Abstract
Background
Associations between reproductive factors and risk of breast cancer differ by subtype defined by joint estrogen receptor (ER), progesterone receptor (PR), and HER2 expression status. Racial and ethnic differences in the incidence of breast cancer subtypes suggest etiologic heterogeneity, yet data are limited because most studies have included non-Hispanic White women only.
Methods
We analyzed harmonized data for 2,794 breast cancer cases and 4,579 controls, of whom 90% self-identified as African American, Asian American or Hispanic. Questionnaire data were pooled from three population-based studies conducted in California and data on tumor characteristics were obtained from the California Cancer Registry. The study sample included 1,530 luminal A (ER-positive and/or PR-positive, HER2-negative), 442 luminal B (ER-positive and/or PR-positive, HER2-positive), 578 triple-negative (TN; ER-negative, PR-negative, HER2-negative), and 244 HER2-enriched (ER-negative, PR-negative, HER2-positive) cases. We used multivariable unconditional logistic regression models to estimate subtype-specific ORs and 95% confidence intervals associated with parity, breast-feeding, and other reproductive characteristics by menopausal status and race and ethnicity.
Results
Subtype-specific associations with reproductive factors revealed some notable differences by menopausal status and race and ethnicity. Specifically, higher parity without breast-feeding was associated with higher risk of luminal A and TN subtypes among premenopausal African American women. In contrast, among Asian American and Hispanic women, regardless of menopausal status, higher parity with a breast-feeding history was associated with lower risk of luminal A subtype. Among premenopausal women only, luminal A subtype was associated with older age at first full-term pregnancy (FTP), longer interval between menarche and first FTP, and shorter interval since last FTP, with similar OR estimates across the three racial and ethnic groups.
Conclusions
Subtype-specific associations with reproductive factors overall and by menopausal status, and race and ethnicity, showed some differences, underscoring that understanding etiologic heterogeneity in racially and ethnically diverse study samples is essential. Breast-feeding is likely the only reproductive factor that is potentially modifiable. Targeted efforts to promote and facilitate breast-feeding could help mitigate the adverse effects of higher parity among premenopausal African American women.
Similar content being viewed by others
Introduction
Racial and ethnic differences in the incidence of breast cancer subtypes are well documented in the Surveillance, Epidemiology, and End Results (SEER) Program [1]. Among incident cases with known subtype defined by estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) [1], luminal A (ER-positive and/or PR-positive and HER2-negative) is the most common subtype, accounting for 72.7% of breast cancers, with the highest incidence among non-Hispanic White (NHW) women. Triple negative (TN) subtype (ER-negative and PR-negative and HER2-negative) accounts for 12.2% of breast cancers, and, among women diagnosed under age 50 years, the incidence is highest among African American and Hispanic women. Luminal B (ER-positive and/or PR-positive and HER2-positive) and HER2-enriched (ER-negative and PR-negative and HER2-positive) subtypes account for 4.6% and 10.3% of breast cancers, respectively. Racial and ethnic differences in the incidence of breast cancer subtypes suggest etiologic heterogeneity. Most epidemiologic studies, however, included NHW women only [2,3,4,5,6,7]. There is a need to better understand risk factors for breast cancer subtypes among racially and ethnically minoritized populations who have a greater burden of the clinically more aggressive subtypes that have poorer prognosis compared to luminal A subtype [8].
We investigated subtype-specific associations with reproductive characteristics which are well established risk factors for breast cancer [9, 10]. Heterogeneity by subtypes has been reported, although results are not consistent [2,3,4,5,6,7]. Furthermore, most findings on subtype-specific associations with reproductive factors are based on cohort and case-control studies [11,12,13,14,15,16,17,18,19] and pooled analyses [4, 6, 20, 21] that included mostly NHW women; few studies have been conducted among African American women [21,22,23,24,25], and subtype-specific analyses among Asian American or Hispanic women are lacking. We previously examined associations between reproductive factors and risk of breast cancer defined by joint ER/PR status in the Breast Cancer Etiology in Minorities (BEM) Study, a population-based pooled dataset with 90% of study participants who self-identified as African American, Asian American, or Hispanic [26, 27]. Building upon this previous work, the present analysis was based on a subset of women with breast cancer who had complete data on ER/PR/HER2 status. There is some evidence that age at diagnosis or menopausal status may modify some subtype-specific associations with reproductive factors, but findings are not consistent [6, 7, 17, 27,28,29,30,31,32]. Given that younger women are more likely to be diagnosed with more aggressive breast cancer subtypes compared with older women [1], an evaluation of menopause-specific associations with reproductive factors is warranted. To fill these gaps in knowledge, we conducted subtype-specific case-control analyses overall and by menopausal status and race and ethnicity.
Materials and methods
Study sample
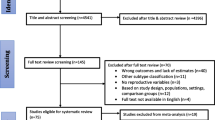
The analysis was based on harmonized data from three population-based studies included in the BEM Study [26]: the Los Angeles County Asian American Breast Cancer Study (AABCS), a case-control study of Chinese, Japanese, and Filipina women [33]; the San Francisco Bay Area Breast Cancer Study (SFBCS), a case-control study of Hispanic, African American, and NHW women [34]; and the Northern California Breast Cancer Family Registry (NC-BCFR), a multiethnic family study that oversampled African American, Chinese, Filipina, Japanese, and Hispanic women and also included population controls [35] (Additional file 1: Table S1). Briefly, the three studies ascertained incident female breast cancer cases through regional population-based cancer registries that are part of the California Cancer Registry and the SEER Program. In AABCS, Chinese, Japanese, and Filipina cases aged 25–74 years, diagnosed with invasive breast cancer from 1995 to 2001 or 2003 to 2006, were ascertained through the Los Angeles County Cancer Surveillance Program. In SFBCS, African American, Hispanic and NHW women diagnosed with invasive breast cancer at age 35–79 years from 1995 to 1999 (all African American women and a 10% random sample of NHW women) or 1995 to 2002 (all Hispanic women) were ascertained through the Greater Bay Area Cancer Registry. In NC-BCFR, women diagnosed with invasive breast cancer at age 18–64 years were ascertained through the Greater Bay Area Cancer Registry (diagnoses 1995 to 2009) or the Sacramento and Sierra Cancer Registry (diagnoses 2005 to 2006). Details on the eligibility criteria and sampling in NC-BCFR are provided in Additional file 1: Table S1. Population controls were identified through random digit-dialing in SFBCS and NC-BCFR or neighborhood block-walking in AABCS, and frequency-matched to cases on race and ethnicity and age group. The Institutional Review Boards of the participating institutions approved the studies, and study participants provided signed informed consent.
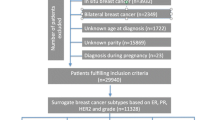
The present analysis included women with a first primary invasive breast cancer defined by joint ER/PR/HER2 status obtained from the regional cancer registries at each study site. Reporting of HER2 expression was not required before 1999 in California. Thus, HER2 data were available for only a subset of cases diagnosed during the early years of case ascertainment in the three studies. For 108 NC-BCFR cases diagnosed from 1995 to 1998 with data on ER/PR status, stored tumor slides were used to determine HER2 expression status by immunohistochemistry (by T.L.). Of 5,243 available controls, 20% were NHW, compared to 10% NHW cases. To achieve a more balanced pooled dataset for NHW women, we selected a random sample of available NHW controls frequency-matched to NHW cases at a 1:1.5 case-control ratio by 1-year age group. The current study sample comprised 2,840 cases and 4,653 controls, of whom 90% self-identified as non-Hispanic African American, non-Hispanic Asian American, or Hispanic (White or Black).
Data collection and harmonization
The three studies collected information on breast cancer risk factors using structured questionnaires that were administered in English, Spanish, Cantonese or Mandarin by trained staff in home visits. Risk factors were assessed up to the reference year which was defined as the calendar year before diagnosis for cases or before the interview for controls in AABCS and NC-BCFR or the calendar year before selection into the study for controls in SFBCS. Height and weight during the reference year were assessed by self-report in the three studies, and height and weight were measured at the interview in AABCS and SFBCS.
Questionnaire data were harmonized according to common definitions [26]. Race and ethnicity were based on self-report and categorized as non-Hispanic African American, non-Hispanic Asian American, Hispanic (White or Black), or NHW. Parity was defined as the number of full-term pregnancies (FTP). Lifetime duration of breast-feeding was calculated by summing duration of breast-feeding reported as a continuous measure for each live birth, except for NC-BCFR. In that study, breast-feeding was assessed as a categorical measure (0, < 1, 1–5, 6–11, 12–24, ≥ 25 months) for each pregnancy, and the midpoint of the reported category was used, or 0.5 and 30 months for the categories < 1 month and ≥ 25 months, respectively, to calculate lifetime duration of breast-feeding. To assess the joint association of breast-feeding and parity, we generated a composite variable (1–2 FTP/never breast-fed; 1–2 FTP/ever breast-fed; ≥3 FTP/never breast-fed; ≥3 FTP/ever breast-fed) that we and others have used previously [18, 27, 36,37,38]. Given that the lower breast cancer risk associated with higher parity is apparent only about 10 years after the last FTP [6], we also used a composite variable to assess the impact of time since last FTP on parity (< 10 years/1–2 FTP; <10 years/≥3 FTP; ≥10 years/1–2 FTP; ≥10 years/≥3 FTP). Women who still had menstrual periods or were pregnant, breast-feeding or perimenopausal during the reference year, and under age 55 years were classified as premenopausal. Women who reported that their periods had stopped naturally or due to surgery, medical treatment, or other reasons prior to the reference year were classified as postmenopausal. Women who still had periods when they started using menopausal hormone therapy were classified as postmenopausal if they were ≥ 55 years of age; otherwise, their menopausal status was classified as unknown. Body mass index (BMI) was calculated as self-reported weight (kg) in the reference year divided by measured or self-reported height (m) squared. If self-reported weight in the reference year was missing, measured weight was used. If measured height was missing, self-reported height was used.
Statistical analyses
We used unconditional logistic regression models to calculate odds ratios (OR) as estimates of relative risks, in accordance with the rare disease assumption, particularly for breast cancer subtypes. We calculated OR and 95% confidence intervals (CI) for associations of breast cancer subtypes with parity, lifetime duration of breast-feeding, a composite parity/breast-feeding variable, age at menarche, age at first FTP, interval between age at menarche and first FTP, interval between last FTP and diagnosis, and a composite variable of interval between last FTP and diagnosis/parity. Because of smaller sample sizes, analyses for luminal B, TN, and HER2-enriched subtypes were based on broader exposure categories. Regression models were adjusted for race and ethnicity, study, age, education, first-degree family history of breast cancer, personal history of benign breast disease, history of oral contraceptive use, BMI in the reference year, and alcohol consumption in the reference year. Categories of the covariates are shown in the footnotes of the tables. Because the association between BMI and breast cancer risk differs by menopausal status [39], regression models for all women combined were additionally adjusted for a composite variable of menopausal status/BMI (premenopausal BMI < 25 kg/m2, premenopausal BMI 25-29.9, premenopausal BMI ≥ 30, postmenopausal BMI < 25, postmenopausal BMI 25-29.9, postmenopausal BMI ≥ 30, unknown menopausal status).
Among premenopausal women, we also adjusted the parity analyses for interval between last FTP and diagnosis. The OR estimates changed very minimally (results not shown) and we did not adjust for years since last FTP in the multivariable models presented in the tables. Linear trends were assessed across ordinal values of categorical variables. Separate analyses were performed for premenopausal and postmenopausal women. For comparison of findings with other studies, most of which did not stratify the analyses by menopausal status or age, we also performed analyses for all women combined that included those with unknown menopausal status. To assess heterogeneity in associations by subtype, we used polytomous regression models, and tested for differences in subtype-specific ORs using a Wald statistic p value. We tested for heterogeneity by menopausal status by including interaction terms for reproductive factors and menopausal status in unconditional logistic regression models, excluding women with unknown menopausal status. To test for heterogeneity by race and ethnicity, we included an interaction term of each exposure variable with race and ethnicity, and tested for heterogeneity using a Wald statistic p value. Among all women combined, we evaluated between-study heterogeneity in subtype-specific associations, separately for premenopausal and postmenopausal women, by including interaction terms for reproductive factors and study. We excluded 46 cases and 74 controls with missing covariate data, leaving 2,794 cases and 4,579 controls in the analytic dataset. NHW cases were only included in the TN analyses as there were only a small number of NHW cases with information on all three markers (84 luminal A, 14 luminal B, 10 HER2-enriched cases). However, because NC-BCFR recruited all TN cases diagnosed from 2007 to 2009 (see Additional file 1: Table S1), the TN case group included 165 NHW cases and analyses were stratified by the four racial and ethnic groups. Counts of controls and cases by subtype, menopausal status, race and ethnicity, and parity status are shown in Additional file 2: Table S2. Two-sided p values were used for tests of trend, with a p < 0.05 considered statistically significant. Statistical analyses were conducted using SAS version 9.4 software (SAS Institute, Inc., Cary, NC).
Results
Of 2,794 breast cancer cases in the analysis, 17% self-identified as African American, 39% Asian American, 34% Hispanic, and 10% NHW (Table 1). Hispanic cases were mostly White; only 17 Hispanic cases self-identified as Black. Compared to controls, higher proportions of cases had a higher education, a first-degree family history of breast cancer, nulliparity or low parity, older age at first FTP, no breast-feeding or for ≤ 12 months, premenopausal status, and higher alcohol consumption. Distributions of reproductive factors among controls varied widely by race and ethnicity (all p < 0.05) (Additional file 3: Table S3). Among premenopausal controls, proportions ranged from 6 to 30% for ≥ 4 FTP, 6 to 26% for breast-feeding ≥ 24 months, 4 to 34% for first FTP at age < 20 years; and 20 to 55% for ≥ 15-year interval between menarche and first FTP.
Associations between reproductive factors and breast cancer subtypes among all women
Among all women combined, heterogeneity in associations with parity status, parity, and age at first FTP was observed across subtypes (p < 0.05) (Table 2). For luminal A and luminal B subtypes, parity vs. nulliparity (OR = 0.64 and 0.68) and ≥ 4 vs. 1 FTP (OR = 0.55 and 0.46) were associated with lower risk. Longer breast-feeding (> 12 vs. 0 months) was associated with lower risk of luminal A (OR = 0.69) and HER2-enriched (OR = 0.60) subtypes. For the composite of parity/breast-feeding, lower risks were observed for women with ≥ 3 FTP and a history of breast-feeding compared to those with lower parity who never breast-fed, for all subtypes, with ORs ranging from 0.55 to 0.76 and all 95% CIs excluded the null except for TN subtype. Age at menarche was not associated with risk of any subtype. Higher risk of luminal A subtype was associated with older age at first FTP (OR per year = 1.02, p-heterogeneity by subtype = 0.02).
In analyses stratified by menopausal status (Table 3; Additional files 7–10: Figures S1-S4), associations of parity with risk of luminal A and luminal B subtypes were consistent by menopausal status. Parity was associated with lower risk of TN subtype among postmenopausal women only. Longer breast-feeding was associated with lower risk of both premenopausal (OR = 0.64, p trend = 0.02) and postmenopausal (OR = 0.76, p trend = 0.02) luminal A subtype and lower risk of HER2-enriched subtype among postmenopausal women only (OR = 0.54, p trend = 0.05). Among premenopausal women, the composite ≥ 3 FTP/ever breast-fed (vs. 1–2 FTP/never breast-fed) was associated with lower risk of luminal A subtype only (OR = 0.66), whereas among postmenopausal women, lower risks were associated with all subtypes, with ORs ranging from 0.46 to 0.64, although of borderline statistical significance for TN subtype.
Associations with timing of reproductive events were limited to luminal A subtype among premenopausal women, although heterogeneity by menopausal status did not reach statistical significance. Younger age at menarche was associated with higher risk of all subtypes, with ORs per year ranging from 1.06 to 1.10, although the p trend reached statistical significance only for luminal A subtype. Two-fold elevated risks were associated with older age at first FTP (≥ 30 vs. <20 years: OR = 2.09, p-heterogeneity by subtype = 0.01), longer interval between menarche and first FTP (≥ 15 vs. <10 years: OR = 2.41, p-heterogeneity by subtype = 0.04), and shorter interval since last FTP (< 10 vs. ≥20 years: OR = 1.74).
The assessment of between-study variation in subtype-specific associations, separately for premenopausal and postmenopausal women, showed no significant heterogeneity by study.
Associations between reproductive characteristics and breast cancer subtypes by menopausal status and race and ethnicity
Luminal A subtype (African American, Asian American, and Hispanic women)
Premenopausal women. Associations of parity status, parity, and the composite parity/breast-feeding history with risk of luminal A subtype were generally of similar magnitude across Asian American and Hispanic participant groups (Table 4; Fig. 1). Risk of luminal A subtype was not associated with age at menarche among premenopausal African American women, whereas for Asian American and Hispanic women, OR per year were 1.10 and 1.16, respectively. Higher risks were associated with older age at first FTP, longer interval between menarche and first FTP, and shorter interval since last FTP across the three racial and ethnic groups, with estimates of OR per year generally of similar magnitude. For the composite < 10 years since last FTP/1–2 FTP (vs. ≥10 years/≥3 1FTP), suggestive higher risks were observed among Asian American (OR = 1.85, 95% CI = 0.99–3.46) and Hispanic (OR = 2.36, 95% CI = 1.00-5.57) women, with no association among African American women.
Postmenopausal women. For parity status, parity, and breast-feeding, no heterogeneity by race and ethnicity was observed (Fig. 2). Higher parity (≥ 3 vs. 1 FTP) was associated with lower risk of luminal A subtype across racial and ethnic groups, with ORs ranging from 0.48 to 0.59. Lower risk was associated with the composite of higher parity with breast-feeding (vs. low parity without breast-feeding) across groups, with OR estimates ranging from 0.39 to 0.56. For age at menarche, we observed heterogeneity by race and ethnicity (p < 0.01). Earlier menarche (< 12 vs. ≥14 years) was associated with higher risk of luminal A subtype among postmenopausal Hispanic women only (OR = 2.00); no association was observed among African American women, whereas among Asian American women, there was an inverse association (OR = 0.52).
Luminal B subtype (African American, Asian American, and Hispanic women)
Few reproductive factors were associated with risk of luminal B subtype (Table 5). Among premenopausal women, heterogeneity by race and ethnicity was observed for parity (p = 0.04), breast-feeding history (p < 0.01), and interval between last FTP and diagnosis (p = 0.03). Higher parity was associated with lower risk among premenopausal Asian American (OR = 0.45) and Hispanic (OR = 0.33) women, but not among premenopausal African American women. Among postmenopausal women, higher parity (≥ 3 vs. 1–2 FTP) was associated with lower risk overall (OR = 0.57), with OR estimates of similar magnitude across the three racial and ethnic groups, ranging from 0.56 to 0.66. Lower risk was associated with older age at first FTP among Hispanic women and earlier menarche among Asian American women.
Triple-negative subtype (African American, Asian American, Hispanic women, and NHW women)
No significant heterogeneity in associations by race and ethnicity was observed among premenopausal women (Table 6; Fig. 3); however, patterns of association were different with respect to TN subtype among premenopausal African American women. Higher parity was associated with higher risk of TN subtype (≥ 3 vs. 1 FTP: OR = 5.75, 95% CI = 1.39–23.8), and an even higher OR for the composite of higher parity without breast-feeding (OR = 16.1, 95% CI = 2.64–97.8). While the OR was attenuated for the composite of higher parity with breast-feeding, it remained elevated (OR = 4.58, 95% CI = 1.02–20.5).
Among postmenopausal women, the composite of higher parity with breast-feeding was associated with lower risk of TN subtype, although the association was statistically significant among Asian American women only (OR = 0.38) (Fig. 4). Heterogeneity by race and ethnicity was observed for the interval between menarche and first FTP (p = 0.01), with a higher risk associated with longer interval observed among Asian American women only (≥ 11 vs. <11 years: OR = 2.31).
HER2-enriched subtype (African American, Asian American, and Hispanic women)
Analyses of HER2-enriched subtype stratified by menopausal status and race and ethnicity were based on small sample sizes (Table 7). Among premenopausal Hispanic women, lower risk was associated with parity vs. nulliparity (OR = 0.19, p-heterogeneity by race and ethnicity < 0.01), and higher risk was associated with longer interval between menarche and first FTP (≥ 11 vs. <11 years: OR = 4.87). Among African American women, higher risk was associated with parity vs. nulliparity, higher parity, and a breast-feeding history, but OR estimates were based on very small case counts. Among postmenopausal women, higher parity was associated with lower risk among African American women (≥ 3 vs. 1–2 FTP: OR = 0.23), and younger age at menarche was associated with higher risk among Hispanic women (< 13 vs. ≥13 years: OR = 2.26).
Discussion
To our knowledge, this is the only U.S. pooled study of breast cancer subtypes enriched with African American, Asian American, and Hispanic women. In the pooled dataset that comprised over 2,700 women with breast cancer, subtype-specific associations with reproductive factors were generally of similar magnitude across racial and ethnic groups and consistent with associations reported for NHW women. For luminal A subtype, lower risk associated with higher parity combined with a breast-feeding history was observed, regardless of menopausal status, with one exception. Among premenopausal African American women, higher parity without a breast-feeding history was associated with a higher risk of luminal A and TN subtypes; these higher risks, however, were attenuated by breast-feeding. For luminal A subtype among premenopausal women only, higher risk was associated with older age at first FTP, longer interval between menarche and first FTP, and shorter interval since last FTP, with similar OR estimates across the three racial and ethnic groups.
The two largest pooled analyses of breast cancer subtypes include an NCI Cohort Consortium analysis by Gaudet et al. (11,741 cases) [4] and an analysis of the Breast Cancer Association Consortium (BCAC) by Jung et al. (23,353 cases, 71,072 controls) [6]. Neither study presented racial- and ethnic-specific subtype results. Data are sparse for African American women on associations of reproductive factors with specific subtypes [21, 24, 25] or TN subtype [22, 23, 38]. The largest study for African American women to date is the African American Breast Cancer and Risk (AMBER) consortium (1,128 cases, 2,932 controls) [24]. To our knowledge, no prior studies have evaluated case-control associations with subtypes defined by joint ER/PR/HER2 status among Asian American and U.S. Hispanic women. Due to the diversity of the study sample (90% African American, Asian American, or Hispanic) and the over-sampling of TN cases in NC-BCFR, the proportions of women with luminal B (16%) and TN (21%) subtypes were higher in our study compared to U.S. population estimates [1].
For all women combined, the present findings of lower risk associated with parous status and higher parity (luminal A and luminal B) and longer breast-feeding (luminal A, HER2-enriched subtype, and TN of borderline statistical significance), and higher risk associated with older age at first FTP (luminal A subtype) were generally consistent with other studies [2, 4, 6, 7]. While some studies of breast cancer subtypes included only younger [12, 16] or older [13, 20] women, only a few studies stratified the analysis by menopausal status [17] or age [4, 6, 11, 21] for select reproductive factors. The present findings of heterogeneity by menopausal status for some reproductive variables highlight its importance, as associations could be masked without stratification. Among premenopausal African American women, we found no evidence of benefit associated with being parous or higher parity; in fact, higher ORs associated with higher parity were observed for all four subtypes, and the OR was statistically significant for TN subtype. For African American women overall, some studies found no evidence of higher risk of luminal A subtype associated with higher parity [21, 24], whereas other studies observed a higher risk of TN or basal-like subtypes [37, 38], likely reflecting the higher risk among premenopausal women only, since we found a strong inverse association with parity among postmenopausal African American women.
Although breast-feeding has been associated with lower risk of breast cancer, regardless of menopausal status [36], associations with breast cancer subtypes have not been consistent [3, 6, 40]. Some studies found similar risk reductions for luminal A and TN subtypes [21], or associations that were stronger for or limited to TN or basal-like subtypes [6, 12, 17, 24, 37]. Notably, in BCAC, a clear inverse association with breast-feeding was observed for TN subtype only [6]. In the present study, longer breast-feeding was associated with lower risk of luminal A, TN (borderline statistical significance), and HER2-enriched subtypes, although in analyses by race and ethnicity, none of the associations reached statistical significance. In agreement with a large pooled analysis of breast cancer overall [36], the risk reduction associated with higher parity was greater in the presence of a breast-feeding history among postmenopausal women for all four subtypes and among premenopausal women for luminal A and luminal B subtypes. Importantly, for luminal A, the most common subtype, this added benefit of breast-feeding was observed among all racial and ethnic and menopausal groups.
Our findings add to the growing evidence that breast-feeding may mitigate the higher risk of TN or ER-negative subtypes associated with higher parity [6, 18, 24, 37, 41]. It has been suggested that the mitigating effect of breast-feeding is more difficult to detect in populations with a high prevalence of breast-feeding [42]. We observed a mitigating effect among premenopausal African American women only who had the lowest prevalence of breast-feeding (48%) compared with 80% among premenopausal Hispanic control women. Pregnancy-associated breast cancer has been attributed to changes in pregnancy-related hormones, as well as immune factors and inflammatory processes triggered during postpartum involution that resemble the pro-tumorigenic process of wound healing. Specifically, the tissue microenvironment of involution, which includes the influx of immune cells, activated fibroblasts, extracellular matrix deposition, elevated matrix metalloproteinase levels, and bioactive matrix fragments, promotes tumorigenesis [43, 44].
We found that early menarche was associated with higher risk of luminal A subtype only and limited to premenopausal women, in agreement with two other pooled analyses that observed an association among younger women only [6, 21]. In contrast, early menarche was also associated with higher risk of non-luminal A subtypes, and in particular with TN subtype among younger women in BCAC [6]. Unlike some studies that observed a higher risk of luminal A subtype associated with earlier menarche among African American women [21, 24, 25], we found no association among African American women, although a longer interval between menarche and first FTP was associated with a suggestive higher risk of borderline statistical significance. The positive associations with luminal A subtype observed among Asian American and Hispanic women are consistent with other studies of NHW women [4, 17].
The exposure measure integrating two early reproductive events (age at menarche, age at first FTP) may be a more relevant risk factor for luminal A subtype, as this represents a window of increased susceptibility when breast tissue undergoes rapid cellular proliferation and rapid accumulation of risk until terminal differentiation occurs during a first pregnancy [45, 46]. The more than two-fold higher risk of premenopausal luminal A subtype associated with ≥ 15 vs. <10 years between menarche and first FTP is of particular concern given trends of delayed childbearing. We did not have data on exposures during this critical time window to further explore what factors might underlie this association, but additional research is warranted.
Pregnancy is associated with a transient increase in breast cancer risk that follows an FTP, wanes over time, and then shifts to a long-term reduction in breast cancer risk [47, 48], about 10 years after a last birth [6]. Consistent with these observations and the large BCAC analysis [6], a shorter interval (< 10 years) between last FTP and diagnosis was associated with a higher risk of luminal A subtype among premenopausal women. The overall OR estimate of 1.03 per year was the same across the three racial and ethnic groups, but reached statistical significance only for women overall.
Comparisons across different subtype classifications
In analyses of mostly NHW women, associations with reproductive factors were generally of similar magnitude for subtypes defined by joint ER/PR/HER2 status or joint ER/PR status [4, 6, 18], and for ER-negative and TN subtypes [4, 6, 22]. Similarly, in our earlier BEM Study analysis [27], associations for ER/PR-positive breast cancer were similar to those for luminal A subtype in the present study, particularly for Asian American and Hispanic women. Larger studies will need to confirm the distinct associations we observed for luminal A vs. luminal B subtypes (e.g., breast-feeding among premenopausal women) and for TN vs. HER2-enriched subtypes (e.g., parity among postmenopausal women). In BCAC, associations with reproductive factors differed primarily between TN subtype and the other subtypes [6].
Racial and ethnic differences in reproductive risk factors
Subtype-specific associations with reproductive factors among premenopausal and postmenopausal women were in the same direction and generally of similar magnitude across racial and ethnic groups, except for parity and breast-feeding among premenopausal African American women. Variation in OR estimates and very wide confidence intervals were likely due to small numbers, particularly among premenopausal women. Distributions of reproductive factors varied considerably across racial and ethnic groups which may contribute to racial and ethnic differences in the incidence of specific breast cancer subtypes. Palmer [22, 49] and Ambrosone [50] suggested that the higher prevalence of high parity, absence of breast-feeding, and young age at first FTP contributes to the higher incidence of early-onset ER-negative breast cancer among African American women. This constellation of factors may also contribute to the higher incidence of TN subtype among premenopausal African American women.
Study limitations and strengths
The subtype-specific analyses were limited by sample size, especially for analyses of the less common subtypes stratified by menopausal status. Subtype was based on readily available cancer registry records, similar to other pooled analyses where subtype was based on medical records, pathology reports, or cancer registry data [4, 6]. The lack of centralized subtyping, as done in some studies [11, 12, 15, 17, 18, 24, 37], might have introduced some misclassification, but it is unlikely that such misclassification would be differential by reproductive characteristics. The small numbers of luminal A, luminal B, and HER2-enriched cases among NHW women precluded subtype-specific analyses in NHW women for comparison with published data from other studies. Not all eligible women with breast cancer and control women in the parent studies participated in the study interviews, which could have introduced selection bias. Reproductive characteristics were based on self-report, therefore subject to inaccurate recall. Non-differential recall bias could result in exposure misclassification which would bias the OR estimates towards the null. There is the possibility that recall is differential between cases and controls, although that may apply to a lesser extent for reproductive factors. Nevertheless, the associations for luminal A subtype in our study were generally consistent with the literature on breast cancer risk factors, providing support to the validity of our findings.
Study strengths include the population-based design of the three studies that were pooled, and case ascertainment through the regional population-based cancer registries which increases the generalizability of our study findings. The diversity of the study sample and use of harmonized exposure variables allowed the direct comparison of OR estimates for African American, Asian American, and Hispanic women. Detailed information was collected on pregnancy and breast-feeding histories and other risk factors. Lastly, we performed analyses stratified by menopausal status that revealed some important differences in associations.
Implications for breast cancer prevention and risk reduction
Breast-feeding is likely the only reproductive risk factor for breast cancer that is potentially modifiable. Efforts focused on improving knowledge on the benefits of breast-feeding and creating a more supportive environment that facilitates breast-feeding could have major impact on lowering breast cancer risk for all subtypes, particularly among premenopausal African American women who are at higher risk. Breast-feeding disparities are tied at multiple levels to social determinants of health that impose barriers to breast-feeding, particularly among African American women (e.g., shorter parental leave; differential access to breast-feeding programs and lactation support; limited accommodations for pumping and storing breast milk at work; and historical and cultural factors [51,52,53,54]. Effective primary breast cancer prevention efforts focused on increasing breast-feeding need to address these barriers among African American women and implement tailored approaches that overcome them [54, 55]. The interval between menarche and first FTP may be a risk factor of increasing importance, given trends of earlier menarche [56, 57] and delayed childbearing [58]. Consistent with these trends, we saw a higher prevalence of longer mean interval between menarche and first FTP and a higher proportion of women with a first FTP at age ≥ 30 years among premenopausal compared to postmenopausal women. These findings warrant studies focused on identifying etiologic factors during this critical time window. The finding of a higher risk of luminal A subtype after a full-term pregnancy suggests that increased surveillance for breast cancer after a full-term pregnancy may be an important strategy to detect breast cancers at an early stage when they are easier to treat and have better survival.
Conclusions
The higher incidence of TN and HER2-enriched breast cancer in some racial and ethnic groups [1], the worse prognosis for these subtypes [8], and the limited knowledge about risk factors warrant research focused on these less common subtypes. Foremost, larger studies and/or pooled analyses in racially and ethnically diverse populations are needed to evaluate reproductive and other risk factors for breast cancer subtypes with greater precision. The distinct associations with parity and breast-feeding among premenopausal African American women, as well as rising incidence rates of distant-stage breast cancer among women under age 40 years [59] underscore the importance of identifying risk factors for breast cancer subtypes among younger women. Centralized subtyping would minimize potential misclassification, and tumor expression data may further facilitate the detection of etiologic heterogeneity for more refined subtypes. A deeper understanding of subtype-specific risk factors, based on both menopausal status and race and ethnicity, is critical for prevention efforts aimed at reducing breast cancer risk and improving survival.
Data availability
The dataset used for the current study may be obtained from the corresponding author (EMJ) on reasonable request, contingent upon approval by appropriate Institutional Review Boards and study Principal Investigators.
Abbreviations
- AABCS:
-
Los Angeles County Asian American Breast Cancer Study
- BEM:
-
Breast Cancer Etiology in Minorities
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- NC-BCFR:
-
Northern California Breast Cancer Family Registry
- OR:
-
Odds ratio
- PR:
-
Progesterone receptor
- SFBCS:
-
San Francisco Bay Area Breast Cancer Study
- U.S.:
-
United States
References
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5).
Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta. 2015;1856(1):73–85.
Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, Peccatori FA, Azim HA. Jr. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76.
Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, Giles GG, Tamimi RM, Eliassen AH, Rosner B, et al. Pooled analysis of nine cohorts reveals breast cancer risk factors by tumor molecular subtype. Cancer Res. 2018;78(20):6011–21.
Houghton SC, Hankinson SE. Cancer progress and priorities: breast cancer. Cancer Epidemiol Biomarkers Prev. 2021;30(5):822–44.
Jung AY, Ahearn TU, Behrens S, Middha P, Bolla MK, Wang Q, Arndt V, Aronson KJ, Augustinsson A, Beane Freeman LE, et al. Distinct reproductive risk profiles for intrinsic-like breast cancer subtypes: pooled analysis of population-based studies. J Natl Cancer Inst. 2022;114(12):1706–19.
Mao X, Omeogu C, Karanth S, Joshi A, Meernik C, Wilson L, Clark A, Deveaux A, He C, Johnson T, et al. Association of reproductive risk factors and breast cancer molecular subtypes: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):644.
Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–26.
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–51.
Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47.
Ma H, Wang Y, Sullivan-Halley J, Weiss L, Marchbanks PA, Spirtas R, Ursin G, Burkman RT, Simon MS, Malone KE, et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res. 2010;70(2):575–87.
Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas Thompson W, Bernstein JL. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–97.
Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;103(6):470–7.
Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, Li CI. Reproductive history and risk of three breast cancer subtypes defined by three biomarkers. Cancer Causes Control. 2011;22(3):399–405.
Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, Rosner B, Marotti J, Connolly JL, Schnitt SJ, Collins LC. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131(1):159–67.
Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137(2):579–87.
Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: results from the nurses’ Health studies. Int J Cancer. 2016;138(10):2346–56.
Fortner RT, Sisti J, Chai B, Collins LC, Rosner B, Hankinson SE, Tamimi RM, Eliassen AH. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the nurses’ Health studies. Breast Cancer Res. 2019;21(1):40.
McCarthy AM, Friebel-Klingner T, Ehsan S, He W, Welch M, Chen J, Kontos D, Domchek SM, Conant EF, Semine A, et al. Relationship of established risk factors with breast cancer subtypes. Cancer Med. 2021;10(18):6456–67.
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113(7):1521–6.
Ma H, Ursin G, Xu X, Lee E, Togawa K, Duan L, Lu Y, Malone KE, Marchbanks PA, McDonald JA, et al. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: a pooled analysis. Breast Cancer Res. 2017;19(1):6.
Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, Bandera EV, Borges V, McKinnon C, Haiman CA et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. 2014;106(10).
Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, Schedin P, Bethea TN, Borges V, Park SY et al. Important role of menarche in development of estrogen receptor-negative breast cancer in African American women. J Natl Cancer Inst. 2015;107(9).
Benefield HC, Zirpoli GR, Allott EH, Shan Y, Hurson AN, Omilian AR, Khoury T, Hong CC, Olshan AF, Bethea TN, et al. Epidemiology of basal-like and luminal breast cancers among black women in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2021;30(1):71–9.
Friebel-Klingner TM, Ehsan S, Conant EF, Kontos D, Domchek SM, McCarthy AM. Risk factors for breast cancer subtypes among black women undergoing screening mammography. Breast Cancer Res Treat. 2021;189(3):827–35.
John EM, Hines LM, Phipps AI, Koo J, Longacre TA, Ingles SA, Baumgartner KB, Slattery ML, Wu AH. Reproductive history, breast-feeding and risk of triple negative breast cancer: the breast Cancer etiology in minorities (BEM) study. Int J Cancer. 2018;142(11):2273–85.
John EM, Phipps AI, Hines LM, Koo J, Ingles SA, Baumgartner KB, Slattery ML, Wu AH. Menstrual and reproductive characteristics and breast cancer risk by hormone receptor status and ethnicity: the breast Cancer etiology in minorities study. Int J Cancer. 2020;147(7):1808–22.
Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86(5):723–7.
Trentham-Dietz A, Sprague BL, Hampton JM, Miglioretti DL, Nelson HD, Titus LJ, Egan KM, Remington PL, Newcomb PA. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case-control studies. Breast Cancer Res Treat. 2014;145(1):165–75.
Chollet-Hinton L, Anders CK, Tse CK, Bell MB, Yang YC, Carey LA, Olshan AF, Troester MA. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina breast Cancer Study: a case-control study. Breast Cancer Res. 2016;18(1):79.
Jeong SH, An YS, Choi JY, Park B, Kang D, Lee MH, Han W, Noh DY, Yoo KY, Park SK. Risk reduction of breast cancer by childbirth, breastfeeding, and their interaction in Korean women: heterogeneous effects across menopausal status, hormone receptor status, and pathological subtypes. J Prev Med Public Health. 2017;50(6):401–10.
Work ME, John EM, Andrulis IL, Knight JA, Liao Y, Mulligan AM, Southey MC, Giles GG, Dite GS, Apicella C, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the breast Cancer Family Registry. Br J Cancer. 2014;110(5):1367–77.
Wu AH, Vigen C, Lee E, Tseng CC, Butler LM. Traditional breast cancer risk factors in Filipina americans compared with Chinese and Japanese americans in Los Angeles County. Cancer Epidemiol Biomarkers Prev. 2016;25(12):1572–86.
John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2905–13.
John EM, Sangaramoorthy M, Koo J, Whittemore AS, West DW. Enrollment and biospecimen collection in a multiethnic family cohort: the Northern California site of the breast Cancer Family Registry. Cancer Causes Control. 2019;30(4):395–408.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187–95.
Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39.
Ambrosone CB, Zirpoli G, Ruszczyk M, Shankar J, Hong CC, McIlwain D, Roberts M, Yao S, McCann SE, Ciupak G, et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the women’s Circle of Health Study. Cancer Causes Control. 2014;25(2):259–65.
Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:906495.
Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, Boffetta P, Weiss M. Breastfeeding and breast cancer risk by receptor status–a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–407.
Ambrosone CB, Higgins MJ. Relationships between breast feeding and breast cancer subtypes: lessons learned from studies in humans and in mice. Cancer Res. 2020;80(22):4871–7.
Schedin P, Palmer JR. Can breast Cancer Prevention Strategies be tailored to Biologic Subtype and Unique Reproductive Windows? J Natl Cancer Inst. 2022;114(12):1575–6.
Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–91.
Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14(2):87–98.
Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014;145(3):567–79.
Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4(5):567–71.
Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9.
Albrektsen G, Heuch I, Kvale G. The short-term and long-term effect of a pregnancy on breast cancer risk: a prospective study of 802,457 parous Norwegian women. Br J Cancer. 1995;72(2):480–4.
Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1883–91.
Ambrosone CB, Zirpoli GR, Bovbjerg DH, Shankar J, Hong CC, McCann SE, Ruszczyk M, Khoury T, Yao S, Ciupak GL, et al. Associations between estrogen receptor-negative breast cancer and timing of reproductive events differ between African American and European American women. Cancer Epidemiol Biomarkers Prev. 2014;23(6):1115–20.
Jones KM, Power ML, Queenan JT, Schulkin J. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10(4):186–96.
Li R, Perrine CG, Anstey EH, Chen J, MacGowan CA, Elam-Evans LD. Breastfeeding trends by race/ethnicity among US children born from 2009 to 2015. JAMA Pediatr. 2019;173(12):e193319.
Morrow AL, McClain J, Conrey SC, Niu L, Kinzer A, Cline AR, Piasecki AM, DeFranco E, Ward L, Ware J, et al. Breastfeeding disparities and their mediators in an urban birth cohort of Black and White mothers. Breastfeed Med. 2021;16(6):452–62.
Segura-Perez S, Hromi-Fiedler A, Adnew M, Nyhan K, Perez-Escamilla R. Impact of breastfeeding interventions among United States minority women on breastfeeding outcomes: a systematic review. Int J Equity Health. 2021;20(1):72.
Johnson A, Kirk R, Rosenblum KL, Muzik M. Enhancing breastfeeding rates among African American women: a systematic review of current psychosocial interventions. Breastfeed Med. 2015;10(1):45–62.
Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med. 1982;306(17):1033–5.
Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Secular trends in age at menarche in women in the UK born 1908-93: results from the breakthrough generations study. Paediatr Perinat Epidemiol. 2011;25(4):394–400.
Kehm RD, Osypuk TL, Poynter JN, Vock DM, Spector LG. Do pregnancy characteristics contribute to rising childhood cancer incidence rates in the United States? Pediatr Blood Cancer. 2018;65(3).
Kehm RD, Yang W, Tehranifar P, Terry MB. 40 years of change in age- and stage-specific cancer incidence rates in US women and men. JNCI Cancer Spectr. 2019;3(3):pkz038.
Acknowledgements
Not applicable.
Funding
The Breast Cancer Etiology in Minorities Study was funded by grant R03 CA199343 (E.M. John) from the National Cancer Institute. The San Francisco Bay Area Breast Cancer Study was supported by grants R01 CA63446 (E.M. John) and R01 CA77305 (E.M. John) from the National Cancer Institute, grant DAMD17-96-1-6071 (E.M. John) from the U.S. Department of Defense and grant 7 PB-0068 (E.M. John) from the California Breast Cancer Research Program. The Northern California site of the Breast Cancer Family Registry was funded by grant U01 CA164920 (E.M. John) from the National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government or the BCFR. The collection of cancer incidence data used in these studies was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Sect. 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000036C awarded to the Cancer Prevention Institute of California; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The Los Angeles County Asian American Breast Cancer Study was funded by the California Breast Cancer Research Program grants 1RB-0287, 3 PB-0120, 5 PB-0018 and 10 PB-0038 (A.H. Wu). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute or endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Author information
Authors and Affiliations
Contributions
E.M.J., L.M.H., and A.W. conceptualized and designed the study. E.M.J. supervised the study. E.M.J. and A.H.W. collected the data in the three parent studies. T.A.L. performed HER2 analyses for some cases. J.K. harmonized the data, performed data management, and the statistical analysis. S.A.I. advised on the statistical analysis approach. E.M.J. and L.M.H. wrote the main manuscript text, and J.K. contributed to the writing of the statistical analysis section. All authors reviewed the manuscript and provided critical input.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of each participating institution approved the studies, and study participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
John, E.M., Koo, J., Phipps, A.I. et al. Reproductive characteristics, menopausal status, race and ethnicity, and risk of breast cancer subtypes defined by ER, PR and HER2 status: the Breast Cancer Etiology in Minorities study. Breast Cancer Res 26, 88 (2024). https://doi.org/10.1186/s13058-024-01834-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-024-01834-5