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria; 2019. https://www.R-project.org/
Morgan M. BiocManager: access the bioconductor project package repository. R package version 1.3.10; 2019. https://github.com/Bioconductor/BiocManager/
Oleś A. BiocStyle: Standard styles for vignettes and other Bioconductor documents. R package version 2.22.0; 2021. https://github.com/Bioconductor/BiocStyle
McMurdie PJ, Holmes S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217.
Wright ES. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J. 2016;8:352–9.
Schliep K, Potts AJ, Morrison DA, Grimm GW. Intertwining phylogenetic trees and networks. Methods Ecol Evol. 2017;8:1212–20.
Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6:226.
Wickham H. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York; 2016. https://ggplot2.tidyverse.org
Baptiste A. gridExtra: Miscellaneous Functions for "Grid" Graphics. R package version 2.0.0; 2015. http://CRAN.R-project.org/package=gridExtra
Xie Y. knitr: A general-purpose package for dynamic report generation in R. R package version 1.28; 2020. https://cran.r-project.org/web/packages/knitr/index.html
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6.
Yilmaz O, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, et al. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014;42:D643–8.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. R package version 2.5–7; 2020. https://github.com/vegandevs/vegan
McCoy AG, Noel ZA, Sparks AH, Chilvers M. ‘hagis’, an R package resource for pathotype analysis of Phytophtora sojae populations causing stem and root rot of soybean. Mol Plant Microbe Interact. 2019;32:1574–6.
Lahti L, Shetty S. Microbiome R package. R package version 1.16.0; 2019. https://doi.org/10.18129/B9.bioc.microbiome
Wickham H, François R, Henry L, Müller K. Dplyr: A Grammar of Data Manipulation. R package version 1.0.8; 2022. https://cran.r-project.org/package=dplyr
Length RV. Least-squares means: the R package lsmeans. J Stat Softw. 2016;69:1–33.
Ogle DH, Doll JC, Wheeler P, Dinno A. FSA: Fisheries stock analysis. R package version 0.9.3.9000; 2022. https://github.com/fishR-Core-Team/FSA
Paradis E, Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–8.
Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Oleś A. DEFormats: differential gene expression data formats converter. r package version 1.22.0; 2021. https://github.com/aoles/DEFormats
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2018;35:2084–92.
Martin BD, Witten D, Willis AD. Modeling microbial abundances and dysbiosis with beta-binomial regression. Ann Appl Stat. 2020;14:94–115.
Lin H, Peddada S. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020;11:3514.
Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS ONE. 2014;9:e101717.
Paulson JN, Olson ND, Braccia DJ, Wagner J, Talukder H, Pop M, et al. metagenomeSeq: Statistical analysis for sparse high-throughput sequencing. R package version 1.36.0; 2013. http://www.cbcb.umd.edu/software/metagenomeSeq
Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Rese. 2009;19:1141–52.
Shade A, Handelsman J. Beyond the Venn diagram: the hunt for a core microbiome. Environ Microbiol. 2011;14:4–12.
Jousset A, Bienhold C, Chatzinotas A, Gallien L, Gobet A, Kurm V, et al. Where less may be more: how the rare biosphere pulls ecosystems strings. ISME J. 2017;11:853–62.
Hammer TJ, Sanders JG, Fierer N. Not all animals need a microbiome. FEMS Microbiol Lett. 2019;366:fnz117.
Risely A. Applying the core microbiome to understand host-microbe systems. J Anim Ecol. 2020;89:1549–58.
Costa MC, Arroyo LC, Allen-Vercoe E, Stämpfli HR, Kim PT, Sturgeon A, et al. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3–V5 region of the 16S rRNA gene. PLoS ONE. 2012;7:e41484.
O’Donnell MM, Harris HMB, Jeffery IB, Claesson MJ, Younge B, O’Toole PW, et al. The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Lett Appl Microbiol. 2013;57:492–501.
Paßlack N, Vahjen W, Zentek J. Impact of dietary cellulose on the fecal microbiota of horses. J Equine Vet Sci. 2020;91:103106.
Ang L, Vinderola G, Endo A, Kanatanen J, Jingfeng C, Binetti A, et al. Gut microbiome characteristics in feral and domesticated horses from different geographic regions. Commun Biol. 2022;5:172.
Gilroy R, Leng J, Ravi A, Adriaenssens EM, Oren A, Baker D, et al. Metagenomic investigation of the equine faecal microbiome reveals extensive taxonomic diversity. PeerJ. 2022;10:e13084.
Mach N, Midoux C, Leclercq S, Pennarun S, Le Moyec L, Rué O, et al. The first horse gut microbiome gene catalog reveals that rare microbiome ensures better cardiovascular fitness in endurance horses. bioRxiv:2022.01.24.477461 [Preprint]. 2022 [cited 2022 June 23]. https://www.biorxiv.org/content/10.1101/2022.01.24.477461v1
Voss SJ, McGuinness DH, Weir W, Sutton DGM. A study comparing the healthy and diseased equine glandular gastric microbiota sampled with sheathed transendoscopic cytology brushes. J Equine Vet Sci. 2022;104002:Forthcoming.
Li H, Nie C, Xiao B, Chen S, Yu J, Zhu Y. Structure of vaginal microbiome community after perineal disinfection and its effects on neonatal oral microbiome. research square;rs-69318.v1 [Preprint]. 2020 [cited 2022 June 23]. https://www.researchsquare.com/article/rs-69318/v1
Zhang M, Sun Y, Liu Y, Qiao F, Chen L, Liu W, et al. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture. 2016;454:72–80.
Méndez-Pérez R, Garciá-López R, Bautista-López JS, Vázquez-Castellanoa J, Alvarez-González C, Peña-Marín E, et al. High-throughput sequencing of the 16S rRNA gene to analyze the gut microbiome in juvenile and adult tropical gar (Atractosteus tropicus). Lat Am J Aquat Res. 2020;48:456.
Mondal HK, Maji UJ, Mohanty S, Sahoo PK, Maiti NK. Alteration of gut microbiota composition and function of Indian major carp, rohu (Labeo rohita) infected with Argulus siamensis. Microb Pathog. 2022;164:105420.
Pagnier I, Raoult D, La Scola B. Isolation and characterization of Reyranella massiliensis gen. nov., sp. Nov. from freshwater samples by using an amoeba co-culture procedure. Int J Syst Evol Microbiol. 2011;61:2151–4.
Legal E, Estrada-Peña A, Marsot M, Cosson J, Rué O, Mariadassou M, et al. Taxon appearance from extraction and amplification steps demonstrates the value of multiple controls in tick microbiota analysis. Front Microbiol. 2020;11:1093.
Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analysis. BMC Biol. 2014;12:87.
Mackenzie BW, Waite DW, Taylor MW. Evaluating variation in human gut microbiota profiles due to DNA extraction method and inter-subject differences. Front Microbiol. 2015;6:130.
Lim Y, Totsika M, Morrison M, Punyadeera C. The saliva microbiome profiles are minimally affected by collection method of DNA extraction protocols. Sci Rep. 2017;7:8523.
Wasimuddin SK, Ronchi F, Leib SL, Erb M, Ramette A. Evaluation of primer pairs for microbiome profiling from soils to humans within the one health framework. Mol Ecol Resour. 2020;20:1558–71.
Liu YX, Qin Y, Chen T, Lu M, Qian X, Guo X, et al. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell. 2021;12:315–30.
Allali I, Arnold JW, Roach J, Cadenas MB, Butz N, Hassan HM, et al. A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiol. 2017;17:194.
Balvočiūtė M, Huson DH. SILVA, RDP, greengenes, NCBI and OTT - how do these taxonomies compare? BMC Genomics. 2017;18:114.
Sierra MA, Li Q, Pushalkar S, Paul B, Sandoval TA, Kamer AR, et al. The influences of bioinformatics tools and reference databases in analyzing the human oral microbiome community. Genes. 2020;11:878.
Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–5.
Emery DL, Hunt PW, Le Jambre LF. Haemonchus contortus: the then and now, and where to from here? Int J Parasitol. 2016;46:755–69.
Klementowicz JE, Travis MA, Grencis RK. Trichuris muris: a model of gastrointestinal parasite infection. Semin Immunopathol. 2012;34:815–28.
Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396–406.
Khachatryan ZA, Ktsoyan ZA, Manukyan GP, Kelly D, Ghazaryan KA, et al. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS ONE. 2008;3:e3064.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–90.
Eichmiller JJ, Hamilton MJ, Staley C, Sadowsky MJ, Sorensen PW. Environment shapes the fecal microbiome of invasive carp species. Microbiome. 2016;4:44.
Su S, Zhao Y, Liu Z, Liu G, Du M, Wu J, et al. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments of Mongolian horses. Microbiologyopen. 2020;9:1085–101.
Costa MC, Silva G, Ramos RV, Staempfli HR, Arroyo LG, Kim P, et al. Characterization and comparison of the bacterial microbiota in different gastrointestinal compartments in horses. Vet J. 2015;205:74–80.
Ericsson AC, Johnson PJ, Lopes MA, Perry SC, Lanter HR. A microbiological map of the healthy equine gastrointestinal tract. PLoS ONE. 2016;11:e0166523.
Acknowledgements
The authors would like to thank the faculty and staff at the University of Kentucky Veterinary Diagnostic Laboratory, staff at the University of Kentucky Genomics Core Laboratory, and farm crew at the Main Chance Research Farm for all of their technical support. Jennifer Cain would also like to thank Christopher Cain for all of his help and support.
Funding
This work was supported by grants from Zoetis, Inc. (https://www.zoetis.com/), Parsippany-Troy Hills, New Jersey and the National Center for Veterinary Parasitology (https://www.ncvetp.org/), Stillwater, Oklahoma, both obtained by J.L.C. and M.K.N. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
JLC and MKN contributed to conceptualization; JKN contributed to data curation; JLC and JKN contributed to formal analysis; JLC and MKN contributed to funding acquisition; JLC, NRR, PS, CAF, and HSG contributed to investigation and methodology; MKN contributed to supervision; JLC and MKN contributed to writing—original draft preparation and writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was conducted under University of Kentucky IACUC protocol 2012-1046.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Relative abundances of all genera, families, and phyla for each whole worm sample.
Additional file 2: Table S2.
Relative abundances of all genera, families, and phyla for each organ sample.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cain, J.L., Norris, J.K., Ripley, N.E. et al. The microbial community associated with Parascaris spp. infecting juvenile horses. Parasites Vectors 15, 408 (2022). https://doi.org/10.1186/s13071-022-05533-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05533-y