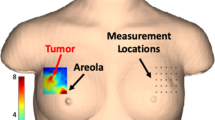
Abstract
Background
Breast cancer neoadjuvant chemotherapy (NACT) allows for assessing tumor sensitivity to systemic treatment, planning adjuvant treatment and follow-up. However, a sufficiently large number of patients fail to achieve the desired level of pathological tumor response while optimal early response assessment methods have not been established now. In our study, we simultaneously assessed the early chemotherapy-induced changes in the tumor volume by ultrasound (US), the tumor oxygenation by diffuse optical spectroscopy imaging (DOSI), and the state of the tumor vascular bed by Doppler US to elaborate the predictive criteria of breast tumor response to treatment.
Methods
A total of 133 patients with a confirmed diagnosis of invasive breast cancer stage II to III admitted to NACT following definitive breast surgery were enrolled, of those 103 were included in the final analysis. Tumor oxygenation by DOSI, tumor volume by US, and tumor vascularization by Doppler US were determined before the first and second cycle of NACT. After NACT completion, patients underwent surgery followed by pathological examination and assessment of the pathological tumor response. On the basis of these, data regression predictive models were created.
Results
We observed changes in all three parameters 3 weeks after the start of the treatment. However, a high predictive potential for early assessment of tumor sensitivity to NACT demonstrated only the level of oxygenation, ΔStO2, (ρ = 0.802, p ≤ 0.01). The regression model predicts the tumor response with a high probability of a correct conclusion (89.3%). The “Tumor volume” model and the “Vascularization index” model did not accurately predict the absence of a pathological tumor response to treatment (60.9% and 58.7%, respectively), while predicting a positive response to treatment was relatively better (78.9% and 75.4%, respectively).
Conclusions
Diffuse optical spectroscopy imaging appeared to be a robust tool for early predicting breast cancer response to chemotherapy. It may help identify patients who need additional molecular genetic study of the tumor in order to find the source of resistance to treatment, as well as to correct the treatment regimen.
Similar content being viewed by others
Background
Neoadjuvant chemotherapy (NACT) as the standard of care for patients with newly diagnosed breast cancer (BC) is now widely used to shrink the tumor before surgical treatment and reduce the volume of axillary lymph node dissections [1, 2]. This approach allows for assessing tumor sensitivity to systemic treatment, planning adjuvant treatment and follow-up [3]. Significant advances in the management of breast cancer are associated primarily with the personalization of the therapy depending on the molecular characteristics of the neoplasm [4, 5]. A pathologic complete response (pCR) to chemotherapy which means the absence of viable tumor cells in the breast tissue after surgery is a significant criterion that correlates with a favorable prognosis and good survival rates [1]. However, in case of the absence of a known molecular target, a sufficiently large number of patients fail to achieve the desired level of pathological tumor response (PTR) [1, 6]. Those patients may need additional genetic tests to find the cause of resistance to chemotherapy and their therapy should be altered to avoid unjustified toxicity, surgery delay and high cost of ineffective treatment [7, 8]. Unfortunately, optimal early response assessment methods have not been established now.
It would be attractive to use the dynamic of tumor volume as a simple and easily detectable biomarker. Standard cost-effective imaging modality ultrasound (US) investigation seems to be very proper for solving this problem, but earlier studies showed low efficacy of the method [9, 10]. Studies conducted in recent years demonstrated better efficacy of the US for predicting pCR but not the lack of response to treatment. The accuracy of predicting PTR to treatment depended on the cancer subtype [11,12,13,14,15]. Contrast-enhanced MRI [16] and 18F-FDG PET/CT [7, 17, 18] have demonstrated a good predicting ability by evaluation of the metabolic activity of tumor tissue. Both methods suffer from high costs and require the administration of contrast agents or radiopharmaceuticals, which limits their application for treatment monitoring.
Optical methods based on the difference in the scattering and absorption properties of normal and tumor tissues have been actively developed for diagnosis [19, 20] and monitoring of breast pathology [21,22,23,24]. Diffuse optical spectroscopy imaging (DOSI) utilizes light of far-red and near-infrared spectral range (~ 650 to 1000 nm), which has a low attenuation rate in tissue. The main chromophores in this range are oxyhemoglobin and deoxyhemoglobin, the concentration of which indirectly reflects oxygen delivery and consumption by tissues [25,26,27]. Evaluating their concentration, one can assess the level of tumor oxygenation (hypoxia) which appears to be an important characteristic of tumor biology [28]. Multiple studies have demonstrated DOSI data to be a prospective marker that reflects pathological tumor response at various times from the NACT starts [21, 24, 29,30,31,32,33]. The combined use of DOSI and assessment of the dynamics of tumor volume by the US, as well as taking into account tumor immunophenotype, made it possible to increase the predictive capabilities of the method [34].
Tumor oxygenation is inextricably linked with the state of its vascular bed [35, 36]. Antitumor treatment can affect both the cells and the stroma of the tumor, including its microvessels [37]. So, according to the reaction of the tumor vascular bed, it is possible to indirectly assess the sensitivity of the neoplasm to drug exposure. Doppler ultrasound investigation is a noninvasive, widespread, and relatively inexpensive vascular imaging method, which can be safely used for repeated measurements [38]. Change in the number of registered vessels turned out to be the independent factor reflecting the tumor response to treatment [39,40,41].
In our study, for the first time, we simultaneously assessed the early chemotherapy-induced changes in the tumor volume (by US), tumor oxygenation (by DOSI), and the state of the vascular bed (by Doppler US) to elaborate the predictive criteria of breast tumor response to treatment.
Methods
Patients’ data
The study was performed at Nizhny Novgorod Regional Clinical Oncology Hospital (Russia) from June 2016 to August 2021. A total of 133 patients with a confirmed diagnosis of invasive breast cancer stage II to III admitted to NACT following definitive breast surgery were enrolled, and 108 of them completed the therapy. Patients with tumors larger than 8 cm, fungating breast cancers, and age under 18 were excluded. Patient informed consent and study methods were reviewed by the local Research Ethics Border.
Study design
Before treatment, all patients were examined in accordance with the guidelines of the European Society for Medical Oncology (ESMO) [42] including mammography, ultrasound examination, and core biopsy of the tumor which provided the tumor receptor status. Tumor oxygenation by DOSI, tumor volume by US and tumor vascularization by Doppler US were determined twice, 1–3 days before the start of NACT and 1–3 days before the second cycle of NACT. The average interval between the first and second cycles was 21 days (± 2 days). After NACT completion, patients underwent surgery followed by pathological examination and assessment of the pathological tumor response to treatment (Fig. 1).
Histopathology
After surgery, the tumor was assigned a response status according to Miller–Payne scale [43] which implies the five grades depending on the residual number of viable tumor cells assessed after chemotherapy. For binary classification of response, an accurate evaluation with a calculation of the number of viable malignant cells was performed. In order to build a regression logistic model, we introduced a criterion for a “high” response (reducing the number of viable tumor cells by more than 50% or “low” response (reducing the number of viable tumor cells by less than 50%).
Imaging procedures
Doppler US
Scanning of the area of interest which was chosen to fully cover the tumor with proper margins was carried out by a Medison Accuvix-V20 US scanner (Samsung, Korea) by a 5.0–13.0 MHz multifrequency linear sensor in power Doppler mode. An original ultrasound technique was developed to assess breast tumor vascularization [44]. We calculated the average number of colored pixels (corresponding to the vessels) relative to the total number of pixels in all the images—a vascularization index (Fig. 2c). Then the difference between the two values (ΔVascularization index) was calculated. To assess the tumor blood flow changes during the treatment, we entered the value of Δrel(relative) of the vascularization index (Δrel Vascularization index = ΔVascularization index × 100%/Vascularization index before the NACT).
Changes in the tumor oxygen saturation, tumor vascularization, and tumor volume of patient B, aged 33. Diagnosis: multifocal (2 nodes) right BC, stage IIa (T2mN0M0), invasive breast carcinoma of no special type G3 (d, × 100) HER2neu-positive cancer (estrogen receptors = 0 points, progesterone receptors = 0 points, Ki67 = 60% (e, × 100)). After 1 cycle of NACT, DOSI showed an increase in the oxygenation level by 10% (a). US revealed a 10% decrease in tumor volume (b) as well as a 30% decrease in tumor vascularization (c). A pathologic investigation after six NACT cycles and surgery failed to find vital tumor cells, equivalent to the 5 grade of pathological tumor response (f, × 100)
Tumor volume
Tumor volume was determined automatically using the calculation options of the Medison Accuvix-V20 by the following formula:
Tumor volume (cm3) = length × width × thickness × ellipsoid correction factor (0.479)
It was measured before the start of the treatment and before the second cycle of NACT. Then, the difference between the two values was calculated, i.e., ΔTumor volume was determined. To assess the tumor volume changes during the treatment, we entered the value of Δrel(relative) of the tumor volume (Δrel Tumor volume = ΔTumor volume × 100%/Tumor volume before the NACT) (Fig. 2b).
Diffuse optical spectroscopy imaging
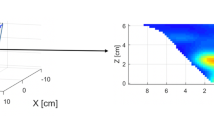
DOSI was performed on the setup (Institute of Applied Physics RAS, Nizhny Novgorod, Russia) [45] utilizing a frequency-domain approach and parallel plane trans-illumination scanning geometry. Radiation from three lasers is coupled in a single fiber bundle which illuminates the studied volume at three wavelengths: 684 nm corresponding to the maximum absorption of deoxygenated hemoglobin, 850 nm corresponding to the maximum absorption of oxyhemoglobin, and 794 nm at which absorption coefficients of oxygenated and deoxygenated hemoglobin coincide. The images were obtained by simultaneous scanning by moving the source and the detector located along the sagittal axis from the opposite sides of the studied subject with a step of 1–2 mm synchronously. In each position, data were detected from all three sources. High-frequency modulation of the laser radiation intensity 140 MHz was used to evaluate reduced scattering and absorption coefficient independently, thus improving the quantitative data on concentrations of oxy- and deoxyhemoglobin.
Image analysis
The numerical processing of DOSI images was described in our previous study [44]. The tumor region was selected manually by combining the tumor area on mammograms and DOSI images, as well as taking into account ultrasound data. Tumor chromophore concentrations were calculated by taking the mean over the tumor ROI. Blood oxygen saturation was calculated as StO2 = [HbO2]/[HbO2 + HHb] × 100% where HbO2 and HHb are the concentrations of oxy- and deoxyhemoglobin reconstructed from absorption coefficients at three wavelengths. StO2 was calculated for each spatial position, thus creating a DOSI image (optical mammogram) as a 2-D map of tissue oxygenation in the scanning area. The oxygen state of tumor tissue was calculated from the DOSI image by averaging StO2 in the tumor area using the ImageJ program (NIH, USA) (Fig. 2a). StO2 was calculated twice before the first and before the second cycle of NACT. The difference between the two values (ΔStO2) was determined and compared with tumor response to treatment.
Statistical analysis
For statistical data processing, the SPSS Statistics (v. 27) software package was used. The distributions were checked for normality using the Shapiro–Wilk test. Since there was no normal distribution of data, the nonparametric Spearman correlation coefficient was used. The bond strength was assessed using the Chaddock scale. For statistical modeling of the probability of an event, the logit model (binary logistic model) was used.
Separate models were run on each of three indicators: ΔStO2, Δrel Vascularization index, and Δrel Tumor volume. The fact of tumor response (decrease in the number of tumor cells after NACT by more than 50%—a “high” response) was taken as a binary event.
Besides, two supplementary models for ΔStO2 were developed for the prediction of the tumor complete response (fifth grade of PTR as a binary event) [see Additional file 1]. Model 4 was created for predicting the pCR in patients with triple-negative and HER2-positive breast cancer and Model 5 for predicting the pCR in all 103 patients included in the study.
The probability of an event occurring for a particular case was calculated by the formula:
where \(z=b\times X+a\)
X—independent variable value, a, b—regression coefficients.
With a p value of less than 0.5, it can be assumed that the event will not occur (Low response); otherwise, an event is expected (High response).
The critical significance level was taken as p ≤ 0.05.
To assess the predictive value of ΔStO2, ΔrelVascularization index, Δrel Tumor volume to the early BC response to NACT, the sensitivity, specificity, and accuracy of the methods were calculated according to the logit model.
Results
After the first cycle of NACT, five patients showed a significant (more than 100% compared to baseline) increase in the number of tumor vessels. We assumed this was associated with severe peritumoral inflammation around necrotic areas, and therefore these patients were not included in the final analysis. The characteristics of all analyzed subjects (103 patients included in further analysis) are given in Table 1. When studying the pathologic tumor response (PTR), the first grade was detected in 13 patients (13%), the second grade in 21 patients (20%), the third grade in 24 patients (23%), and the fourth grade in 20 patients (20%). The fifth grade (pCR) was detected in 25 patients (24%). In accordance with the proposed criterion for tumor response (> 50% or < 50% of cells loss), 57 (55%) patients demonstrated a “high” response to treatment and 46 (45%) patients demonstrated a “low” response.
Doppler US
In 46 patients who demonstrated a “low” response, a decrease in the number of tumor vessels was observed in 33 (72%), and an increase in 13 (28%) (Fig. 3a). Among 57 “high” responders, 56 showed a decrease in tumor vascularization. Statistical analysis showed a moderate negative correlation between ΔrelVascularization index and PTR (ρ = − 0.502, p ≤ 0.01).
Tumor volume
In the vast majority (94 patients, 91%), a decrease in tumor volume has been observed after the first cycle of NACT (Fig. 3b). Among 46 patients with a “low” response, a tumor volume increased in 6 cases and has not changed in 2. Among 57 patients with a “high” response, 56 patients showed a decrease in the tumor volume. A moderate negative correlation was revealed between Δrel Tumor volume and PTR (ρ = − 0.416, p ≤ 0.01).
Diffuse optical spectroscopy imaging
Breast tumors showed multidirectional dynamics of oxygenation depending on the PTR. Among 57 patients with a “high” response, 53 (93%) showed an increase in StO2 after the 1st cycle of NACT. The oxygenation decreased in three patients, but in one it did not change (Fig. 3c). Twenty-nine of the 46 patients with a “low” response experienced a decrease in tumor oxygenation after the 1st cycle of NACT. In 12 cases, the level of oxygenation did not change, and in 5 cases it increased. A positive strong correlation was found between the change in the oxygenation (ΔStO2) and the PTR (ρ = 0.802, p ≤ 0.01).
Binary logistic model
Model 1 "Δrel Tumor volume"
The overall number of patients correctly assigned to the groups of "high” response and "low” response using the variable "Δrel Tumor volume" was 70.9% (Table 2).
The sensitivity, specificity, and accuracy of the index for predicting PTR to NACT were 78.9%, 60.9%, and 70.9%, respectively.
Despite the presence of a fairly large number of false positive cases, all variables in the regression equation appeared to be statistically significant (Table 3): a = − 0.831 (p = 0.027) и b = − 0.052 (p = 0.001).
To test Model 1, a patient was randomly selected with “ΔrelTumor volume” = -3.
According to the model, the patient will respond to treatment with a probability of 33.7%. This was confirmed by a pathomorphological tumor study of the residual tumor (the number of viable tumor cells was 90% which corresponds to the second grade of PTR).
Model 2 "ΔrelVascularization index"
When using the "Δrel Vascularization index" model, the total percentage of correct predictions decreases up to 68% (Table 4).
The sensitivity, specificity, and accuracy of the indicator ΔrelVascularization index for predicting PTR were 75.4%, 58.7%, and 68.0%, respectively.
In Model 2 (Table 5), only the variable b = − 0.043 (p = 0.001) turned out to be statistically significant. For the constant a = − 0.989, the critical level of significance was not overcome (p = 0.07), so it is not advisable to test Model 2.
z = − 0.043 × X(Δrel Vascularization index)—0.989 (Model 2 (“Δrel Vascularization index”)).
Model 3 "ΔStO2"
The last step of the regression analysis was the introduction of the variable “the oxygenation level” (ΔStO2) into the model.
The “ΔStO2” variable significantly increases the number of correct predictions of low response to treatment. The overall percentage of correct predictions was 89.3% (Table 6). Early changes in the tumor oxygenation for prediction of the PTR demonstrated high sensitivity, specificity, and accuracy—89.5%, 89.1%, and 89.3%, respectively.
Both variables of the regression equation turned out to be statistically significant: a = − 0.773 (p = 0.035) and b = 0.318 (p = 0.001) (Table 7).
z = 0.318 × X(ΔStO2) − 0.773 (Model 3 “ΔStO2”)
To test the oxygenation level model, we have randomly used data from patients from the two groups.
Patient 1 (ΔStO2 = − 15)
z = 0.318 × ( − 15) − 0.773 = − 4.91
This patient will have a low response to treatment with a probability of 99.27%, which was confirmed by a pathological tumor response (the number of viable malignant cells was 94% which corresponded to the second grade of PTR).
Patient 2 (ΔStO2 = 18)
z = 0.318 × (18) − 0.773 = 4.95
The second patient with a probability of 99.3% could be attributed to the high response group, which was confirmed by the fifth grade of PTR.
Thus, Model 1 "Δrel Tumor volume" and Model 2 "Δrel Vascularization index" have the same limitation: they do not accurately predict a lack of response to treatment, while predicting a positive response is relatively good. The combined inclusion of all of the above variables (ΔStO2, Δrel Vascularization index, and Δrel Tumor volume) into the model did not show an improvement in the predictive values.
According to Model 4, the sensitivity, specificity, and accuracy of the «ΔStO2» indicator for predicting pCR in patients with triple-negative and HER2-positive breast cancer were 60.9%, 80.9%, and 74.3%, respectively (65 patients). According to Model 5, the sensitivity, specificity, and accuracy of the ΔStO2 indicator for predicting pCR were 20%, 95.2%, and 77.8%, respectively (103 patients). A detailed description of Model 4 and Model 5 is presented in Additional file 1.
Discussion
Starting the study of tumor oxygen state in the course of chemotherapy, we proceeded to that the effects of chemotherapy on tumors are due to both direct death of tumor cells and the delicate interplay between tumor vasculature responses and the inextricably linked changes in oxygen state [see Additional file 2] as well as its contribution to the processes driving the tumor cells death [46, 47]. Accordingly, for the development of criteria predicting the sensitivity of breast tumors to chemotherapy, we used complementary methods: assessment of the dynamics of tumor volume and blood flow using US and the dynamics of tumor oxygenation by DOSI.
Our study confirmed that ΔStO2 correctly reflects tumor changes under the “pressure” of cytostatic therapy 3 weeks after treatment starts. The sensitivity, specificity, and accuracy of this indicator in relation to predicting tumor response appeared to be 89.5%, 89.1%, and 89.3%, respectively. Changes in oxygenation when constructing a logistic regression (Model 3) made it possible to attribute patients to the group of both “high response” and “low response” (89.3%) with a high probability and predicting the response with a high accuracy when validating the model. Similar results were obtained in [48], where the use of logistic regression based on the ratio of saturation of normal and tumor tissues (z-score) within 10 days after the start of treatment turned out to be a significant predictor of the complete response. The main parameter reflecting the sensitivity of the tumor to chemotherapy was the improvement in the oxygenation of the neoplasm after the chemotherapy administration. Apparently, this appears to be due to a well-known radiation biology phenomenon of reoxygenation as a result of a decrease in oxygen consumption by damaged tumor cells [49, 50].
The study of the vascular bed of the tumor using the power Doppler method revealed a decrease in the number of tumor vessels after the first cycle of chemotherapy in the vast majority of cases. All the tumors with a “high” response to treatment demonstrated a decrease in the vascularization of the neoplasm. In general, this indicator demonstrated relatively high sensitivity (75.4%), but low specificity (58.7%) and low accuracy (68.0%). This may be explained by the fact that tumors contain predominantly small (less than 100 µm) and immature blood vessels with low blood flow velocity [35]. Apparently, the resolution of power Doppler ultrasound is not sufficient to identify such vessels and correctly assess the blood filling of the tumor tissue and its changes in the course of chemotherapy. The study demonstrated the limitations of noninvasive imaging biomarkers of breast cancer response, when tumors with extended necrotic areas cannot be evaluated correctly because of unpredictable vessel’s reaction.
The change in the volume of tumor tissue also did not demonstrate a high predictive ability in relation to the pathological tumor response (70.9% of correct predictions). However, in the case of “low” response, the vast majority of tumors showed a decrease in tumor volume after the first cycle of NACT. It can be assumed that in this case, chemotherapy reduces the inflammatory component of the tumor or death of the drug-sensitive fraction of tumor cells occurs.
In an earlier work [44], we concluded that it is necessary to calculate an accurate number of the residual viable tumor cells after NACT in order to study in more detail the changes in grade 3 of PTR tumors. We used a loss of 50% cellularity as a threshold, and not a standard histopathological score, since a pronounced heterogeneity of changes, which are attributed to the third grade of tumor response. It includes tumors with a loss of cellularity in the range from 30 to 90%, in which multidirectional changes in oxygenation were detected (Fig. 3). From this point of view, our approach (an accurate calculation of cell loss) seems to be more reasonable than a formalized assignment a tumor to any grade of pathological response. A similar approach was used by [30], where the 50% change in the volume of the tumor node was chosen as a criterion of response.
Our study did not allow for identifying tumors that completely responded to treatment with sufficient accuracy. Model 5, where a pCR was chosen as a binary event, showed only 20% correct predictions of complete response. A low ability of the method to predict a complete response is its characteristic feature, since it is very difficult to separate grade 4 (10% of viable tumor cells) and grade 5 (0% of viable tumor cells) in the early time after the start of a treatment. Our approach does not allow capturing such minor differences; however, it makes it possible to predict the trend of tumor resistance to treatment very well, which is also very important information regarding escalation or de-escalation of therapy, also a need of additional genetic study of the tumor.
Conclusion
We observed changes in all three parameters (oxygenation, tumor volume, and blood flow) at the early time after the start of the treatment. However, a high predictive potential for early assessment of tumor sensitivity to NACT was found only in changes in the level of oxygenation, ΔStO2, (ρ = 0.802, p ≤ 0.01). The regression model can be used for the prediction of the response to treatment with a high probability of a correct conclusion. The analysis showed that the “Tumor volume” model and the “Vascularization index” model did not accurately predict the absence of a pathological tumor response to treatment (60.9 and 58.7%, respectively), while predicting a positive response to treatment was relatively better (78.9 and 75.4%, respectively).
Availability of data and materials
These data that support the findings of this manuscript can be made available from the corresponding author upon reasonable request.
Change history
07 February 2023
A Correction to this paper has been published: https://doi.org/10.1186/s13058-023-01614-7
Abbreviations
- BC:
-
Breast cancer
- DOSI:
-
Diffuse optical spectroscopy imaging
- ECOG:
-
Eastern cooperative oncology group
- HER2neu:
-
Human epidermal growth factor receptor-2
- HbO2 :
-
Oxyhemoglobin
- HHb:
-
Deoxyhemoglobin
- NACT:
-
Neoadjuvant chemotherapy
- pCR:
-
Pathologic complete response
- PTR:
-
Pathological tumor response
- Rel:
-
Relative
- StO2 :
-
Oxygen saturation
- US:
-
Ultrasound
- ROI:
-
Region of interest
References
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thürlimann B, St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017, André F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Hussein K, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B. De-escalating and escalating treatments for early-stage breast cancer the St Gallen International expert consensus conference on the primary therapy of early breast cancer. Ann Oncol. 2017;28(8):1700–12. https://doi.org/10.1093/annonc/mdx308.
Gunther JE, Lim EA, Kim HK, Flexman M, Altoé M, Campbell JA, Hibshoosh H, Crew KD, Kalinsky K, Hershman DL, Hielscher AH. Dynamic diffuse optical tomography for monitoring neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2018;287(3):778–86. https://doi.org/10.1148/radiol.2018161041.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Isakoff SJ, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch M, Lyons J, Matro J, Mayer IA, Mortimer J, O’Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Wisinski KB, Young JS, Burns JL, Kumar R. NCCN guidelines® insights: breast cancer, version 4.2021. J Natl Compr Cancer Netw. 2021;19(5):484–93. https://doi.org/10.6004/jnccn.2021.0023.
Jackisch C, Cortazar P, Geyer CE Jr, Gianni L, Gligorov J, Machackova Z, Perez EA, Schneeweiss A, Tolaney SM, Untch M, Wardley A, Piccart M. Risk-based decision-making in the treatment of HER2-positive early breast cancer: Recommendations based on the current state of knowledge. Cancer Treat Rev. 2021;99:102229. https://doi.org/10.1016/j.ctrv.2021.102229.
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Flippo-Morton T, Hunt KK. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14. https://doi.org/10.1097/SLA.0000000000000924. (discussion 614-6).
Rauch GM, Adrada BE, Kuerer HM, van la Parra RF, Leung JW, Yang WT. Multimodality imaging for evaluating response to neoadjuvant chemotherapy in breast cancer. AJR Am J Roentgenol. 2017;208(2):290–9. https://doi.org/10.2214/AJR.16.17223.
Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, Brufsky AM, Lembersky BC, Ahrendt GM. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116(6):1431–9. https://doi.org/10.1002/cncr.24876.
Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, Halpern E, Moore R, Kuter I, Taghian A. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184(3):868–77. https://doi.org/10.2214/ajr.184.3.01840868.
Baumgartner A, Tausch C, Hosch S, Papassotiropoulos B, Varga Z, Rageth C, Baege A. Ultrasound-based prediction of pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2018;39:19–23. https://doi.org/10.1016/j.breast.2018.02.028.
Adrada BE, Candelaria R, Moulder S, Thompson A, Wei P, Whitman GJ, Valero V, Litton JK, Santiago L, Scoggins ME, Moseley TW, White JB, Ravenberg EE, Yang WT, Rauch GM. Early ultrasound evaluation identifies excellent responders to neoadjuvant systemic therapy among patients with triple-negative breast cancer. Cancer. 2021;127(16):2880–7. https://doi.org/10.1002/cncr.33604.
Marinovich ML, Houssami N, Macaskill P, von Minckwitz G, Blohmer JU, Irwig L. Accuracy of ultrasound for predicting pathologic response during neoadjuvant therapy for breast cancer. Int J Cancer. 2015;136(11):2730–7. https://doi.org/10.1002/ijc.29323.
Scheel JR, Kim E, Partridge SC, Lehman CD, Rosen MA, Bernreuter WK, Pisano ED, Marques HS, Morris EA, Weatherall PT, Polin SM, Newstead GM, Esserman LJ, Schnall MD, Hylton NM, ACRIN 6657 Trial Team and I-SPY Investigators Network. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 Trial. AJR Am J Roentgenol. 2018;210(6):1376–85. https://doi.org/10.2214/AJR.17.18323.
Park J, Chae EY, Cha JH, Shin HJ, Choi WJ, Choi YW, Kim HH. Comparison of mammography, digital breast tomosynthesis, automated breast ultrasound, magnetic resonance imaging in evaluation of residual tumor after neoadjuvant chemotherapy. Eur J Radiol. 2018;108:261–8. https://doi.org/10.1016/j.ejrad.2018.09.032.
Candelaria RP, Bassett RL, Symmans WF, Ramineni M, Moulder SL, Kuerer HM, Thompson AM, Yang WT. Performance of mid-treatment breast ultrasound and axillary ultrasound in predicting response to neoadjuvant chemotherapy by breast cancer subtype. Oncologist. 2017;22(4):394–401. https://doi.org/10.1634/theoncologist.2016-0307.
Wu J, Gong G, Cui Y, Li R. Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. J Magn Reson Imaging. 2016;44(5):1107–15. https://doi.org/10.1002/jmri.25279.
Humbert O, Riedinger JM, Vrigneaud JM, Kanoun S, Dygai-Cochet I, Berriolo-Riedinger A, Toubeau M, Depardon E, Lassere M, Tisserand S, Fumoleau P, Brunotte F, Cochet A. 18F-FDG PET-derived tumor blood flow changes after 1 cycle of neoadjuvant chemotherapy predicts outcome in triple-negative breast cancer. J Nucl Med. 2016;57(11):1707–12. https://doi.org/10.2967/jnumed.116.172759.
Avril S, Muzic RF Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. 18F-FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med. 2016;57(1):34S-S39. https://doi.org/10.2967/jnumed.115.157875.
Ohmae E, Yoshizawa N, Yoshimoto K, Hayashi M, Wada H, Mimura T, Asano Y, Ogura H, Yamashita Y, Sakahara H, Ueda Y. Comparison of lipid and water contents by time-domain diffuse optical spectroscopy and dual-energy computed tomography in breast cancer patients. Appl Sci. 2019;9(7):1482. https://doi.org/10.3390/app9071482.
Vavadi H, Mostafa A, Zhou F, Uddin KMS, Althobaiti M, Xu C, Bansal R, Ademuyiwa F, Poplack S, Zhu Q. Compact ultrasound-guided diffuse optical tomography system for breast cancer imaging. J Biomed Opt. 2018;24(2):1–9. https://doi.org/10.1117/1.JBO.24.2.021203.
Tromberg BJ, Zhang Z, Leproux A, O’Sullivan TD, Cerussi AE, Carpenter PM, Mehta RS, Roblyer D, Yang W, Paulsen KD, Pogue BW, Jiang S, Kaufman PA, Yodh AG, Chung SH, Schnall M, Snyder BS, Hylton N, Boas DA, Carp SA, Isakoff SJ, Mankoff D, ACRIN 6691 investigators. Predicting responses to neoadjuvant chemotherapy in breast cancer: ACRIN 6691 Trial of diffuse optical spectroscopic imaging. Cancer Res. 2016;76(20):5933–44. https://doi.org/10.1158/0008-5472.CAN-16-0346.
Zhu Q, DeFusco PA, Ricci A Jr, Cronin EB, Hegde PU, Kane M, Tavakoli B, Xu Y, Hart J, Tannenbaum SH. Breast cancer: assessing response to neoadjuvant chemotherapy by using US-guided near-infrared tomography. Radiology. 2013;266(2):433–42. https://doi.org/10.1148/radiol.12112415.
Ueda S, Saeki T. Early therapeutic prediction based on tumor hemodynamic response imaging: clinical studies in breast cancer with time-resolved diffuse optical spectroscopy. Appl Sci. 2019;9(1):3. https://doi.org/10.3390/app9010003.
Altoe ML, Kalinsky K, Marone A, Kim HK, Guo H, Hibshoosh H, Tejada M, Crew KD, Accordino MK, Trivedi MS, Hershman DL, Hielscher AH. Changes in diffuse optical tomography images during early stages of neoadjuvant chemotherapy correlate with tumor response in different breast cancer subtypes. Clin Cancer Res. 2021;27(7):1949–57. https://doi.org/10.1158/1078-0432.CCR-20-1108.
Choe R, Putt ME, Carlile PM, Durduran T, Giammarco JM, Busch DR, Jung KW, Czerniecki BJ, Tchou J, Feldman MD, Mies C, Rosen MA, Schnall MD, DeMichele A, Yodh AG. Optically measured microvascular blood flow contrast of malignant breast tumors. PLoS ONE. 2014;9(6):e99683. https://doi.org/10.1371/journal.pone.0099683.
Larusson F, Anderson PG, Rosenberg E, Kilmer ME, Sassaroli A, Fantini S, Miller EL. Parametric estimation of 3D tubular structures for diffuse optical tomography. Biomed Opt Express. 2013;4(2):271–86. https://doi.org/10.1364/BOE.4.000271.
Pakalniskis MG, Wells WA, Schwab MC, Froehlich HM, Jiang S, Li Z, Tosteson TD, Poplack SP, Kaufman PA, Pogue BW, Paulsen KD. Tumor angiogenesis change estimated by using diffuse optical spectroscopic tomography: demonstrated correlation in women undergoing neoadjuvant chemotherapy for invasive breast cancer? Radiology. 2011;259(2):365–74. https://doi.org/10.1148/radiol.11100699.
Multhoff G, Radons J, Vaupel P. Critical role of aberrant angiogenesis in the development of tumor hypoxia and associated radioresistance. Cancers (Basel). 2014;6(2):813–28. https://doi.org/10.3390/cancers6020813.
Tromberg BJ, Cerussi A, Shah N, Compton M, Durkin A, Hsiang D, Butler J, Mehta R. Imaging in breast cancer: diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005;7(6):279–85. https://doi.org/10.1186/bcr1358.
Tank A, Peterson HM, Pera V, Tabassum S, Leproux A, O’Sullivan T, Jones E, Cabral H, Ko N, Mehta RS, Tromberg BJ, Roblyer D. Diffuse optical spectroscopic imaging reveals distinct early breast tumor hemodynamic responses to metronomic and maximum tolerated dose regimens. Breast Cancer Res. 2020;22(1):29. https://doi.org/10.1186/s13058-020-01262-1.
Zhu Q, Tannenbaum S, Kurtzman SH, DeFusco P, Ricci A Jr, Vavadi H, Zhou F, Xu C, Merkulov A, Hegde P, Kane M, Wang L, Sabbath K. Identifying an early treatment window for predicting breast cancer response to neoadjuvant chemotherapy using immunohistopathology and hemoglobin parameters. Breast Cancer Res. 2018;20(1):56. https://doi.org/10.1186/s13058-018-0975-1.
Tran WT, Gangeh MJ, Sannachi L, Chin L, Watkins E, Bruni SG, Rastegar RF, Curpen B, Trudeau M, Gandhi S, Yaffe M, Slodkowska E, Childs C, Sadeghi-Naini A, Czarnota GJ. Predicting breast cancer response to neoadjuvant chemotherapy using pretreatment diffuse optical spectroscopic texture analysis. Br J Cancer. 2017;116(10):1329–39. https://doi.org/10.1038/bjc.2017.97.
Roblyer D, Ueda S, Cerussi A, Tanamai W, Durkin A, Mehta R, Hsiang D, Butler JA, McLaren C, Chen WP, Tromberg B. Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment. Proc Natl Acad Sci U S A. 2011;108(35):14626–31. https://doi.org/10.1073/pnas.1013103108.
Zhu Q, Ademuyiwa FO, Young C, Appleton C, Covington MF, Ma C, Sanati S, Hagemann IS, Mostafa A, Uddin KMS, Grigsby I, Frith AE, Hernandez-Aya LF, Poplack SS. Early assessment window for predicting breast cancer neoadjuvant therapy using biomarkers, ultrasound, and diffuse optical tomography. Breast Cancer Res Treat. 2021;188(3):615–30. https://doi.org/10.1007/s10549-021-06239-y.
Ehling J, Lammers T, Kiessling F. Non-invasive imaging for studying anti-angiogenic therapy effects. Thromb Haemost. 2013;109(3):375–90. https://doi.org/10.1160/TH12-10-0721.
Lukina MM, Sirotkina MA, Orlova AG, Dudenkova VV, Komarova AD, Plekhanov AA, Snopova LB, Zagaynova EV, Shcheslavskiy VI, Shirmanova MV. Effects of irinotecan on tumor vasculature and oxygenation: an in vivo study on colorectal cancer model. IEEE J Sel Top Quantum Electron. 2021;27(4):1–8. https://doi.org/10.1109/JSTQE.2020.3047518.
Guérin E, Raffelsberger W, Pencreach E, Maier A, Neuville A, Schneider A, Bachellier P, Rohr S, Petitprez A, Poch O, Moras D, Oudet P, Larsen AK, Gaub MP, Guenot D. In vivo topoisomerase I inhibition attenuates the expression of hypoxia-inducible factor 1α target genes and decreases tumor angiogenesis. Mol Med. 2012;18(1):83–94. https://doi.org/10.2119/molmed.2011.00120.
Kedar RP, Cosgrove DO, Smith IE, Mansi JL, Bamber JC. Breast carcinoma: measurement of tumor response to primary medical therapy with color Doppler flow imaging. Radiology. 1994;190(3):825–30. https://doi.org/10.1148/radiology.190.3.81156347.
Singh S, Pradhan S, Shukla RC, Ansari MA, Kumar A. Color Doppler ultrasound as an objective assessment tool for chemotherapeutic response in advanced breast cancer. Breast Cancer. 2005;12(1):45–51. https://doi.org/10.2325/jbcs.12.45.
Kumar A, Singh S, Pradhan S, Shukla RC, Ansari MA, Singh TB, Shyam R, Gupta S. Doppler ultrasound scoring to predict chemotherapeutic response in advanced breast cancer. World J Surg Oncol. 2007;5:99. https://doi.org/10.1186/1477-7819-5-99.
Singh G, Kumar P, Parshad R, Seith A, Thulkar S, Hosten N. Role of color Doppler indices in predicting disease-free survival of breast cancer patients during neoadjuvant chemotherapy. Eur J Radiol. 2010;75(2):158–62. https://doi.org/10.1016/j.ejrad.2009.12.027.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, Cardoso MJ, Cufer T, El Saghir N, Fallowfield L, Fenech D, Francis P, Gelmon K, Giordano SH, Gligorov J, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Krop I, Kyriakides S, Lin UN, Mayer M, Merjaver SD, Nordström EB, Pagani O, Partridge A, Penault-Llorca F, Piccart MJ, Rugo H, Sledge G, Thomssen C, Van’t Veer L, Vorobiof D, Vrieling C, West N, Xu B, Winer E. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)†. Ann Oncol. 2014;25(10):1871–88. https://doi.org/10.1093/annonc/mdu385.
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7. https://doi.org/10.1016/s0960-9776(03)00106-1.
Pavlov MV, Kalganova TI, Lyubimtseva YS, Plekhanov VI, Golubyatnikov GY, Ilyinskaya OY, Orlova AG, Subochev PV, Safonov DV, Shakhova NM, Maslennikova AV. Multimodal approach in assessment of the response of breast cancer to neoadjuvant chemotherapy. J Biomed Opt. 2018;23(9):1–11. https://doi.org/10.1117/1.JBO.23.9.091410.
Orlova AG, Turchin IV, Plehanov VI, Shakhova NM, Fiks II, Kleshnin MI, Konuchenko NYu, Kamensky VA. Frequency-domain diffuse optical tomography with single source-detector pair for breast cancer detection. Laser Phys Lett. 2008;5(4):321–7. https://doi.org/10.1002/lapl.200710131.
Orlova A, Pavlova K, Kurnikov A, Maslennikova A, Myagcheva M, Zakharov E, Skamnitskiy D, Perekatova V, Khilov A, Kovalchuk A, Moiseev A, Turchin I, Razansky D, Subochev P. Noninvasive optoacoustic microangiography reveals dose and size dependency of radiation-induced deep tumor vasculature remodeling. Neoplasia. 2022;26:100778. https://doi.org/10.1016/j.neo.2022.100778.
Tilsed CM, Fisher SA, Nowak AK, Lake RA, Lesterhuis WJ. Cancer chemotherapy: insights into cellular and tumor microenvironmental mechanisms of action. Front Oncol. 2022;12:960317. https://doi.org/10.3389/fonc.2022.960317.
Cochran JM, Busch DR, Leproux A, Zhang Z, O’Sullivan TD, Cerussi AE, Carpenter PM, Mehta RS, Roblyer D, Yang W, Paulsen KD, Pogue B, Jiang S, Kaufman PA, Chung SH, Schnall M, Snyder BS, Hylton N, Carp SA, Isakoff SJ, Mankoff D, Tromberg BJ, Yodh AG. Tissue oxygen saturation predicts response to breast cancer neoadjuvant chemotherapy within 10 days of treatment. J Biomed Opt. 2018;24(2):1–11. https://doi.org/10.1117/1.JBO.24.2.021202.
Harryman WL, Cress AE. Basic principles of radiobiology and cancer metastasis prevention. In: Leong SP, Nathanson SD, Zager JS, editors. Cancer metastasis through the lymphovascular system. Cham: Springer; 2022. https://doi.org/10.1007/978-3-030-93084-4_62.
Wang HW, Putt ME, Emanuele MJ, Shin DB, Glatstein E, Yodh AG, Busch TM. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 2004;64(20):7553–61. https://doi.org/10.1158/0008-5472.CAN-03-3632.
Acknowledgements
The authors thank Sergei V. Gamayunov personally and the staff of Nizhny Novgorod Regional Oncology Hospital for helping in organizing the study. The authors thank the patients that graciously volunteered their time to participate in this study.
Funding
Clinical study was supported by the Governmental Project of the Institute of Applied Physics RAS (Project #0030-2021-0014), and data processing was supported by Center of Excellence «Center of Photonics» funded by The Ministry of Science and Higher Education of the Russian Federation, Contract No. 075-15-2022-316, DOSI setup development and service by Russian Foundation for Basic Research (Project #19-34-60034)
Author information
Authors and Affiliations
Contributions
MVP, IVT, and AVM were responsible for study design. MVP, VIP, GYuG, AGO, PVS, and DAD were responsible for data collection and imaging. MVP, VIP, GYuG, AGO, PVS, and DAD were responsible for data processing and image analysis. APB was responsible for statistical analysis. MVP, IVT, and AVM were responsible for data interpretation. MVP, IVT, and AVM wrote the manuscript with assistance from the other authors. AVM was responsible for the overall study supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In accordance with the Declaration of Helsinki on research involving human participants, this study was reviewed and approved by the local Research Ethics Border (protocol #2, 18.05.2015) at Nizhny Novgorod Regional Clinical Oncology Hospital. All patients provided written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Binary logistic models for prediction the tumor complete response (the 5th grade of PTR as a binary event) using ΔStO2 indicator.
Additional file 2.
Oxyhemoglobin and deoxyhemoglobin tumor concentration before NACT and before the 2nd cycle of NACT (103 patients).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pavlov, M.V., Bavrina, A.P., Plekhanov, V.I. et al. Changes in the tumor oxygenation but not in the tumor volume and tumor vascularization reflect early response of breast cancer to neoadjuvant chemotherapy. Breast Cancer Res 25, 12 (2023). https://doi.org/10.1186/s13058-023-01607-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-023-01607-6