Abstract
Background
Associations between reproductive factors and breast cancer (BC) risk vary by molecular subtype (i.e., luminal A, luminal B, HER2, and triple negative/basal-like [TNBC]). In this systematic review and meta-analysis, we summarized the associations between reproductive factors and BC subtypes.
Methods
Studies from 2000 to 2021 were included if BC subtype was examined in relation to one of 11 reproductive risk factors: age at menarche, age at menopause, age at first birth, menopausal status, parity, breastfeeding, oral contraceptive (OC) use, hormone replacement therapy (HRT), pregnancy, years since last birth and abortion. For each reproductive risk factor, BC subtype, and study design (case–control/cohort or case-case), random-effects models were used to estimate pooled relative risks and 95% confidence intervals.
Results
A total of 75 studies met the inclusion criteria for systematic review. Among the case–control/cohort studies, later age at menarche and breastfeeding were consistently associated with decreased risk of BC across all subtypes, while later age at menopause, later age of first childbirth, and nulliparity/low parity were associated with increased risk of luminal A, luminal B, and HER2 subtypes. In the case-only analysis, compared to luminal A, postmenopausal status increased the risk of HER2 and TNBC. Associations were less consistent across subtypes for OC and HRT use.
Conclusion
Identifying common risk factors across BC subtypes can enhance the tailoring of prevention strategies, and risk stratification models can benefit from subtype specificity. Adding breastfeeding status to current BC risk prediction models can enhance predictive ability, given the consistency of the associations across subtypes.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer type and a leading cause of cancer death among women globally [1, 2]. Established risk factors for BC include genetic, reproductive, and lifestyle-related factors, contributing to variations in worldwide BC incidence rates [3]. Reproductive factors that have been linked with BC risk include age at menarche, age at menopause, menopausal status, pregnancy-related factors (age at first birth, parity, breastfeeding, years since last birth), oral contraceptive (OC) use, and hormone replacement therapy (HRT) [3,4,5,6]. These factors likely influence breast cancer risk through alterations in circulating levels of hormones such as estrogen [4]. Certain reproductive risk factors such as HRT and pregnancy may directly change hormonal levels, while other risk factors such as age at menarche and age at menopause are markers for the lifetime duration of hormonal exposures [5].
BC is recognized as a heterogeneous cancer due to differences in tumor and genomic features that indicate different etiology and prognosis [6]. Based on hormone receptors (estrogen (ER) and progesterone (PR)) and expression level of human epidermal growth factor receptor 2 (HER2), BC can be classified as luminal A (ER + and/or PR + , HER2 −), luminal B (ER + and/or PR + , HER2 +), HER 2-overexpression (ER − , PR − , and HER2 +), triple-negative, and basal-like (ER − , PR − , and HER2-),–luminal A being the most common of all the subtypes [7]. Although several studies have evaluated the associations between reproductive factors and molecular subtypes, results have been inconsistent [8,9,10]. A 2016 meta-analysis that evaluated 15 studies reported that parity was associated with decreased risk of luminal subtype and later age at first birth was associated with increased risk; while breastfeeding was associated with decreased risk of both luminal and triple-negative BC subtypes [6]. The meta-analysis included studies published up to 2014 and evaluated only three reproductive factors; age at first birth, parity, and breastfeeding. In the present systematic review and meta-analysis, we extend the review period through 2021 and examine 11 separate reproductive factors associated with each BC molecular subtype: age at menarche, age at menopause, age at first birth, menopausal status, parity, breastfeeding, OC use, HRT use, pregnancy, years since last birth, and abortion. The purpose of this study is to: i) comprehensively summarize the published literature on reproductive risk factors and molecular BC subtypes, and ii) generate summary estimates of the associations between reproductive risk factors and BC molecular subtypes.
Methods
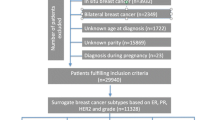
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (Fig. 1) [11, 12]. Primary studies published in the English language between January 2000 and April 2021 were retrieved from PubMed, Scopus, and Embase. We focused on studies published after 2000 to account for the updated research based on breast tumor classifications and different subtypes. For instance, a study conducted by Perou et al., 2000 demonstrated how tumors can be categorized into different subtypes of breast cancer based on their unique patterns of gene expression, which may contribute to differential analysis of molecular gene expression patterns of BC tumors in studies conducted after 2000 [13]. The search strategy included MeSH and non-MeSH key terms for 1) “Breast Neoplasms”, and specific subtypes evaluated which included: luminal A, luminal B, HER-2- overexpression (HER2), basal-like, and triple-negative/basal-like (TNBC) breast neoplasms; 2)“Reproductive behavior” as well as specific reproductive factors, including menarche, abortion, parity (children born alive), breastfeeding, pregnancy (including live pregnancies and abortions), contraceptives, menopause, menstruation, menstrual period, age at first birth, birth control, birth intervals, and hormone replacement therapy. The specific search strategy is presented in the Supplementary materials.
Study eligibility
Included studies focused on BC subtypes and reproductive factors of interest. Studies were excluded if they were published in languages other than English; full texts were not available; examined BC subtypes other than luminal A, luminal B, HER-2, TNBC, or outcomes other than BC subtypes; only analyzed non-reproductive risk factors or none of our a priori reproductive risk factors of interest; if effect estimates (i.e., odds ratios, relative risks, hazard ratios) were unavailable; or were study designs other than case–control, case-case, or cohort.
Selection
Two authors (CO, SK) reviewed the titles, abstracts, and full text of all studies retrieved from electronic databases. Discrepancies in selection were resolved by consensus, and disagreements were resolved in consultation with a third author (XM). A total of 12,243 studies were retrieved from database searches. We excluded 7,702 duplicate articles, and 4,396 articles after the title and abstract review, resulting in 145 articles for full-text review. Of these, 40 studies were excluded due to non-relevant outcomes (e.g., percentages, proportions, means rather than OR or RR estimates); 11 studies due to other subtype classifications; 12 studies due to study design; 4 full texts were not available in English; and 3 studies lacked reproductive variables, resulting in 75 eligible studies. Of these, 56 studies reported estimates for the association between reproductive factors and BC subtypes and were included in meta-analysis (Fig. 1).
Data extraction
Data was abstracted by one author (CO) and independently reviewed and verified based on original full texts by the two other authors (SK and AJ). Information on author, year, study design, study characteristics (country of study, sample size, data source, patient race, etc.), BC subtypes (luminal A, luminal B, HER-2, and TNBC), reproductive factors, covariates, and corresponding risk estimates and their 95% confidence intervals (CI) were recorded.
Statistical analysis
Odds ratios (OR), relative risks (RR), and hazard ratios (HR) for reproductive factors comparing the most extreme (e.g., highest vs. lowest) categories were extracted. To ensure consistency in the meta-analysis, if needed, ratio measures were inverted to ensure reference categories matched across studies. Odds ratios and hazard ratios were converted to approximate RRs and 95% CIs. Studies were included in the meta-analysis when at least three studies of the same exposure-outcome combination were available. The meta-analysis was conducted separately for each combination of exposure and BC subtype. All 11 reproductive factors were included in the systematic review, but only eight that were examined in at least three studies were included in the meta-analysis: 1) age at menarche, 2) age at menopause, 3) age at first birth, 4) menopausal status, 5) parity, 6) breastfeeding, 7) OC use and 8) HRT use. Pregnancy, years since last birth, and abortion were qualitatively summarized. Study-level results are presented in supplemental materials (Supplementary Figs. 1-16). Random effects models were used to compute pooled estimates with 95% CIs for each reproductive factor and molecular subtype for both case–control/cohort, and case-case studies separately. We combined cohort and case–control studies together in our analysis based on similarity in results and strength of study design. In the case-case analysis, luminal A was the reference group as it was the most common subtype reported as a reference in the studies. The Q-statistic was used to assess the presence of between-study heterogeneity; the I2-statistic was used to examine the proportion of variation between studies due to heterogeneity (p-values reported with effect estimates correspond to the I2 p-value); [14] and the Egger test was used to assess publication bias [15]. Analyses were conducted using STATA version 15 (Stata Corp LLC, College Station, TX).
Results
Seventy-five studies met the inclusion criteria and were deemed suitable for the systematic review (Fig. 1). The characteristics of included studies are summarized in Table 1 and Supplementary Table 1. Most eligible studies were published between 2011 and 2021 (n = 59), and about 40% of the studies were conducted in the United States. Of the seventy-five studies, thirteen were cohort studies, thirty-six studies were case–control, twenty-two were case-case studies, and four studies were both case–control and case-case study designs. Nineteen of the seventy-five studies reported data that could not be meta-analyzed, resulting in fifty-six studies included in meta-analysis. Four cohort studies [16,17,18,19] that reported hazard ratios to evaluate the association of reproductive factors and subtypes were combined with case–control studies in the meta-analysis.
Age at menarche
Forty-six studies evaluated the association between age at menarche and BC subtypes (twenty-four case–control studies [9, 22, 28, 36, 37, 39,40,41,42, 44, 46, 48, 53, 57, 61, 63, 65, 66, 72, 79, 80, 83, 85, 86], fourteen case-only studies [20, 27, 30, 35, 43, 59, 69, 71, 74,75,76,77, 81, 89], three case–control/case-case studies [24, 52, 60], and five cohort studies [17, 18, 55, 70, 88]), and were included in the systematic review. Among the cohort and case–control studies, later age at menarche was associated with lower risk of BC in the majority of studies regardless of subtype [9, 17, 18, 22, 24, 28, 36, 37, 39,40,41,42, 44, 46, 48, 52, 53, 55, 57, 60, 61, 63, 65, 66, 70, 72, 79, 80, 83, 85, 86, 88], of which fourteen studies [17, 18, 24, 39, 40, 42, 52, 57, 63, 72, 79, 83, 85, 86] were luminal A, nine studies [17, 24, 39, 42, 52, 57, 63, 85, 86] were luminal B, twelve studies [17, 22, 24, 39, 42, 44, 53, 63, 65, 79, 83, 85] were HER2, and eighteen studies were TNBC [9, 18, 22, 37, 39, 40, 46, 52, 53, 57, 60, 63, 65, 66, 72, 79, 85, 86]. Among the case-only studies [20, 24, 27, 30, 35, 43, 52, 59, 60, 69, 71, 74,75,76,77, 81, 89], compared to luminal A, later age of menarche was associated with lower risk of luminal B in seven studies [20, 24, 30, 43, 52, 69, 89], HER2 subtype in six studies [20, 30, 59, 74, 76, 89], and TNBC in eight studies [27, 30, 52, 60, 71, 74, 76, 89]. Sung et.al. compared luminal A basal-positive with luminal A basal-negative cases but reported a non-significant lower association between age at menarche and BC subtypes [77]. Yaun et. al., reported younger age at menarche was more often observed in patients with HER2-positive compared to patients with HER2- negative status, while Ly et. al., and Rauh et. al., observed no significant differences between mean age of menarche and BC subtypes [55, 70, 88].
In meta-analysis of two cohort and twenty-seven case–control studies (Fig. 2; Supplementary Fig. 1), later vs earlier age at menarche was associated with a 12% lower risk of luminal A (RR: 0.88, 95% CI: 0.83, 0.93, I2 = 64.1%, p < 0.001) and 14% lower risk of TNBC (RR:0.86, 95% CI: 0.79, 0.95, I2 = 57.6%, p < 0.001). Associations with luminal B (RR: 0.95, 95% CI: 0.90, 1.00, I2 = 12.1%, p = 0.315) and HER2-overexpressing (RR: 0.95, 95% CI: 0.86, 1.05, I2 = 37.2%, p = 0.038) subtypes were lower but not significant. There was evidence of heterogeneity and publication bias among the studies (Egger test: -0.631, p = 0.004). In analysis of thirteen case-case studies (Fig. 2; Supplementary Fig. 2), compared to luminal A, other subtypes were not significantly associated with age at menarche. There was some heterogeneity among the case-case studies, but no evidence of publication bias (Egger test:0.43, p = 0.309).
Age at menopause
Twenty-one studies evaluated the association between age at menopause and BC subtypes (ten case–control studies, seven case-case studies, one case–control/case-case study, and three cohort studies). In the cohort studies and eleven case–control studies [9, 17, 18, 36, 38, 40, 46, 48, 52, 53, 63, 65, 66, 72, 85,86,87,88], later age at menopause was associated with higher risk among nine luminal A studies [17, 18, 38, 40, 63, 72, 85,86,87], four luminal B studies [17, 18, 40, 86], ten HER2-overexpressing studies [17, 18, 38, 40, 46, 63, 65, 72, 86, 87], and eleven TNBC/basal-like studies [17, 18, 36, 38, 46, 63, 65, 66, 72, 85, 86]. Ye et al. [87] and Yuan et al. [88] observed no significant differences in mean age at menopause and BC subtypes. Among the eight case-case studies [35, 59, 69, 74, 76, 77, 87, 89], compared to luminal A, later age of menopause was associated with higher risk of HER2 subtype in three studies [35, 74, 87], but conflicting associations with risk of TNBC in four studies [35, 71, 76, 81].
In meta-analysis of two cohort and eleven case–control studies (Fig. 2; Supplementary Fig. 3), later vs. earlier age at menopause was associated with a 7% higher risk of luminal A (RR:1.07, 95% CI: 1.03, 1.10, I2 = 56.1%, p = 0.025), 6% higher risk of luminal B (RR:1.06, 95% CI: 1.03, 1.08, I2 = 9.2%, p = 0.359) and 13% higher risk of HER2-overexpressing (RR:1.13, 95% CI: 1.04, 1.23, I2 = 69.6%, p = 0.001), while the association with TNBC subtype (RR:1.02, 95% CI: 0.94, 1.11, I2 = 58.8% p = 0.005 was not significant. There was no evidence of publication bias (Egger’s test: 0.615, p = 0.074). In the case-case analyses (Fig. 2; Supplementary Fig. 4), later age at menarche was not significantly associated with any of the subtypes compared to luminal A. There was some heterogeneity in the studies, but no evidence of publication bias (Egger’s test -1.04, p = 0.06).
Age at first birth
Forty-three studies evaluated the association between age at first birth and BC subtypes (twenty-three case–control studies, fourteen case-case studies, three case–control/case-case studies, and three cohort studies). In the cohort and case–control analysis [9, 17, 18, 22, 24, 28, 37,38,39,40, 42, 44, 46, 52, 53, 56, 57, 61, 65, 67, 72, 79, 80, 83, 85, 88], later age at first birth/nulliparity vs. earlier age /parity was associated with higher risk in fifteen studies [9, 17, 18, 24, 38,39,40, 42, 52, 56, 57, 60, 63, 79, 86] except one [85] evaluating luminal A, ten [17, 24, 38,39,40, 42, 52, 57, 79, 86] studies evaluating luminal B, thirteen [17, 18, 24, 28, 38, 39, 42, 53, 61, 67, 72, 80, 85] studies evaluating HER2 and twelve [17, 18, 37,38,39,40, 42, 52, 56, 67, 72, 79] studies evaluating TNBC. Yuan et al. did not find any difference in the mean age at first birth comparing HER2-positive vs. HER2-negative [88]. In case-case analysis [20, 24, 27, 30, 35, 43, 49, 52, 59, 60, 71, 74,75,76,77, 81, 82, 89], compared to luminal A, later age/nulliparity was associated with lower risk of luminal B in five [24, 43, 52, 81, 89] studies, three studies [24, 49, 60] evaluating HER2-overexpression, and six TNBC studies [27, 43, 49, 71, 74, 89]. Five studies had other comparison groups other than luminal A [43, 75, 77, 81, 82], and found later age at first birth was associated with a higher risk of luminal B, lower risk of HER2 and a mixed association with TNBC.
In the meta-analysis of two cohort and twenty-six case–control studies (Fig. 2; Supplementary Fig. 7), later age at first birth/nulliparity vs. younger age was associated with a 19% higher risk of luminal A (RR:1.19 95% CI: 1.13, 1.26, I2 = 87.6%, p = 0.000), 19% for luminal B (RR:1.19, 95% CI: 1.10, 1.30, I2 = 76.6%, p < 0.001) and 15% for HER2 (RR:1.15, 95% CI: 1.05, 1.26, I2 = 45.2%, p = 0.009), while there was no difference in risk for TNBC (RR:0.99, 95% CI: 0.92, 1.07, I2 = 51.3%, p = 0.002). There was heterogeneity among the studies and evidence of publication bias (Egger 0.778, p = 0.001). In meta-analysis of case-case studies (Fig. 2; Supplementary Fig. 8), compared to luminal A, later age at first birth/nulliparity vs. younger age was associated with a 18% lower risk of HER2 subtype (RR: 0.82, 95% CI: 0.73, 0.92, I2 = 0.0%, p = 0.517) and 16% lower risk of TNBC (RR: 0.84, 95% CI: 0.72, 0.97, I2 = 55.6%, p = 0.004). Lower risk was also observed in studies evaluating luminal B but the association was not significant (RR: 0.83, 95% CI: 0.68, 1.01, I2 = 60.6%, p = 0.007). There was heterogeneity among the studies but no evidence of publication bias (Egger’s test -1.043, p = 0.059).
Menopausal status
Eighteen studies evaluated the association between menopausal status and BC subtypes (five case–control studies, seven case-case studies, three case–control/case-case studies, and three cohort studies). In the cohort and case–control study analysis [9, 18, 22, 29, 38, 45, 52, 60, 65, 85, 87], post/peri vs pre/natural menopause was associated with a lower risk of luminal A in four studies [18, 38, 52, 85], luminal B in four studies [18, 38, 52, 85], and TNBC in seven studies [9, 18, 38, 52, 65, 85, 87], but higher risk in five HER-2 overexpressing studies [22, 38, 45, 52, 65]. Ihemelandu et al. observed that molecular subtypes did not differ by menopausal status, however basal cell-like subtype also showed an age-specific bimodal distribution with a peak in the < 35 and 51 to 65 years age groups [45]. Chauhan et. al., reported a higher percentage of Her2-neu receptor in post-menopausal compared to pre-menopausal women, while the opposite was seen in TNBC patients [29]. In the ten case-case analysis studies [35, 49, 52, 58, 60, 71, 75, 76, 87, 89], compared to luminal A, post/perimenopausal status was associated with a higher risk in four [52, 58, 60, 89] luminal B studies, six HER2-overexpressing studies [49, 52, 58, 60, 87, 89], and six TNBC studies [49, 52, 58, 60, 75, 89].
In the meta-analysis of one cohort and six case–control studies (Fig. 3; Supplementary Fig. 5) post/peri menopausal status was associated with a 39% lower risk of luminal A (RR: 0.61, 95% CI: 0.49, 0.76, I2 = 79.0%, p = 0.001), 20% lower risk of luminal B (RR:0.80, 95% CI: 0.68, 0.94, I2 = 0%, p = 0.630); however the association was statistically non-significantly higher for HER2-overexpressing (RR: 1.07, 95% CI: 0.86, 1.33, I2 = 0.0%, p = 0.967) and lower for TNBC (RR: 0.99, 95% CI: 0.84, 1.15, I2 = 0%, p = 0.844). There was heterogeneity among luminal A studies, as well as evidence of publication bias (Egger’s test 2.17, p < 0.001). In the case-case meta-analysis (Fig. 3; Supplementary Fig. 6), compared to luminal A, post/peri-menopausal status was associated with 61% higher risk of HER2-overexpressing (RR: 1.61 95% CI: 1.04, 2.49, I2 = 78.8%, p < 0.001), though the association was not significant with luminal B (RR: 1.10, 95% CI: 0.86, 1.40, I2 = 41.0%, p < 0.001) or TNBC (RR: 1.07, 95% CI: 0.74, 1.56, I2 = 60.5%, p = 0.019). There was significant heterogeneity but no evidence of publication bias (Egger -0.138, p = 0.910).
Parity
Fifty-three studies evaluated the association between parity and BC subtypes (twenty-three case–control studies, nineteen case-case studies, three case–control/case-case studies, and eight cohort studies). In the cohort and case–control studies [16,17,18, 21, 22, 24, 26, 31, 34, 39, 40, 42, 46,47,48, 51,52,53, 55,56,57, 60,61,62, 64,65,66,67, 72, 79, 80, 83, 85, 86], higher parity was associated with a lower risk in fifteen studies [16,17,18, 26, 39, 40, 42, 47, 52, 56, 57, 60, 79, 83, 86] evaluating luminal A, twelve [16, 17, 39, 40, 42, 47, 52, 56, 57, 79, 83, 86] studies evaluating luminal B, twelve [18, 26, 39, 42, 53, 56, 57, 61, 65, 67, 79, 83] studies evaluating HER2-overexpression, and higher risk in eleven [22, 26, 37, 42, 46, 47, 53, 57, 65, 79, 83] studies evaluating TNBC. In 22 case-case studies [20, 24, 27, 30, 35, 43, 49, 50, 52, 59, 60, 69, 71, 74,75,76,77,78, 81, 82, 84, 89] compared with luminal A, higher parity was associated with higher risk in five luminal B studies, [24, 27, 52, 69, 78] seven HER2-overexpressing studies, [24, 43, 59, 69, 74, 78, 81], and six TNBC studies [49, 69, 75, 76, 78, 81]. Ten studies had either different comparison groups other than luminal A or reported proportions to evaluate associations [31, 34, 43, 50, 51, 55, 75, 77, 81, 82]. These studies found a lower risk of HER2 and mixed associations between higher parity and luminal B and TNBC.
In the meta-analysis of three cohort and twenty-six case–control studies (Fig. 3; Supplementary Fig. 9), higher parity was associated with a 39% lower risk of luminal A (RR: 0.59, 95% CI: 0. 49, 0.71, I2 = 92.4%, p < 0.001) and 39% lower risk of luminal B (RR: 0.61, 95% CI: 0.47, 0.80, I2 = 91.9%, p < 0.001). There was no significant association for HER2-overexpressing BC (RR: 0.92, 95% CI: 0.79, 1.07, I2 = 17.7%, p = 0.225) or TNBC (RR: 1.03, 95% CI: 0.93, 1.15, I2 = 21.0%, p = 0.165). There was no heterogeneity between the studies and no evidence of publication bias (Egger’s test 0. 465, p = 0.304). For the sixteen case-case studies (Fig. 3; Supplementary Fig. 10), compared to luminal A, higher parity was associated with a 32% higher risk of TNBC (RR: 1.32, 95% CI: 1.19, 1.46, I2 = 45.7%, p = 0.003). No significant association was found in luminal B (RR: 1.06, 95% CI: 0.96, 1.18) and HER2-overexpressing BC (RR: 1.13, 95% CI: 0.99, 1.30). There was heterogeneity between the groups and evidence of publication bias (Egger’s test: 1.07, p = 0.007).
Breastfeeding
Forty-seven studies evaluated the association between breastfeeding and BC subtypes (twenty-five case–control studies, fifteen case-case studies, three case–control/case-case studies, and four cohort studies). In the cohort and case–control studies [9, 16,17,18, 21, 22, 24, 28, 36,37,38,39, 41, 42, 44, 46,47,48, 52, 53, 56, 57, 60,61,62,63, 65, 72, 79, 83, 88], breastfeeding was associated with lower risk in all subtypes: thirteen studies [17, 18, 39, 42, 47, 56, 57, 60, 63, 72, 79, 83, 85] evaluating luminal A, thirteen studies [17, 18, 24, 39, 42, 47, 56, 57, 63, 72, 79, 83, 85] evaluating luminal B, 15 studies [9, 18, 24, 28, 39, 42, 44, 47, 52, 61, 63, 72, 79, 83, 85] evaluating HER2-overexpression, and twenty-three studies evaluating TNBC [9, 17, 18, 21, 22, 24, 39, 42, 46,47,48, 52, 53, 56, 57, 60, 62, 63, 65, 72, 79, 83, 85]. In the case-case analysis [24, 30, 35, 43, 49, 52, 59, 60, 71, 74,75,76,77,78, 81, 82, 84, 89], ever/higher duration of breastfeeding was associated with lower risk in all subtypes: five [30, 52, 60, 84, 89] luminal B studies, ten HER2-overexpressing studies [24, 43, 49, 52, 59, 60, 74, 76, 81, 84], and 13 evaluating TNBC [24, 30, 35, 49, 52, 60, 71, 74,75,76, 78, 81, 84] vs. luminal A. Six studies had different comparison groups or used proportions [43, 75, 77, 81, 82, 88], and found a higher association between longer duration of breastfeeding with HER2 subtype and mixed associations between longer duration of breastfeeding and luminal B and TNBC.
In the meta-analysis of thirty studies (Fig. 3; Supplementary Fig. 11), ever/longer duration of breastfeeding was associated with lower risk of all subtypes compared to the controls; 28% lower risk of luminal A (RR: 0.72, 95% CI: 0.60, 0.86, I2 = 93.6%, p < 0.001), 29% lower risk of luminal B (RR: 0.71, 95% CI: 0.59, 0.85, I2 = 82.5%, p < 0.001), 23% lower risk of HER2-overexpressing (RR: 0.77, 95% CI: 0.64, 0.92, I2 = 74.3%, p < 0.001), and 42% lower risk of TNBC (RR: 0.66, 95% CI: 0.58, 0.74, I2 = 58.8%, p < 0.001) with significant heterogeneity between the studies, but no evidence of publication bias (Egger: -0.333, p = 0.499). In case-case studies (Fig. 3; Supplementary Fig. 12) compared to luminal A, there was 15% lower risk of TNBC (RR: 0.85, 95% CI: 0.74, 0.99, I2 = 58.8%, p < 0.001), while luminal B breast cancer (RR: 0.90 95% CI: 0.80, 1.00) and HER2-overexpressing BC (RR: 0.92 95% CI: 0.79, 1.07) results were not significant. There was heterogeneity between the studies reporting HER2 subtype but no evidence of publication bias (Egger’s test -0.27, p = 0.529).
Oral contraceptive (OC) use
Twenty-three studies evaluated the association between OC use and BC subtypes (thirteen case–control studies, nine case-case studies, and two case–control/ case-case studies). In the case–control analysis [23,24,25, 28, 37,38,39,40,41, 44, 56, 60, 63, 66, 80], OC use was associated with higher risk in all subtypes: three [24, 38, 63] luminal A studies, six [24, 38,39,40, 56, 63] luminal B studies, seven [24, 37,38,39, 44, 56, 63] HER2-overexpressing studies and eight [23,24,25, 37,38,39, 63, 66] TNBC studies. In the case-case analysis in comparison to luminal A subtype [24, 49, 50, 54, 59, 60, 74, 76, 81, 84, 89], OC use was associated with a higher risk in one [81] study evaluating luminal B, six [24, 49, 60, 74, 76, 84] studies evaluating HER2 and six [24, 50, 54, 74, 76, 84] studies evaluating TNBC. Two studies had different comparison groups [50, 81]. Turkoz et al. observed no significant difference with OC use (< 2 years, ≥ 2 to 5 years and ≥ 5 years vs. no use) among breast cancer subtypes comparing luminal A to non-luminal A and luminal B to non-luminal B [81]. Lee et al. did not find any risk of TNBC with OC use compared to non-TNBC [50].
In the meta-analysis of fifteen case–control studies (Fig. 4; Supplementary Fig. 13), ever/longer duration OC use was associated with a 16% higher risk of TNBC (RR: 1.16, 95% CI: 1.05, 1.29, I2 = 26.0%, p = 0.174). The higher association was not statistically significant for luminal A (RR:1.02, 95% CI: 0.94, 1.11), luminal B (RR: 1.07, 95% CI: 0.97, 1.17) or HER2 (RR: 1.14, 95% CI: 0.95, 1.38). There was no heterogeneity between these studies, but there was publication bias (Egger 0.545, p = 0.030). In the meta-analysis of nine case-case studies (Fig. 4; Supplementary Fig. 14) compared to luminal A, ever/longer duration OC use was associated with a 17% lower risk of luminal B (RR: 0.83, 95% CI: 0.73, 0.94, I2 = 9.4%, p = 0.358). There was a higher risk of HER2-overexpression (RR: 1.02, 95% CI: 0.92, 1.14) and TNBC subtypes (RR: 1.04, 95% CI: 0.93, 1.16), but these were not significant. There was no evidence of heterogeneity or publication bias (Egger: -0.36, p = 0.527).
Hormone replacement therapy (HRT)
Twenty-one studies evaluated the association between HRT use and BC molecular subtypes (ten case–control studies, six case-case studies, one case–control/case-case study, and four cohort studies). In the cohort and case–control analysis [17, 18, 28, 33, 36, 38, 42, 44, 60, 65, 70, 72, 73, 80], any use of HRT was associated with higher risk in all BC subtypes: all seven luminal A studies [17, 33, 38, 42, 70, 72, 73] and lower risk among subgroups of estrogen and progesterone therapy (≤ 5 years vs. never) [17] and women with BMI < 25 [33], higher risk in three [17, 38, 72] luminal B studies, four [36, 42, 60, 72] HER2-overexpressing studies and six [17, 19, 33, 38, 42, 72] TNBC studies. In the case-case analysis [49, 59, 60, 74, 75, 81, 89], ever use of HRT was associated with a lower risk in three luminal B studies [60, 81, 89], while higher risk was observed in five [59, 60, 74, 81, 89] HER2 studies, and one [74] TNBC study compared to luminal A. Three studies that were excluded from the meta-analysis because they had different comparison groups or reported frequencies [70, 75, 81] found an association between higher duration of HRT use with higher risk of luminal B and HER2, and mixed results with TNBC.
In the meta-analysis of eleven case–control studies and two cohort studies (Fig. 4; Supplementary Fig. 15), ever use of HRT was associated with a 50% higher risk of luminal A (RR: 1.50, 95% CI: 1.27, 1.77, I2 = 90.3%, p < 0.001) and 23% higher risk of luminal B (RR: 1.23, 95% CI: 1.07, 1.40, I2 = 60.0%, p = 0.001), however no significant association with HER2-overexpressing (RR: 1.01, 95% CI: 0.81, 1.25) and TNBC (RR: 1.10, 95% CI: 0.97, 1.25) with significant heterogeneity between the studies, but no publication bias (Egger -0.77, p = 0.108). For the five case-case studies (Fig. 4; Supplementary Fig. 16), any use of HRT was not associated with any BC molecular subtypes. There was some heterogeneity but no evidence of publication bias: (Egger: -1.61, p = 0.123).
Pregnancy
Four studies [38, 57, 72, 89] evaluated the association between pregnancy and BC molecular subtypes (three case–control studies and one case-case study). However, we did not have a sufficient number of studies for meta-analysis; studies by Rojas-Lima, Ma, and Ellingford-Dale et al. showed a similar association between the number of pregnancies with lower risk of luminal B, HER2-overexpressing, and TNBC [38, 57, 72]. In Zhang et al., the results show a higher risk of luminal B breast cancer and lower risk of HER2-overexpressing and TNBC with number of pregnancies [89].
Years since last birth/pregnancy
Six studies [31, 32, 34, 53, 59, 68] evaluated the association between years since last birth/pregnancy and BC molecular subtypes (two case–control studies, one case-case, and two cohort studies). DeMudler et. al., reported that diagnosis of BC within 5 years after last birth was proportionally more likely to be TNBC and HER2-overexpressing BC compared to luminal A breast cancer subtype [34]. Pilewskie et. al., found BCs diagnosed in parous women 0–2 years from last parity were more likely to be diagnosed with TNBC and HR negative compared to nulliparous women [68]. However, Li et. al [53], and Martinez et al. [59] did not find any significant association between years since last birth and any BC molecular subtypes. Martinez et al. adjusted for age at menopause, though the four other studies did not adjust for age or menopausal status (potential confounding variables).
Abortion
Five studies [37, 44, 80, 85, 87] evaluated the association between abortion and BC molecular subtypes (four case–control studies and one case–control/case-only study), which were included in the systematic review. However, we did not have enough studies to conduct a meta-analysis. Ye et al. reported no significant association with lower risk for all subtypes except TNBC, which showed a non-significant higher risk [87], while Huang et al. reported no significant association with HER2 + subtype but the studies had different reference groups [44]. Dolle et al. reported a non-significant association for higher risk in TNBC [37]. Xing et al. reported a significant association for lower risk of luminal A and luminal B breast cancer, but a lower non-significant association for HER2 + and TNBC for spontaneous abortion [85]. For induced abortion, Xing et al. reported a significant association for higher risk of luminal A, and a higher non-significant association for luminal B, HER2 overexpressing, and TNBC subtypes [85]. Tsakountakis et. al. reported that history of ever abortion was associated with a lower risk of HER-2/neu + tumors among postmenopausal women [80].
Summary of meta-analyses
Out of eight reproductive factors included in this meta-analysis, three were consistently associated with BC risk across subtypes: age at menopause, age at first birth, and breastfeeding, with the strongest associations observed in the luminal A subtype among case–control and cohort studies (Table 2). The associations for parity, age at menarche, OC use, HRT use, and menopausal status were less consistent, and the strongest associations were observed for the luminal B subtype (Table 2). In the case-case analysis, the strongest associations were menopausal status and age at first birth for HER2-enriched and TNBC subtypes, but not for luminal B breast cancer (Table 3). The associations for parity, age at menarche, OC use, HRT use, and breastfeeding were less consistent and the strongest associations overall were observed in TNBC (Table 3).
Discussion
This systematic review and meta-analysis summarizes the current published literature examining eleven reproductive factors and the risk of breast cancer molecular subtypes. To ensure consistency in contemporary classifications of BC based on molecular subtypes, the scope of this review was limited to luminal A, luminal B, HER2-overexpressing, and TNBC/basal-like breast cancer. In the 75 included studies, we observed that the strength and consistency of associations across subtypes differed by risk factors.
Late age at menarche was protective for all four BC subtypes. However, the other factors were most consistently associated with the luminal A subtype. Except for OC use, most of the associations between reproductive risk factors were observed for the luminal subtypes, similar to a prior systematic review showing stronger associations among hormone receptor-positive BC [3].
The prior meta-analysis on this topic evaluated three risk factors: parity, age at first birth, and breastfeeding [6]. Our meta-analysis updates these previous findings by including more recently conducted studies, and includes additional reproductive factors (age at menarche, age at menopause, menopausal status, OC use, and HRT use) for evaluation. Consistent with these mechanisms, we observed that later age at menarche was associated with reduced risk of all four subtypes regardless of study design, although the association was weakest for luminal B and HER2-overexpressing BC. Post/peri-menopausal status was also associated with reduced BC risk for luminal A and luminal B, but not HER-2 overexpressing or TNBC in case–control studies. Misclassification of menopausal status may be possible here because most post-menopausal breast cancers are hormone-receptor positive. In case-only analysis, there was an increased risk of HER2-overexpressing and TNBC compared with luminal A breast cancer. Older age at menopause was associated with increased BC risk, with the strongest association seen in HER2-overexpressing BC. In the case-only analysis, younger vs. older age at menopause was associated with increased TNBC risk compared with luminal A [30, 59].
Higher parity was protective against BC risk regardless of study design, except for TNBC, where no significant association was observed across 27 studies. Lambertini et al. and Islami et al. observed that breastfeeding was associated with a lower risk of luminal and TNBC [6, 8], consistent with our findings showing significantly lower risk for luminal A, luminal B, HER2 + and TNBC subtypes with ever breastfeeding and with longer duration of breastfeeding. Breastfeeding is associated with additional prolonged reductions in estrogen levels locally in breast fluid by increased secretion of hormones like prolactin, glucocorticoids, and insulin [90]. Parous women experience reduced breast fluid estrogen due to the destruction of alveolar lobular breast tissue during post-partum [90]. Increased parity, younger age at first pregnancy, and lactation in breastfeeding women are found to be protective reproductive risk factors and restriction of these factors can cause prolonged exposure to endogenous estrogens and lead to breast cancer [90, 91]. We observed the strongest associations of OC use with TNBC subtype [23, 25, 37, 38]. In case-only studies, duration of OC use was associated with increased risk of HER2-overexpressing and TNBC, but not luminal B breast cancer [54, 76, 84, 89]. For HRT use, there was a strong association between duration of HRT use and ever use with all BC subtypes, [17, 19, 33, 36, 38, 42, 72], although the strongest association was observed in luminal A breast cancer [16, 17]. OC and HRT may drive BC risk via estrogen and/or progesterone-related pathways [92], as estrogens accelerate the mitotic rates of both normal and cancerous breast epithelial cells, and metabolites of estradiol are carcinogenic in this tissue type [93,94,95].
This review identified several gaps in the literature on luminal A, luminal B, HER2-overexpressing, and TNBC/basal-like subtypes and reproductive factors. History of abortion, pregnancy, and years since last birth could not be included in the meta-analysis due to an insufficient number of studies examining risk associations by molecular subtype. However, the existing studies indicate that women with a history of abortion have an increased risk of BC regardless of subtype, even though studies on abortion with BC subtypes are lacking [87]. Pregnancy has been shown to have a protective effect against BC; pregnancy has a shortterm increase in exposure to estrogen and progesterone, but overall reduces risk of breast cancer [93]. However, this also must be considered in relation to age at first pregnancy, because at later age of first pregnancy there is a higher probability of existing abnormal cells in the breast epithelium (due to accumulation of genomic errors with aging), and the burst of estrogens during pregnancy stimulates the growth of these pre-existing abnormal cells. This will then contribute to cancer progression in the breast tissue. Additional studies of subtype-specific risk associations for the history of abortion (spontaneous vs. induced), years since last pregnancy, and age at pregnancy at which abortion occurred are needed. Risk factors for breast cancer are thought to differ across menopausal status [96], however, due to the insufficient number of studies, a meta-analysis of reproductive risk factors by menopausal status could not be conducted. This review also reported that certain reproductive factors have a protective effect against BC; these factors include increased breastfeeding, higher parity and early age at first birth. We report that OC and HRT use have a high probability of increasing risk of BC and are non-protective risk factors. This review also includes a meta-analysis of eight reproductive risk factors, more than previously published systematic reviews and meta-analyses.
According to the American Cancer Society preventative guidelines, not having children, not breastfeeding, use of OC, and use of HRT after menopause increase the risk of breast cancer [97]. However, BC preventative guidelines lack information specific to molecular breast cancer subtypes, and breastfeeding information is not included in the most commonly used BC risk prediction model [98, 99]. This study demonstrates the potential utility of targeting interventions for modifiable reproductive factors such as breastfeeding to populations with high incidences of luminal A, luminal B, HER2-overexpressing, and TNBC/basal-like subtypes, which have a significant association with breastfeeding. Future studies might benefit from including reproductive risk factor associations combined with family history and other known risk factors to predict the risk of developing specific breast cancer subtypes.
Potential risk of biases of included studies should be considered. Meta-analytic associations between age at menarche, age at first birth, menopausal status, and parity with BC subtypes were often in opposing directions for case–control/cohort studies compared to case-case studies. Selection bias in the case–control/cohort studies is possible; additionally, interpretation of case-case studies has its limitations, as true risks relative to a cancer-free population cannot be directly estimated. Further, some of the results were unexpected in regards to the hypothesized associations in relation to biological mechanisms. For instance, hormone-dependent subtypes are more common in post-menopausal women, though we observed the opposite in case–control/cohort studies. Misclassification of menopausal status may be a contributing factor to these findings. Additionally, exposure to OC is expected to increase hormone-dependent BC, though we found a protective effect for luminal B in the case-case studies. Comprehensive data on OC formulation was not available for our analysis, though warrants further investigation to assess heterogeneity of effect by estrogen and progestin dosages. This review has several limitations: first, eligible studies were restricted to papers published in English, which may have excluded relevant studies from other populations. We did not conduct a formal risk of bias assessment, though we have discussed potential biases in the observed associations. Additionally, there were not enough studies examining pregnancy, abortion, and time since last birth associated with luminal breast cancer and TNBC subtypes, and we only included studies from the year 2000 to 2021. However, this study serves as the first comprehensive review and meta-analysis of the association between luminal A, luminal B, HER2-overexpressing, and TNBC subtypes and a wide range of reproductive factors.
Conclusions
This study offers a comprehensive and up-to-date evaluation of the scientific literature on the associations between reproductive factors and luminal A, luminal B, HER2-overexpressing, and TNBC molecular subtypes. We identified a need for additional studies examining abortion as a risk factor and studies examining reproductive risk factors for BC subtypes stratified by patient menopausal status. Across all reproductive factors examined, age at menarche, age at first birth, parity, pregnancy, and breastfeeding showed relatively consistent risk associations across breast cancer subtypes. Considering this finding, common risk prediction models may be improved upon with the inclusion of breastfeeding status as a predictor.
Availability of data and materials
Data were taken from publicly available publications and as such can be widely accessed. All data generated or analyzed during this study are included in this published article [and its supplementary information files]. The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin Jul-Aug. 2014;64(4):252–71. https://doi.org/10.3322/caac.21235.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144(1):1–10. https://doi.org/10.1007/s10549-014-2852-7.
Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–68.
Kapil U, Bhadoria AS, Sareen N, Singh P, Dwivedi SN. Reproductive factors and risk of breast cancer: a review. Indian J Cancer. 2014;51(4):571–6. https://doi.org/10.4103/0019-509x.175345.
Lambertini M, Santoro L, Del Mastro L, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76. https://doi.org/10.1016/j.ctrv.2016.07.006.
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–91. https://doi.org/10.1200/jco.2009.24.9284.
Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status–a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–407. https://doi.org/10.1093/annonc/mdv379.
Li H, Sun X, Miller E, et al. BMI, reproductive factors, and breast cancer molecular subtypes: a case-control study and meta-analysis. J Epidemiol. 2017;27(4):143–51. https://doi.org/10.1016/j.je.2016.05.002.
Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. https://doi.org/10.1186/bcr1525.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London, England). 2010;8(5):336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. https://doi.org/10.1038/35021093.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med. 2009;151(4):264–9.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Fortner RT, Sisti J, Chai B, et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses’ Health Studies. Breast Cancer Res. 2019;21(1):40. https://doi.org/10.1186/s13058-019-1119-y.
Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: results from the nurses’ health studies. Int J Cancer. 2016;138(10):2346–56. https://doi.org/10.1002/ijc.29968.
Tamimi RM, Colditz GA, Hazra A, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131(1):159–67. https://doi.org/10.1007/s10549-011-1702-0.
Saxena T, Lee E, Henderson KD, et al. Menopausal hormone therapy and subsequent risk of specific invasive breast cancer subtypes in the California teachers study. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2366–78. https://doi.org/10.1158/1055-9965.Epi-10-0162.
Abubakar M, Sung H, Bcr D, et al. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018;20(1):114. https://doi.org/10.1186/s13058-018-1033-8.
Ambrosone CB, Zirpoli G, Ruszczyk M, et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the Women’s Circle of Health Study. Cancer Causes Control. 2014;25(2):259–65. https://doi.org/10.1007/s10552-013-0323-9.
Atkinson RL, El-Zein R, Valero V, et al. Epidemiological risk factors associated with inflammatory breast cancer subtypes. Cancer Causes Control. 2016;27(3):359–66. https://doi.org/10.1007/s10552-015-0712-3.
Beaber EF, Malone KE, Tang MT, et al. Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiol Biomarkers Prev. 2014;23(5):755–64. https://doi.org/10.1158/1055-9965.Epi-13-0944.
Benefield HC, Zirpoli GR, Allott EH, et al. Epidemiology of Basal-like and Luminal Breast Cancers among Black Women in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2021;30(1):71–9. https://doi.org/10.1158/1055-9965.Epi-20-0556.
Bethea TN, Rosenberg L, Hong CC, et al. A case-control analysis of oral contraceptive use and breast cancer subtypes in the African American Breast Cancer Epidemiology and Risk Consortium. Breast Cancer Res. 2015;17(1):22. https://doi.org/10.1186/s13058-015-0535-x.
Brandão M, Guisseve A, Damasceno A, et al. Risk factors for breast cancer, overall and by tumor subtype, among women from mozambique. Sub-Saharan Africa Cancer Epidemiol Biomarkers Prev. 2021;30(6):1250–9. https://doi.org/10.1158/1055-9965.Epi-20-1730.
Brouckaert O, Rudolph A, Laenen A, et al. Reproductive profiles and risk of breast cancer subtypes: a multi-center case-only study. Breast Cancer Res. 2017;19(1):119. https://doi.org/10.1186/s13058-017-0909-3.
Cerne JZ, Ferk P, Frkovic-Grazio S, Leskosek B, Gersak K. Risk factors for HR- and HER2-defined breast cancer in Slovenian postmenopausal women. Climacteric. 2012;15(1):68–74. https://doi.org/10.3109/13697137.2011.609286.
Chauhan R, Trivedi V, Rani R, Singh U. A comparative analysis of body mass index with estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 status in pre- and postmenopausal breast cancer patients. J Midlife Health. 2020;11(4):210–6. https://doi.org/10.4103/jmh.JMH_97_20.
Chen L, Li CI, Tang MT, et al. Reproductive factors and risk of luminal, HER2-overexpressing, and triple-negative breast cancer among multiethnic women. Cancer Epidemiol Biomarkers Prev. 2016;25(9):1297–304. https://doi.org/10.1158/1055-9965.Epi-15-1104.
Collins LC, Gelber S, Marotti JD, et al. Molecular Phenotype of Breast Cancer According to Time Since Last Pregnancy in a Large Cohort of Young Women. Oncologist. 2015;20(7):713–8. https://doi.org/10.1634/theoncologist.2014-0412.
Cruz GI, Martínez ME, Natarajan L, et al. Hypothesized role of pregnancy hormones on HER2+ breast tumor development. Breast Cancer Res Treat. 2013;137(1):237–46. https://doi.org/10.1007/s10549-012-2313-0.
Cui Y, Deming-Halverson SL, Beeghly-Fadiel A, et al. Interactions of hormone replacement therapy, body weight, and bilateral oophorectomy in breast cancer risk. Clin Cancer Res. 2014;20(5):1169–78. https://doi.org/10.1158/1078-0432.Ccr-13-2094.
De Mulder H, Laenen A, Wildiers H, et al. Breast cancer subtype and survival by parity and time since last birth. Breast Cancer Res Treat. 2018;169(3):481–7. https://doi.org/10.1007/s10549-018-4701-6.
Devi CR, Tang TS, Corbex M. Incidence and risk factors for breast cancer subtypes in three distinct South-East Asian ethnic groups: Chinese, Malay and natives of Sarawak. Malaysia Int J Cancer. 2012;131(12):2869–77. https://doi.org/10.1002/ijc.27527.
Dogan L, Kalaylioglu Z, Karaman N, Ozaslan C, Atalay C, Altinok M. Relationships between epidemiological features and tumor characteristics of breast cancer. Asian Pac J Cancer Prev. 2011;12(12):3375–80.
Dolle JM, Daling JR, White E, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1157–66. https://doi.org/10.1158/1055-9965.Epi-08-1005.
Ellingjord-Dale M, Vos L, Tretli S, Hofvind S, Dos-Santos-Silva I, Ursin G. Parity, hormones and breast cancer subtypes - results from a large nested case-control study in a national screening program. Breast Cancer Res. 2017;19(1):10. https://doi.org/10.1186/s13058-016-0798-x.
Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–97. https://doi.org/10.1007/s10549-011-1616-x.
Gaudet MM, Gierach GL, Carter BD, et al. Pooled analysis of nine cohorts reveals breast cancer risk factors by tumor molecular subtype. Cancer Res. 2018;78(20):6011–21. https://doi.org/10.1158/0008-5472.Can-18-0502.
Giudici F, Scaggiante B, Scomersi S, Bortul M, Tonutti M, Zanconati F. Breastfeeding: a reproductive factor able to reduce the risk of luminal B breast cancer in premenopausal White women. Eur J Cancer Prev. 2017;26(3):217–24. https://doi.org/10.1097/cej.0000000000000220.
Holm J, Eriksson L, Ploner A, et al. Assessment of breast cancer risk factors reveals subtype heterogeneity. Cancer Res. 2017;77(13):3708–17. https://doi.org/10.1158/0008-5472.Can-16-2574.
Horn J, Opdahl S, Engstrøm MJ, et al. Reproductive history and the risk of molecular breast cancer subtypes in a prospective study of Norwegian women. Cancer Causes Control. 2014;25(7):881–9. https://doi.org/10.1007/s10552-014-0388-0.
Huang WY, Newman B, Millikan RC, et al. Risk of breast cancer according to the status of HER-2/neu oncogene amplification. Cancer Epidemiol Biomarkers Prev. 2000;9(1):65–71.
Ihemelandu CU, Leffall LD Jr, Dewitty RL, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res. 2007;143(1):109–18. https://doi.org/10.1016/j.jss.2007.03.085.
Islam T, Matsuo K, Ito H, et al. Reproductive and hormonal risk factors for luminal, HER2-overexpressing, and triple-negative breast cancer in Japanese women. Ann Oncol. 2012;23(9):2435–41. https://doi.org/10.1093/annonc/mdr613.
Jeong SH, An YS, Choi JY, et al. Risk reduction of breast cancer by childbirth, breastfeeding, and their interaction in korean women: heterogeneous effects across menopausal status, hormone receptor status, and pathological subtypes. J Prev Med Public Health. 2017;50(6):401–10. https://doi.org/10.3961/jpmph.17.152.
John EM, Hines LM, Phipps AI, et al. Reproductive history, breast-feeding and risk of triple negative breast cancer: the Breast Cancer Etiology in Minorities (BEM) study. Int J Cancer. 2018;142(11):2273–85. https://doi.org/10.1002/ijc.31258.
Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. https://doi.org/10.1186/bcr2261.
Lee E, McKean-Cowdin R, Ma H, et al. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J Clin Oncol. 2011;29(33):4373–80. https://doi.org/10.1200/jco.2010.33.6446.
Lee SK, Kim SW, Han SA, Kil WH, Lee JE, Nam SJ. The protective effect of parity in hormone receptor-positive, Ki-67 expressing breast cancer. World J Surg. 2014;38(5):1065–9. https://doi.org/10.1007/s00268-014-2468-4.
Lee PMY, Kwok CH, Chan WC, et al. Heterogeneous associations between obesity and reproductive-related factors and specific breast cancer subtypes among hong kong chinese women. Horm Cancer. 2020;11(3–4):191–9. https://doi.org/10.1007/s12672-020-00386-2.
Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137(2):579–87. https://doi.org/10.1007/s10549-012-2365-1.
Lorona NC, Cook LS, Tang MC, Hill DA, Wiggins CL, Li CI. Recent use of oral contraceptives and risk of luminal B, triple-negative, and HER2-overexpressing breast cancer. Horm Cancer. 2019;10(2–3):71–6. https://doi.org/10.1007/s12672-019-00362-5.
Ly M, Antoine M, Dembélé AK, et al. High incidence of triple-negative tumors in sub-saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncology. 2012;83(5):257–63. https://doi.org/10.1159/000341541.
Ma H, Wang Y, Sullivan-Halley J, et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res. 2010;70(2):575–87. https://doi.org/10.1158/0008-5472.Can-09-3460.
Ma H, Ursin G, Xu X, et al. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: a pooled analysis. Breast Cancer Res. 2017;19(1):6. https://doi.org/10.1186/s13058-016-0799-9.
Mane A, Khatib KI, Deshmukh SP, Nag SM, Sane SP, Zade BP. A comparison of clinical features, pathology and outcomes in various subtypes of breast cancer in Indian women. J Clin Diagn Res. 2015;9(9):Pc01–4. https://doi.org/10.7860/jcdr/2015/15253.6461.
Martinez ME, Wertheim BC, Natarajan L, et al. Reproductive factors, heterogeneity, and breast tumor subtypes in women of mexican descent. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1853–61. https://doi.org/10.1158/1055-9965.Epi-13-0560.
Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39. https://doi.org/10.1007/s10549-007-9632-6.
Nichols HB, Trentham-Dietz A, Love RR, et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):41–7.
Palmer JR, Viscidi E, Troester MA, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. Oct 2014;106(10)doi:https://doi.org/10.1093/jnci/dju237
Park B, Choi JY, Sung HK, et al. Attribution to heterogeneous risk factors for breast cancer subtypes based on hormone receptor and human epidermal growth factor 2 receptor expression in Korea. Medicine (Baltimore). 2016;95(14):e3063. https://doi.org/10.1097/md.0000000000003063.
Park S, Moon BI, Oh SJ, et al. Clinical subtypes and prognosis in breast cancer according to parity: a nationwide study in Korean breast cancer society. Breast Cancer Res Treat. 2019;173(3):679–91. https://doi.org/10.1007/s10549-018-5032-3.
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113(7):1521–6. https://doi.org/10.1002/cncr.23786.
Phipps AI, Chlebowski RT, Prentice R, et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;103(6):470–7. https://doi.org/10.1093/jnci/djr030.
Phipps AI, Buist DS, Malone KE, et al. Reproductive history and risk of three breast cancer subtypes defined by three biomarkers. Cancer Causes Control. 2011;22(3):399–405. https://doi.org/10.1007/s10552-010-9709-0.
Pilewskie M, Gorodinsky P, Fought A, et al. Association between recency of last pregnancy and biologic subtype of breast cancer. Ann Surg Oncol. 2012;19(4):1167–73. https://doi.org/10.1245/s10434-011-2104-6.
Pizzato M, Carioli G, Rosso S, Zanetti R, La Vecchia C. The impact of selected risk factors among breast cancer molecular subtypes: a case-only study. Breast Cancer Res Treat. 2020;184(1):213–20. https://doi.org/10.1007/s10549-020-05820-1.
Rauh C, Gass P, Heusinger K, et al. Association of molecular subtypes with breast cancer risk factors: a case-only analysis. Eur J Cancer Prev. 2015;24(6):484–90. https://doi.org/10.1097/cej.0000000000000111.
Redondo CM, Gago-Domínguez M, Ponte SM, et al. Breast feeding, parity and breast cancer subtypes in a Spanish cohort. PLoS One. 2012;7(7):e40543. https://doi.org/10.1371/journal.pone.0040543.
Rojas-Lima E, Gamboa-Loira B, Cebrián ME, Rothenberg SJ, López-Carrillo L. A cumulative index of exposure to endogenous estrogens and breast cancer by molecular subtypes in northern Mexican women. Breast Cancer Res Treat. 2020;180(3):791–800. https://doi.org/10.1007/s10549-020-05562-0.
Salagame U, Banks E, O’Connell DL, Egger S, Canfell K. Menopausal Hormone Therapy use and breast cancer risk by receptor subtypes: Results from the New South Wales Cancer Lifestyle and EvaluAtion of Risk (CLEAR) study. PLoS One. 2018;13(11):e0205034. https://doi.org/10.1371/journal.pone.0205034.
Sanderson M, Pal T, Beeghly-Fadiel A, et al. A Pooled Case-only Analysis of Reproductive Risk Factors and Breast Cancer Subtype Among Black Women in the Southeastern United States. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1416–23. https://doi.org/10.1158/1055-9965.Epi-20-1784.
Shinde SS, Forman MR, Kuerer HM, et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer. 2010;116(21):4933–43. https://doi.org/10.1002/cncr.25443.
Song N, Choi JY, Sung H, et al. Heterogeneity of epidemiological factors by breast tumor subtypes in Korean women: a case-case study. Int J Cancer. 2014;135(3):669–81. https://doi.org/10.1002/ijc.28685.
Sung H, Garcia-Closas M, Chang-Claude J, et al. Heterogeneity of luminal breast cancer characterised by immunohistochemical expression of basal markers. Br J Cancer. 2016;114(3):298–304. https://doi.org/10.1038/bjc.2015.437.
Sung H, Devi BCR, Tang TS, Rosenberg PS, Anderson WF, Yang XR. Divergent breast cancer incidence trends by hormone receptor status in the state of Sarawak. Malaysia Int J Cancer. 2020;147(3):829–37. https://doi.org/10.1002/ijc.32812.
Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20(7):1071–82. https://doi.org/10.1007/s10552-009-9331-1.
Tsakountakis N, Sanidas E, Stathopoulos E, et al. Correlation of breast cancer risk factors with HER-2/neu protein overexpression according to menopausal and estrogen receptor status. BMC Womens Health. 2005;5(1):1. https://doi.org/10.1186/1472-6874-5-1.
Turkoz FP, Solak M, Petekkaya I, et al. Association between common risk factors and molecular subtypes in breast cancer patients. Breast. 2013;22(3):344–50. https://doi.org/10.1016/j.breast.2012.08.005.
von Au A, Klotzbuecher M, Uhlmann L, et al. Impact of reproductive factors on breast cancer subtypes in postmenopausal women: a retrospective single-center study. Arch Gynecol Obstet. 2017;295(4):971–8. https://doi.org/10.1007/s00404-017-4298-8.
Wang JM, Wang J, Zhao HG, Liu TT, Wang FY. Reproductive risk factors associated with breast cancer molecular subtypes among young women in Northern China. Biomed Res Int. 2020;2020:5931529. https://doi.org/10.1155/2020/5931529.
Work ME, John EM, Andrulis IL, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the breast cancer family registry. Br J Cancer. 2014;110(5):1367–77. https://doi.org/10.1038/bjc.2013.807.
Xing P, Li J, Jin F. A case-control study of reproductive factors associated with subtypes of breast cancer in Northeast China. Med Oncol. 2010;27(3):926–31. https://doi.org/10.1007/s12032-009-9308-7.
Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439–43. https://doi.org/10.1158/1055-9965.Epi-06-0806.
Ye DM, Li Q, Yu T, Wang HT, Luo YH, Li WQ. Clinical and epidemiologic factors associated with breast cancer and its subtypes among Northeast Chinese women. Cancer Med. 2019;8(17):7431–45. https://doi.org/10.1002/cam4.2589.
Yuan X, Song X, Jiang M, Li Q, Liu X, Wang M. Reproductive factors, steroid receptor status, and tumour markers of HER2-positive breast cancer in Northern China. Breast Care (Basel). 2009;4(5):315–8. https://doi.org/10.1159/000236467.
Zhang L, Huang Y, Feng Z, et al. Comparison of breast cancer risk factors among molecular subtypes: a case-only study. Cancer Med. 2019;8(4):1882–92. https://doi.org/10.1002/cam4.2012.
Petrakis NL, Wrensch MR, Ernster VL, et al. Influence of pregnancy and lactation on serum and breast fluid estrogen levels: implications for breast cancer risk. Int J Cancer. 1987;40(5):587–91. https://doi.org/10.1002/ijc.2910400502.
Russo IH, Russo J. Pregnancy-induced changes in breast cancer risk. J Mammary Gland Biol Neoplasia. 2011;16(3):221–33. https://doi.org/10.1007/s10911-011-9228-y.
Kawai M, Kakugawa Y, Nishino Y, Hamanaka Y, Ohuchi N, Minami Y. Reproductive factors and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women. Cancer Sci. 2012;103(10):1861–70. https://doi.org/10.1111/j.1349-7006.2012.02379.x.
Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24(1):29–43. https://doi.org/10.1016/0277-5379(88)90173-3.
Russo J, Lareef MH, Tahin Q, et al. 17Beta-estradiol is carcinogenic in human breast epithelial cells. J Steroid Biochem Mol Biol. 2002;80(2):149–62. https://doi.org/10.1016/s0960-0760(01)00183-2.
Russo J, Russo IH. Genotoxicity of steroidal estrogens. Trends Endocrinol Metab. 2004;15(5):211–4. https://doi.org/10.1016/j.tem.2004.05.007.
Parsa P, Parsa B. Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pac J Cancer Prev Oct-Dec. 2009;10(4):545–50.
ACS. Lifestyle-related Breast Cancer Risk Factors. https://www.cancer.org/cancer/breastcancer/risk-and-prevention/lifestyle-related-breast-cancer-risk-factors.html.
Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. https://doi.org/10.1093/jnci/81.24.1879.
Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction modelincorporating genetic and nongenetic risk factors. Gen Med. 2019;21(8):1708–18. https://doi.org/10.1038/s41436-018-0406-9.
Acknowledgements
We thank James Burgett for assistance with the literature search as well as Nimish Valvi, Machayla Grace Gatsos, Xiangya Meng for assistance with data verification.
Role of funders
None.
Disclosures
The authors do not have any conflicts of interest to disclose.
Funding
This work was supported by the National Institutes of Health, National Cancer Institute, Fogarty International Center (K01TW010271, T.A).
Author information
Authors and Affiliations
Contributions
Conceptualization: XM, TA. Data Curation: XM, CO, SK, AJ, TA. Formal analysis: XM, CO, SK. Investigation: XM, CO, TA, AD. Supervision: TA, AD, AC, CH. Writing-original draft: XM, CO, SK. Writing-review and editing: all authors.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.and TNBC subtype.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental Table 1. Summary statistics for studies included after full-text review (N=75). Supplemental Figure 1. Age at menarche by molecular subtypes of Breast cancer. Supplemental Figure 2. Age at menarche by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 3. Age at menopause by molecular subtypes of Breast cancer. Supplemental Figure 4. Age at menopause by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 5. Menopausal status by molecular subtypes of Breast cancer. Supplemental Figure 6. Menopausal status by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 7. Age at first birth by molecular subtypes of Breast cancer. Supplemental Figure 8. Age at first birth by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 9. Parity by molecular subtypes of Breast cancer. Supplemental Figure 10. Parity by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 11. Breastfeeding by molecular subtypes of Breast cancer. Supplemental Figure 12. Breastfeeding by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 13. OC use by molecular subtypes of Breast cancer. Supplemental Figure 14. OC use by molecular subtypes of Breast cancer (Case vs Luminal A). Supplemental Figure 15. HRT use by molecular subtypes of Breast cancer. Supplemental Figure 16. HRT use by molecular subtypes of Breast cancer (Case vs Luminal A).
Additional file 2.
Supplemental Appendix.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mao, X., Omeogu, C., Karanth, S. et al. Association of reproductive risk factors and breast cancer molecular subtypes: a systematic review and meta-analysis. BMC Cancer 23, 644 (2023). https://doi.org/10.1186/s12885-023-11049-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11049-0