Abstract
Cefoperazone (Cfz) is a member of the third generation of parenteral cephalosporin antibiotics. It is used on a wide scale in prescribed antibiotic drugs as anti-infection, especially for Gram-negative and also against Gram-positive microorganisms. The current study aimed to find a rapid RP-HPLC method of Cfz analysis with high linearity, repeatability, sensitivity, selectivity, and inexpensive. In our developed method, there is no need to use special chemical reagents, a high percentage of organic solvent, a high flow rate, further guard column. The chromatographic system comprises an ODS column (150 mm × 4.6 mm × 5 μm). The mobile phase was prepared by mixing KH2PO4 solution: acetonitrile (80:20) with a flow rate of 1.0 mL/min at detection wavelength 230 nm, at room temperature using injection volume 20 μL. The method manifested a satisfied linearity regression R2 (0.9993) with a good repeatability range (0.34–0.92%) with LOD and LOQ; 4.04 μg/mL and 12.24 μg/mL respectively. The method proved its efficiency via system suitability achievement in the robustness and ruggedness conduction according to the validation guidelines. The shorter analysis time makes the method very valuable in quality control to quantify the commercial Cfz in pharmaceutical preparations. This improved HPLC method has been successfully applied for Cfz analysis for Peracef and Peractam vials in our routine finished and stability studies testing laboratories. Additionally, the detection limit of Cfz has been tested in our quality control lab to detect the smallest amount of traces that may be present after the cleaning process of the production machines for cephalosporins preparations. In a precedent for the first time, we were able to use the current analysis method to determine the minimum inhibitory concentration (MIC) and minimum bacteriostatic concentration (MBC). The conventional broth micro-dilution tube method was used to determine MIC at 250 µg/mL and MBC at 125 µg/mL of Cfz against the standard strain of Burkholderia cepacia (B. cepacia) ATCC 25416 as Gram-negative bacteria in vitro.
Similar content being viewed by others
Introduction
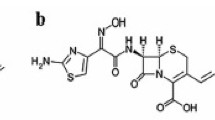
Cefoperazone(Cfz) is a semisynthetic, broad-spectrum antibiotic, and it has the IUPAC name [(6R,7R)-7-[[(2R)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-(4-hydroxy-phenyl)acetyl]amino]-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabi-cyclo[4.2.0]oct-2-ene-2-carboxylic acid]. It is the third-generation cephalosporin family [1] and it has the chemical molecular formula C25H27N9O8S2 in molar mass "645.7 g/mole" as manifested in Fig. 1.
Cfz could be taken by injection into a muscle, a vein, or an intravenous drip. Cfz is used in cases of infections of the respiratory tract, urinary tract, female reproductive system, and skin infections and it is known as an antibacterial, especially Gram-negative, as it is very effective in particular Pseudomonas and Haemophilus influenza. Where Cfz prevents the production of bacterial cell walls [2]. Also, it is used in Gram-positive infections such as Staphylococcus aureus and Streptococcus pneumonia [3]. Although the use of antibiotics is important in our lives, some antibiotics, especially cefoperazone, have an effective effect on some important enzymes in the metabolism as Glutathione S-transferase and acetylcholinesterase. As a result, utilizing this drug may have certain negative consequences for some people. Drug dosages need to be appropriately controlled to reduce negative effects. Patients who lack these enzymes due to a hereditary condition should be closely watched [4].
Cfz is sold under more than one brand name, such as Cefobid, Peracef, Cefazon, and Cefrazon with different strengths of 2.0 g, 1.0 g & 0.5 g vials. Cfz also can be found in mixed forms (cefoperazone sodium: sulbactam sodium) such as Trexotaz 2.0 g vials in the ratio (1:1) or as in Peractam, Sulperazon, Sulbacef as 1.5 g vials in a ratio (2:1).
The wide spectrum of this drug makes it important in the field of pharmaceutical trade, which necessitates the need to find effective, simple, easy, and rapid methods for assay determination. In addition, a sensitive method should be conducted at low concentrations of this drug preparation, when this method is used to estimate Cfz after washing cleaning machines and production lines. The sensitive method should be conducted to ensure the effectiveness of the cleaning method to remove the drug residual effects of this drug that may be entered into the next product in the production process, causing a completely unacceptable cross-contamination process [5,6,7,8,9,10,11,12,13,14,15]. This type of contamination is according to the quality standards mentioned in the rules of good manufacturing practice (GMP) [6, 7, 15].
There are many methods with more than one technique in the analysis tools being conducted for the assay determination of Cfz, including flow injection analysis with thermal lens spectrometry [16], HPTLC technique [17,18,19,20], UPLC-MS/MS [21], RP-HPLC [1,2,3, 22,23,24,25], silver nanoparticles spectrophotometrically [26], spectrophotometrically [27,28,29]. HPLC–MS [30].
However, HPLC–UV detection is an easy, accurate, and inexpensive method, both at an academic and commercial level rate. The United States-Pharmacopoeia (USP44-NF 39 2021) and British-Pharmacopoeia (BP-2022-VOL1) issued two different methods for determining Cfz. The USP44-NF39 2021 mobile phase is composed of triethylamine, glacial acetic acid, ACN, 1 N acetic acid, and water with a stationary phase column of 4.0-mm × 30 cm containing packing L1 at a flow rate of about 2 mL per minute. The retention time is about 22 min. On the other hand, the BP-2022-VOL 1 mobile phase consists of triethylammonium acetate, acetic acid, ACN, and water with a stationary phase column as 4.6-mm × 15-cm end-capped octadecylsilyl silica gel for chromatography R (5 µm) at flow rate about 1 mL per minute. The retention time is about 15 min.
Most of the Cfz HPLC conducted analysis methods used a high percentage of the organic modifiers from MeOH or ACN, adjusted pH buffer solutions, or special reagents such as tetrabutylammonium hydroxide. These factors are used to get the optimum peak shape with ideal tailing [19, 25].
The field of scientific research has recently tended to purify industrial wastewater, pharmaceutical factories, and hospitals, especially for antibiotics. So, finding easy, fast, accurate, and economical methods has become an urgent necessity [5,6,7,8,9,10,11, 15, 31,32,33].
In this paper, an efficient, simple, and rapid method for the assay determination of Cfz will be issued. Furthermore, inexpensive chemicals with integration analysis method parameters were used to obtain the detection and identification of Cfz. The current method became the approved analysis method to determine the Cfz in all the quality control lab activities that contain Cfz as starting raw material, finished product, and stability studies. Also, the method was successfully implemented in the cleaning validation and verification after the production of any pharmaceutical preparation containing Cfz. For the first time, we could determine the MIC and MBC using the present Cfz HPLC analysis method against the bacterial standard strain of (B. cepacia).
Materials and methods
Cfz sodium working standard was supplied as a gift sample from UP Pharma (Assuit, Egypt). ACNHPLC-grade, Potassium dihydrogen phosphate, Hydrochloric acid 37%, Sodium hydroxide, and Hydrogen peroxide 30% (Scharlau, Spain). Water for injection (WFI) was used in the analysis and passed through a 0.45 μm nylon membrane filter before use. Phosphate solution was prepared by weighing about 5.82 g of potassium dihydrogen phosphate in 1000 mL of WFI.
Chromatographic system configuration
Compared with the previously conducted HPLC methods and the current analysis method, we did not use a high percentage of the organic modifier of ACN, dedicated pH solution adjustment, or special chemical reagent to realize the optimum separation for the ideal system suitability achievement.
Cfz assay determination was conducted using the HPLC model HP 1100 series with variable wavelength. The current method was conducted with the RP C18 ODS column (150 mm × 4.6 mm × 5 μm) (Thermo Scientific). The mobile phase was prepared as "KH2PO4 solution "(5.82 g in 1000 mL of WFI)": ACN in a ratio (80:20, v/v) at a flow rate of 1.0 mL/min with detection wavelength at 230 nm at room temperature and injection volume 20 μL.
Parameters of method validation
The HPLC validation method was performed according to the International Conference on Harmonization (ICH) guidelines concerning parameters including system suitability, Range of linearity, the limit of detection (LOD), the limit of quantification (LOQ), repeatability (precision), recovery and accuracy, robustness, ruggedness, the stability of the solution, specificity, and selectivity [12,13,14, 34, 35].
System suitability check
System Suitability was performed by injecting six replicate injections of the same sample solution which was prepared by dissolving a quantity of Cfz sodium equivalent to 1.0 g of Cfz [stock solution] in 1000 mL of WFI, then diluting 10 mL into 100 mL volumetric flask using of WFI to get a solution with concentration about 100 µg/mL.
Range and linearity
The analytical approach is deemed to be linear if there is a substantial portion between the response and claimed working concentration starting at the lowest point in the tested range and increasing to the highest point with R2 ≥ 0.999 [6, 7, 12,13,14,15, 34, 35].
Regression linearity equation:
where Y represents the response of the average peak area, X represents the claimed working concentration in (%), a represents the slope and b is the intercept of the calibration curve.
The linearity parameter was submitted using five concentrations in the range (50–150%) of the Cfz working standard. The claimed working concentrations were prepared as, 50, 70, 100, 120, and 150 µg/mL using the WFI as a solvent from the stock solution at a concentration of 1000 µg/mL. Every solution was injected into duplicates.
Limit of detection (LOD)
It was defined as the lowest specific analyte concentration in the matrix that could be identified using the detection of the instrument. Furthermore, it should not be included in the accuracy, precision, and linearity ranges [12,13,14, 34,35,36].
Limit of quantitation (LOQ)
It was defined as the lowest specific analyte concentration in the matrix that could be identified using the detection of the instrument. Furthermore, it must be included in the accuracy, precision, and linearity ranges [12,13,14, 34,35,36].
LOD and LOQ could be calculated according to the slope and standard error data from the linearity of the calibration as the following:
where σ: is the standard error of X & Y arrays and S: represents the slope of the linearity calibration curve.
Accuracy and recovery
Both recovery and accuracy are used alternatively. The measurement’s accuracy is defined as the proximity of the actual concentration (measured value) to the theoretical concentration (true value) [12,13,14, 34, 35].
Accuracy was implemented by weighing three individual Cfz working standards to give theoretical concentrations at (70 µg/mL), (100 µg/mL), and (120 µg/mL). Accuracy % could be estimated using the linearity equation:
Repeatability and precision
Repeatability was conducted using six different preparations individually of the target concentration of the intended method (100% = 100 µg/mL) of using the same equipment on the same day via the same analyst or compared with another analyst as inter precision [12,13,14, 34, 35].
Robustness
Robustness was submitted using designed small changes including slight changes in the temperature, composition of the mobile phase, etc.
The designed small changes were conducted in a different organic solvent ratio (ACN) at (20% ± 2.5%) and KH2PO4 solution (80% ± 2.5%) and a flow rate (1 mL/min ± 0.1 mL/min).
Ruggedness
Ruggedness was submitted using designed and major observable changes including analyst-analyst, column-column, and day-day with maintaining all the analysis method parameters and conditions as it is without changes [37].
Stability of the mobile phase
This test was performed to judge the stability of the mobile phase composition over time. The test was conducted at the target concentration of (100% = 100 µg/mL) over 14 days. The mobile phase is valid to use at room temperature over 14 days the retention time, USP tailing, and theoretical plates within the acceptance criteria of the conducted experiments.
Specificity and selectivity
Forced degradation studies were performed to indicate the stability-indicating properties. Also, to ease verification and distinguishing the active substance under study “Cfz” from the possibility of its presence among many applied catalytic degradation products. Accelerated degradation was implemented using acid hydrolysis of 0.1 M HCl for 30 min, base hydrolysis of 0.1 M of NaOH for 30 min, accentuate oxidation degradation of 3.0% w/v of H2O2 for 30 min, and light degradation over 6 h.
Test of the validated method
Cfz analysis of the different commercial dosage forms in the Egyptian local market
Peracef 1 g vials, Peractam 1.5 g vials, Trexotaz 1.5 g vials, and Sulbacef 1.5 g vials were tested using the validated method of Cfz.
Peracef 1.0 g vials batch number (220001) after the constitution and after dilution stability studies
The after-constitution stability study was conducted using the supplied solvent WFI at zero time, 24 h at room temperature 30 ± 2 °C, and in the refrigerator at temperature 5 ± 3 °C for 24 h. Also, the after-dilution stability study was conducted in the refrigerator at the same previous time interval using the solutions for infusion as shown in Table 1.
The constituted vial was performed using 5 mL of the WFI, then all the contents of the vial were transferred into a 1000 mL volumetric flask. Then a dilution of 20 mL of the constituted solution (1 mg/mL) in a 200 mL volumetric flask using WFI was conducted and introduced to the HPLC for assay in a final theoretical concentration (0.1 mg/mL of Cfz).
On the other hand, the after-dilution study was conducted individually for each solution as the following, all the vial content (theoretically 1000 mg of Cfz) in 500 mL of each pack. Then a dilution of 10 mL of the diluted Cfz solution (2 mg/mL) in a 200 mL volumetric flask was performed using WFI and introduced to the HPLC for assay in a final theoretical concentration (0.1 mg/mL of Cfz).
Determination of minimum inhibitory concentration (MIC) and minimum bacteriostatic concentration (MBC) of Cfz using HPLC-validated method
The conventional broth micro-dilution tube method was used to determine the minimum inhibitory concentration (MIC) and minimum bacteriostatic concentration (MBC) of Cfz against the standard strain of Burkholderia cepacian (B. cepacian) ATCC 25416 as Gram-negative bacteria in vitro [38].
An overnight culture of Gram-negative bacteria, B. cepacian ATCC 25416, was inoculated in Mueller–Hinton broth, adjusted to equivalent turbidity of 0.5 McFarland standards. Five different concentrations of Cfz (1000, 500, 250, 125, and 62.5 μg/mL) were diluted in five tubes (1 to 5) containing Muller–Hinton broth medium and used for testing their antimicrobial activities [38,39,40,41].
Under aseptic conditions, the prepared bacterial suspension was inoculated in the tested tube containing the different concentrations of the tested antibiotic. The tubes were incubated at 37 °C for 24 h.
The antimicrobial activity was assessed by visual observation of turbidity [42], moreover measuring the assay activity for the Cfz peak by the current validated HPLC method.
Results and discussions
System suitability check
Cfz peak appeared about at 5.0 ± 0.1 min at the optimum parameters of the analysis method Fig. 2 and in the range of (2.9–7.2) ± 0.1 min over all the parameter changes shown in Additional file 1: Fig. S1a–g. Table 2 showed high performance for the intended analysis method where the RSD% < 2.0%, USP tailing < 2.0, and theoretical plates ≥ 2000 [6, 7, 12,13,14,15, 34, 35]. So, according to the output data of the system suitability parameters, the method manifested superior validity through a wide range of retention times.
Range and linearity
The results manifested high linearity with R2 = 0.9993 at the working concentrations in the range (50–150%) as we can see in Fig. 3 and Table 3.
LOD and LOQ
LOD and LOQ limits could be determined simply by using the linearity calibration data of Cfz. LOD was found to be 4.04 µg/mL whereas LOQ was 12.24 µg/mL.
Accuracy and recovery
Table 4 showed that the accuracy results of the tested range (70–120% from the target concentration of 100% = 100 µg/mL) were found to be within the acceptance criteria (98–102%) [6, 7, 12,13,14,15, 34, 35].
Repeatability and precision
The RSD% of peak areas was used for judgment on the repeatability of the analyte using six different preparations at the same target (100 µg/mL of Cfz) concentration as in Additional file 1: Table S1. It was found to be 0.39% and 0.34% within intra-precision and 0.92% at the inter-precision as it demanded in repeatability requirements RSD% < 2.0% [6, 7, 12,13,14,15, 34, 35].
Robustness
The results of conscious small changes included a flow rate ± 0.1 mL/min and ACN (± 2.5%) was determined using RDS%. The RSD% was found to be < 2% in all cases as shown in Additional file 1: Tables S2 and S3. It is clear that is a reverse proportion between the retention time and the ratio of the organic modifier of the ACN. Where the retention time increases by decreasing the organic ratio and vice versa. This assures the principle chromatographic rule "likes to dissolve likes or likes attract likes"[12, 14, 34, 35].
Ruggedness
The results of conscious major and observable changes include day-day and column-column. Data was presented as shown in Additional file 1: Tables S4 and S5. RSD% was found to be < 2% in all cases [12,13,14].
Stability of mobile phase composition
The experimental results revealed that the tested solution of Cfz could is given repeatable and precise system suitability data over 14 days at room temperature as shown in Additional file 1: Table S6.
Specificity and selectivity
The current method supplied us with highly specific data about the resolution and separation performance of the adjacent co-eluted peaks for the Cfz principle peak with a resolution parameter of at least 1.84 as shown in Additional file 1: Table S7 and Fig. S2a–2d.
Table 5 shows a comparison among some of the HPLC analysis methods procedures to determine Cfz. As we can see in our method there is no need to use of special chemical reagent, high organic percentage, pH adjustment of the mobile phase, post-column, pre-column, or high temperature. Additionally, the presented retention time is very acceptable with the time-saving analysis method.
Test of the validated method
Cfz analysis of the different commercial dosage forms in the Egyptian local market
The Cfz assay results of Peracef 1 g vials, Peractam 1.5 g vials, Trexotaz 1.5 g vials, and Sulbacef 1.5 g vials revealed good results, as manifested in Additional file 1: Table S8. Where the method was found to be selective, specific, and resoluted for the Cfz peak either in the presence of sulbactam ingredients.
Peracef 1.0 g vials batch number (220001) after the constitution and after dilution stability studies
The tabulated results in Additional file 1: Tables S9–S13 confirmed the stability and validity of the use of the Cfz solutions either after constitution using WFI at 30 ± 2 °C and 5 ± 3 °C for 24 h or after dilution using different solutions such as sodium chloride 0.9% wt/v, Glucose 5% wt/v, Glucose 10% wt/v, and Ringer solutions. Where the assay% was found to be within the acceptance criteria and did not exceed 3.0% from the starting assay at zero time. Also, the results manifested that the method did not affect the composition of the different initiators of the solvent on the retention time over the study.
Observations of B. cepacia
The MIC test demonstrates the lowest level of an antibacterial agent that greatly inhibits growth; the MBC demonstrates the lowest level of an antibacterial agent resulting in microbial death [39,40,41].
After the MIC determination of the Cfz, aliquots of 50 μL from all the tubes which showed no visible bacterial growth were seeded on Tryptic soy agar plates and incubated at 37 °C for 24 h. When the bacterial growth is not observed at the lowest concentration of an antibacterial agent, it is termed an MBC endpoint. This was done by observing the inoculated plate's pre and after-being incubated for the presence or absence of bacterial growth.
After 24 h of incubation as it was manifested in Additional file 1: Fig. S3, no growth was observed in the negative control, turbidity was noticed in test tube no 5 containing a concentration of 62.5 μg/mL, and tube no 4 containing a concentration of 125 μg/mL of Cfz antibiotic, indicating the growth of bacteria. Whereas the tube from (1 to 3) contained conc of 1000, 500, and 250 μg/mL, no turbidity was seen, exhibiting inhibition of bacterial growth, meaning that the MIC was obtained at 250 μg/mL.
The suspension from the tubes of 250, 500, and 1000 μg/mL was inoculated on the Tryptic soy agar plate and incubated for 24 h. The result showed bacterial growth in the plate inoculated from tube no 3 containing the concentrations of 250 μg/mL, whereas no bacterial growth was observed in the other concentrations 500 and 1000 μg/mL, hence confirming it as bactericidal.
Determination of the MIC and MBC of Cfz using the HPLC-validated method
Fortunately, and according to our literature survey, we have reported the determination of the MIC and MBC of Cfz, and it became clear to us that we might be the pioneer to conduct this study for the first time. Positive results with high sensitivity showed the ability of the current validated HPLC method to use in MIC and MBC determination in an accurate and precise method. This method has precedence and superiority over the use of the ordinary and common method for measuring turbidity using spectrophotometry. This is due to the high ability of HPLC to separate the target Cfz peak from all the matrix content, especially at the low concentration levels of Cfz.
HPLC results of the Cfz after the incubation were summarized in Additional file 1: Table S14.
The manifested Cfz assay results confirmed that inhibition of the B. cepacia bacterial growth had a direct proportion with the increase in the Cfz conc. Where it was very clear at the lowest Cfz conc, the Cfz peak area and its assay hadn't been detected. On the contrary, by increasing the Cfz conc, the remaining Cfz peak areas were increased and so the assay was increased. This finding confirmed the obtained visual examinations that were manifested in Additional file 1: Fig. S3.
Also, the data that were tabulated in Additional file 1: Table S14 could be used as an index to determine the effective conc of the Cfz to prevent the B. cepacia bacterial inhibition to grow as was demonstrated in the determination of the MIC and MBC of Cfz.
Limitations of the current analysis method
From our point of view, as it became clear to us through the study of MIC and MBC, the composition of the media affects the cefoperazone drug, and therefore we made the negative control. In addition to the possibility of the emergence of new degradation materials in the case of using this method for determination of the mixture components of cefoperazone and sulbactam. So, we will conduct a supplementary study for the current manuscript that studies the stability degradation effect of cefoperazone in the presence of sulbactam at the shelf life.
Conclusions
The validity of the new analysis method for the quantitation and detection of Cfz at low concentration levels by achieving acceptance criteria was confirmed by the validation guidelines. The use of the current method was verified for the first time in the determination of MIC and MBC especially, for the standard strain of B. cepacia ATCC 25416 at MIC equals 250 μg/mL. Whereas the MBC was given at 500 μg/mL. Also, Cfz was determined alone and in combination with sulbactam, which indicates that the current innovative analysis method has high specificity and selectivity. The importance of this method was also evident in the complete separation of Cfz from any degradation products that may be present. The minimum resolution between the Cfz principal peak and the most adjacent related impurity peak is 1.84.
Availability of data and materials
All data generated or analyzed during this study are included in this article and the raw data is available from the corresponding author if requested.
Abbreviations
- ACN:
-
Acetonitrile
- ATCC:
-
American Type Culture Collection
- B. cepacia :
-
Burkholderia cepacia
- BP:
-
British-Pharmacopoeia
- Cfz:
-
Cefoperazone
- Conc:
-
Concentration
- HPLC:
-
High-performance liquid chromatography
- UV:
-
Ultraviolet
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- MIC:
-
Minimum inhibitory concentration
- MBC:
-
Minimum bacteriostatic concentration
- MeOH:
-
Methanol
- P. As:
-
Peak areas
- RSD:
-
Relative standard deviation
- STDEV:
-
Standard deviation
- USP:
-
United States Pharmacopeia
- WFI:
-
Water for injection
References
Selavka C, Krull I, Bratin K. Analysis for penicillins and cefoperazone by HPLC—photolysis—electrochemical detection (HPLC—hv—EC). J Pharm Biomed Anal. 1986;4(1):83–93.
Tsou TL, Huang YC, Lee CW, Lee AR, Wang HJ, Chen SH. Simultaneous determination of ampicillin, cefoperazone, and sulbactam in pharmaceutical formulations by HPLC with β-cyclodextrin stationary phase. J Sep Sci. 2007;30(15):2407–13.
El-Shanawani AA. HPLC determination of sulbactam, sultamicillin tosylate, cefaclor, ampicillin and cefoperazone in pharmaceutical preparations. Acta Pol Pharm. 1998;55(1):9–14.
Türkan F, Huyut Z, Demir Y, Ertaş F, Beydemir Ş. The effects of some cephalosporins on acetylcholinesterase and glutathione S-transferase: an in vivo and in vitro study. Arch Physiol Biochem. 2019;125(3):235–43.
Saddik MS, Elsayed MMA, Abdel-Rheem AA, El-Mokhtar MA, Mosa ES, Al-Hakkani MF, Al-Shelkamy SA, Khames A, Daha MA, Abdel-Aleem JA. A Novel C@Fe@Cu nanocomposite loaded with doxorubicin tailored for the treatment of hepatocellular carcinoma. Pharmaceutics. 2022;14(9):1845.
Al-Hakkani MF, Gouda GA, Hassan SHA, Mohamed MMA, Nagiub AM. Environmentally azithromycin pharmaceutical wastewater management and synergetic biocompatible approaches of loaded azithromycin@hematite nanoparticles. Sci Rep. 2022;12:10970.
Al-Hakkani MF, Gouda GA, Hassan SHA, Saddik MS, El-Mokhtar MA, Ibrahim MA, Mohamed MMA, Nagiub AM. Cefotaxime removal enhancement via bio- nanophotocatalyst α-Fe2O3 using photocatalytic degradation technique and its echo-biomedical applications. Sci Rep. 2022;12:11881.
Saddik MS, Elsayed M, El-Mokhtar MA, Sedky H, Abdel-Aleem JA, Abu-Dief AM, Al-Hakkani MF, Hussein HL, Al-Shelkamy SA, Meligy FY. Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics. 2022;14(1):111.
Al-Hakkani MF, Hassan SHA, Saddik MS, El-Mokhtar MA, Al-Shelkamy SA. Bioengineering, characterization, and biological activities of C@Cu2O@Cu nanocomposite based-mediated the Vicia faba seeds aqueous extract. J Mater Res Technol. 2021;14(5):1998–2016.
Al-Hakkani MF, Gouda GA, Hassan SHA. A review of green methods for phytofabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon. 2021;7(1): e05806.
Al-Hakkani MF. Biogenic copper nanoparticles and their applications: a review. SN Appl Sci. 2020;2(3):505.
Al-Hakkani MF. A rapid, developed and validated RP-HPLC method for determination of azithromycin. SN Appl Sci. 2019;1(3):222.
Al-Hakkani MF. Guideline of inductively coupled plasma mass spectrometry “ICP–MS”: fundamentals, practices, determination of the limits, quality control, and method validation parameters. SN Appl Sci. 2019;1(7):791.
Al-Hakkani MF. Forced degradation study with a developed and validated RP-HPLC method for determination of cefpodoxime proxetil in the bulk and finished pharmaceutical products. J Iran Chem Soc. 2019;16(7):1571–8.
Al-Hakkani MF, Gouda GA, Hassan SHA, Mohamed MMA, Nagiub AM. Cefixime wastewater management via bioengineered Hematite nanoparticles and the in-vitro synergetic potential multifunction activities of Cefixime@Hematite nanosystem. Surf Interfaces. 2022;30: 101877.
Soto C, Saavedra R, Contreras D, Poza C, Orellana S, Olivares-Rentería GA. Flow injection analysis (FIA) with thermal lens spectrometry (TLS) for the rapid determination of cefoperazone in urine and water. Anal Lett. 2022;55(12):1932–44.
Saraya RE, Abdel Hameed EA. Eco-friendly micellar HPTLC technique for the simultaneous analysis of co-formulated antibiotic cefoperazone and sulbactam in pure form and vial pharmaceutical formulation. J Planar Chromatogr-Mod TLC. 2021;34(2):121–9.
Farrag SA, Rageh AH, Askal HF, Saleh GA. Biocompatible magnetite nanoparticles coated with ionic liquid-based surfactant as a hydrophilic sorbent for dispersive solid phase microextraction of cephalosporins prior to their quantitation by HPTLC. J Chromatogr B. 2022;1205: 123339.
Abdelaleem EA, Naguib IA, Zaazaa HE, Hussein EA. Development and validation of HPLC and HPTLC methods for determination of cefoperazone and its related impurities. J Chromatogr Sci. 2015;54(2):179–86.
Abdelaleem EA, Naguib IA, Zaazaa HE, Hussein EA. Development and validation of HPLC and HPTLC methods for determination of cefoperazone and its related impurities. J Chromatogr Sci. 2016;54(2):179–86.
Sun H, Xing H, Tian X, Zhang X, Yang J, Wang P. UPLC-MS/MS method for simultaneous determination of 14 antimicrobials in human plasma and cerebrospinal fluid: application to therapeutic drug monitoring. J Anal Methods Chem. 2022;2022:7048605.
Nemutlu E, Kır S, Katlan D, Beksac MS. Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: application to validation and quantification of cefepime, cefixime and cefoperazone. Talanta. 2009;80(1):117–26.
Dhandapani B, Thirumoorthy N, Rasheed SH, Kotaiah MR, Chandrasekhar K. RP-HPLC method development and validation for the simultaneous estimation of cefoperazone and sulbactam in parenteral preparation. Int J Chem Tech Res. 2010;3:752–5.
Ivaturi R, Sastry TM, Sunkara S. Development and validation of stability indicating HPLC method for the determination of impurities in the sterile mixture of cefoperazone and sulbactam. Curr Pharm Anal. 2019;15(7):762–75.
Abd El Aziz Shama S, Abd El Azim S, Elham A, Shaimaa HN. A simultaneous, validated RP-HPLC method for determination of eight cephalosporins in pharmaceutical formulations. Sys Rev Pharm J. 2021;12(2):1–12.
Salem H, Samir E. Determination of cefotaxime, cefoperazone, ceftazidime and cefadroxil using surface plasmon resonance band of silver nanoparticles. Braz J Pharm Sci. 2018. https://doi.org/10.1590/s2175-97902018000317565.
Zaky M, Fadel S, Elgendy KM. Spectrophotometric method for determination of some third generation cephalosporins antibiotics using sodium molybdate. Eurasian J Anal Chem. 2020;15(1):59–73.
Ara J, Sharif SR, Nawrin F, Islam MS. Cefoperazone with antacid, metal complexation studied by spectrophotometrically and exploration of antimicrobial activity, in-vitro investigation. Biomed J Sci Tech Res. 2020;31(2):24093–7.
Patel E, Shah D, Vegad K, Patel T, Patel K, Patel Y. UV-visible derivative spectroscopic method for simultaneous estimation of cefoperazone and tazobactam in injection dosage form. Formulary. 2017;6(7):8.
Wu XJ, Huang X, Shi HY, Chen XK, Dong Q, Hao GX, Li Y, Zheng Y, Zhao W. Determination of cefoperazone and sulbactam in serum by HPLC-MS/MS: an adapted method for therapeutic drug monitoring in children. Biomed Chromatogr. 2018;32(4): e4143.
Al-Hakkani MF, Gouda GA, Hassan SHA, Nagiub AM. Echinacea purpurea mediated hematite nanoparticles (α-HNPs) biofabrication, characterization, physicochemical properties, and its in-vitro biocompatibility evaluation. Surf Interfaces. 2021;24: 101113.
Saddik MS, Alsharif FM, El-Mokhtar MA, Al-Hakkani MF, El-Mahdy MM, Farghaly HS, Abou-Taleb HA. Biosynthesis, characterization, and wound-healing activity of phenytoin-loaded Copper nanoparticles. AAPS PharmSciTech. 2020;21(5):1–12.
Saddik MS, Elsayed MMA, Abdelkader MSA, El-Mokhtar MA, Abdel-Aleem JA, Abu-Dief AM, Al-Hakkani MF, Farghaly HS, Abou-Taleb HA. Novel green biosynthesis of 5-fluorouracil chromium nanoparticles using Harpullia pendula extract for treatment of colorectal cancer. Pharmaceutics. 2021;13(2):226.
Al-Hakkani MF, Gouda GA, Hassan SHA, Farghaly OA, Mohamed MMA. Fully investigation of RP- HPLC analytical method validation parameters for determination of Cefixime traces in the different pharmaceutical dosage forms and urine analysis. Acta Pharm Sci. 2021;59(1):97–111.
Al-Hakkani MF. HPLC analytical method validation for determination of Cefotaxime in the bulk and finished pharmaceutical dosage form. Sustain Chem Eng. 2020;1:33–42.
Bhaskaran NA, Kumar L, Reddy MS, Pai GK. An analytical “quality by design” approach in RP-HPLC method development and validation for reliable and rapid estimation of irinotecan in an injectable formulation. Acta Pharm. 2021;71(1):57–79.
Al-Hakkani MF, Ahmed N, Hassan MHA. Rapidly sensitive quantitative assessment of thiopental via forced stability indicating validated RP-HPLC method and its in-use stability activities. Sci Rep. 2023;13(1):10294. https://doi.org/10.1038/s41598-023-37329-0.
Hassan M, Ismail M, Moharram A, Shoreit A. Synergistic effect of biogenic silver-nanoparticles with β lactam Cefotaxime against resistant Staphylococcus arlettae AUMC b-163 isolated from T3A pharmaceutical cleanroom, Assiut, Egypt. Am J Microbiol Res. 2016;4:132–7.
Moharram A, Ismail M, Shoreit A, Hassan M. Biodiversity of microbiota in cephalosporin-manufacturing environments at T3A factory, Assiut, Egypt. J Basic Appl Mycol. 2014;5:1–13.
Hassan MH, Ismail MA, Moharram AM, Shoreit AA. Phytochemical and antimicrobial of latex serum of Calotropis procera and its silver nanoparticles against some reference pathogenic strains. J Ecol Health Environ. 2017;5(3):65–75.
Hassane AMA, Hussien SM, Abouelela ME, Taha TM, Awad MF, Mohamed H, Hassan MM, Hassan MHA, Abo-Dahab NF, El-Shanawany ARA. In vitro and in silico antioxidant efficiency of bio-potent secondary metabolites from different taxa of black seed-producing plants and their derived mycoendophytes. Front Bioeng Biotechnol. 2022;10:1–12.
Hassan M, Moharram A, Ismail M, Shoreit A. Biogenic silver nanoparticles of resistant Aspergillus flavus AUMC 9834 against some pathogenic microorganisms and its synergistic effect with the antifungal fluconazole. J Basic Appl Mycol. 2015;6:1–7.
Acknowledgements
The corresponding authors gratefully acknowledge UP Pharma Industrial for its valuable support.
Experimental work and methods
We confirm that all methods were carried out following relevant guidelines and regulations.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MFA, NA, AAA, MHA: Conceptualization, methodology, software, data curation. MFA, MHA: Visualization, investigation, supervision, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research is not involving human participants or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1a.
Cfz chromatogram at a flow rate 0.9 mL/min, Column—1, Buffer 80% & Acetonitrile 20%. Figure S1b. Cfz chromatogram at a flow rate 1.1 mL/min, Column—1, Buffer 80% & Acetonitrile 20%. Figure S1c. Cfz chromatogram at a flow rate 1.0 mL/min, Column—1, Buffer 82.5% & Acetonitrile 17.5%. Figure S1d. Cfz chromatogram at a flow rate 1.0 mL/min, Column—1, Buffer 77.5% & Acetonitrile 22.5%. Figure S1e. Cfz chromatogram at a flow rate 1.0 mL/min, Column—1, Buffer 80% & Acetonitrile 20%, Day—2. Figure S1f. Cfz chromatogram at a flow rate 1.0 mL/min, Column—2, Buffer 80% & Acetonitrile 20%, Day—2. Figure S1g. Cfz chromatogram at a flow rate 1.0 mL/min, Column—3, Buffer 80% & Acetonitrile 20%, Day—2. Figure S2a. Forced degradation using acid hydrolysis 0.1 M HCl for 30 min. Figure S2b. Forced degradation using base hydrolysis 0.1 M NaOH for 30 min. Figure S2c. Forced degradation using H2O2 3% w/v hydrolysis for 30 min. Figure S2d. Light-forced degradation after 6 h. Figure S3. Visual examination of the B. cepacia bacterial growth after 24 h. Table S1. Repeatability and precision. Table S2. Change in the flow rate results (0.9 mL/min–1.1 mL/min). Table S3. Change in organic ratio results (17.5–22.5%). Table S4. Day-to-day precision results. Table S5. Column-to-Column precision results. Table S6. Mobile phase composition system suitability. Table S7. Resolution factor at different forced degradation states. Table S8. Cfz assay for the different local finished products. Table S9. Cfz after constitution stability study using WFI. Table S10. Cfz after dilution stability study using Sodium chloride 0.9%. Table S11. Cfz after dilution stability study using Glucose 5%. Table S12. Cfz after dilution stability study using Glucose 10%. Table S13. Cfz after dilution stability study using Ringer. Table S14. Cfz assay after incubation with B. cepacia of at 37 °C for 24 h.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Al-Hakkani, M.F., Ahmed, N., Abbas, A.A. et al. Cefoperazone rapidly and sensitive quantitative assessment via a validated RP-HPLC method for different dosage forms, in-use stability, and antimicrobial activities. BMC Chemistry 17, 72 (2023). https://doi.org/10.1186/s13065-023-00989-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-00989-0