Abstract
Background
Triple-negative breast cancer (TNBC) is highly metastatic and lethal. Due to a lack of druggable targets for this disease, there are no effective therapies in the clinic.
Methods
We used TNBC cells and xenografted mice as models to explore triptonide-mediated inhibition of TNBC metastasis and tumor growth. Colony formation assay was used to quantify the tumorigenesis of TNBC cells. Wound-healing and cell trans-well assays were utilized to measure cell migration and invasion. Tube formation assay was applied to access tumor cell-mediated vasculogenic mimicry. Western blot, quantitative-PCR, immunofluorescence imaging, and immunohistochemical staining were used to measure the expression levels of various tumorigenic genes in TNBC cells.
Results
Here, we showed that triptonide, a small molecule from the traditional Chinese medicinal herb Tripterygium wilfordii Hook F, potently inhibited TNBC cell migration, invasion, and vasculogenic mimicry, and effectively suppressed TNBC tumor growth and lung metastasis in xenografted mice with no observable toxicity. Molecular mechanistic studies revealed that triptonide strongly triggered the degradation of master epithelial-mesenchymal transition (EMT)-inducing protein Twist1 through the lysosomal system and reduced Notch1 expression and NF-κB phosphorylation, which consequently diminished the expression of pro-metastatic and angiogenic genes N-cadherin, VE-cadherin, and vascular endothelial cell growth factor receptor 2 (VEGFR2).
Conclusions
Triptonide effectively suppressed TNBC cell tumorigenesis, vasculogenic mimicry, and strongly inhibited the metastasis of TNBC via degradation of Twist1 and Notch1 oncoproteins, downregulation of metastatic and angiogenic gene expression, and reduction of NF-κB signaling pathway. Our findings provide a new strategy for treating highly lethal TNBC and offer a potential new drug candidate for combatting this aggressive disease.
Similar content being viewed by others
Highlights
-
1.
Triptonide effectively suppresses TNBC tumor growth and metastasis in xenografted mice.
-
2.
The migration and angiogenesis of TNBC cells are markedly inhibited by triptonide.
-
3.
Triptonide triggers the degradation of oncoproteins Twist1 and Notch1 in TNBC cells.
-
4.
Our findings provide a new strategy for treating aggressive and metastatic TNBC.
Introduction
Triple-negative breast cancer (TNBC) is characterized by the absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2) and is highly metastatic and lethal [1, 2]. Although substantial efforts have gone into identifying treatments for TNBC in recent decades, the median survival time of TNBC patients is still less than 15 months largely due to the absence of effective drugs in the clinic [3, 4]. Since its discovery, TNBC has been a challenge in the cancer treatment field [3,4,5], warranting the discovery of novel, potent therapeutics against TNBC.
TNBC has multiple genetic and epigenetic abnormalities. It was recently reported that 411 genes were overexpressed in TNBC tumor tissue compared to normal breast tissue and that multiple tumorigenic and metastatic signaling pathways were aberrantly activated [6, 7]. TNBC cells possess high migratory and invasive abilities and easily disseminate from the primary tumor site to multiple distant organs, such as lung, bone, liver, and brain, resulting in organ dysfunction and patient death [8,9,10].
Epithelial–mesenchymal transition (EMT) seems to play a pivotal role in TNBC initiation, metastasis, and drug resistance [9, 11, 12]. Numerous studies have demonstrated that aberrant overexpression of EMT-inducing genes, such as Twist1, Snail1, Snail2, Zeb1, Zeb2, N-cadherin, and the master stemness gene Notch1, triggers cell de-differentiation and cancer stem cell genesis [8, 9, 11]. Particularly, concurrent overexpression of the oncogenic genes Twist1, Snail1, and Notch1 strongly induces EMT and enhances stemness, thereby inducing cancer and metastasis [9, 11,12,13,14].
Twist1 is a master EMT-inducing gene and plays a critical role in cancer metastasis in various malignant tumors [7]. Twist1 overexpression promotes TNBC metastasis and is associated with poor prognosis of TNBC patients [13, 15]. Accordingly, Twist1 has been a target for the development of therapeutics against TNBC [16,17,18,19]. Several small molecules, such as thymoquinone [16] and tamoxifen [17], reduce Twist1 expression in cell and animal models and inhibit TNBC cell migration and invasion in vitro; however, these agents exert only moderate efficacy and have not yet entered clinical trials. Therefore, potent Twist1-targeted therapeutics remain to be explored.
Notch1 is an essential gene for cancer stem cell genesis and is a hallmark of TNBC [20,21,22,23]. Overexpression of Notch1 is closely associated with a poor prognosis in TNBC patients [24]. Accordingly, Notch1 suppression is a potential strategy for cancer therapy [25,26,27,28,29]. However, Notch1-targeted drugs against TNBC have not advanced to clinical trials due to low anti-cancer efficacy in preclinical studies [23]. Thus, there is a critical need for effective Notch1-targeted therapeutics for TNBC. Since multiple oncogenic genes and signal pathways contribute to the progression of TNBC, targeting a single Notch 1 gene appears to run short of ways in dealing with the situation of TNBC progression, suggesting that concurrently targeting multiple tumorigenic genes and signaling pathways are sensible strategy for TNBC therapy.
TNBC cells gain high tumorigenic and metastatic capabilities via gene mutation and also exhibit aberrant gene overexpression. Because multiple oncogenic genes are simultaneously overexpressed in TBNC [6,7,8,9], targeting a single gene is typically insufficient to treat the disease. Therefore, we hypothesized that concurrently targeting multiple oncogenic genes, such as Twist1 and Notch1, may increase anti-TNBC efficacy, and we explored active components with potential anti-TNBC effects from the traditional Chinese medicinal herbs. Our results showed that triptonide (MW:358 Da), a small molecule from the traditional Chinese medicinal herb Tripterygium wilfordii Hook F [30, 31], exerted potent anti-TNBC effects.
It was recently reported that triptonide exerts strong inhibitory effects on acute myeloid leukemia, lymphoma, lung cancer, gastric cancer, and pancreatic cancer via reducing cancer cell stemness and oncogenic signaling pathways, and suppressing cancer cell tumorigenesis and vasculogenic mimicry [32,33,34,35,36,37,38]. More recently, Gao et al. reported that triptonide inhibits TNBC cell tumorigenesis via downregulation of several cancer stem cell-associated genes, up-regulation of Snail1 expression, and induction of a Snail1-associated feedback mechanism for triptonide resistance [39]. Of note, it is well known that Smail1 is aberrantly overexpressed in various types of malignant tumors including TNBC [40,41,42,43], overexpression of Snail triggers EMT [42, 44,45,46,47], carcinogenesis [48,49,50,51], and drug resistance [52, 53], and is closely associated with poor prognosis in cancer patients [40, 43, 46, 47, 50, 51]. Accordingly, inhibition of Snail1 in cancer has become a new strategy for developing cancer therapeutics [52,53,54]. Therefore, the effect of triptonide on Snail expression in TNBC cells remains to be verified, and the mechanisms underlying triptonide-mediated anti-TNBC need to be elucidated.
In the current investigation, we found that triptonide potently suppressed TNBC cell migration, invasion, and angiogenesis in vitro and effectively suppressed tumor growth and metastasis in xenografted mice. This effect was mediated by triptonide-induced Twist1 and Notch1 protein degradation, which consequently inhibited downstream NF-κB signaling, suppressing the expression of angiogenic and metastatic genes VE-cadherin, VEGFR2, and N-cadherin. Of note, triptonide does not increase Snail1 expression in TNBC cell lines we tested. Our findings provide a new strategy for concurrently inhibition of multiple oncogenic genes and tumorigenic signaling pathways in TNBC and offer a novel drug candidate against metastatic TNBC.
Methods
Materials
Triptonide was from Chengdu Must Biotech Ltd. with a purity > 98%. MG132, cycloheximide, and chloroquine diphosphate were from MCE (Shanghai, China). Revert Aid TM First Strand cDNA Synthesis Kit was from Fermentas Life Sciences (Walsham, Massachusetts). Rabbit monoclonal antibody to Notch1 and other signaling proteins were from Cell Signaling Technology (Beverly, MA). Mouse and Rabbit polyclonal antibodies to Twist1 were from ABCAM (Cambridge, UK). Breast cancer cell lines MDA-MB-231, MDA-MB-468, and BT-549 were from ATCC (Rockefeller, Maryland).
Cell culture
TNBC cell lines MDA-MB-231, MDA-MB-468, and BT-549 were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (complete medium). The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 as we described previously [32].
Tumor xenograft mice and triptonide treatment
The tumor xenograft mice were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Soochow University as previously reported [35, 38]. In brief, 107 MDA-MB-231 cells were injected into the breast of NOD-SCID female mice (18–22 g) and the mice were randomly divided into two groups (triptonide treatment group and control group, 5 mice/group). After 10 days when the tumors grew up, triptonide in saline at a dose of 3 mg/kg or saline as a control was intraperitoneally injected daily for 95 days. Tumor size was measured and tumor volume was calculated according to the formula: tumor volume = 0.55 × length × width2. After 95 days of triptonide treatment, the mice were sacrificed. The lungs and other important organs were isolated, weighed, washed with PBS, fixed with formaldehyde, sectioned, and analyzed by hematoxylin–eosin (H&E) staining.
Immunofluorescence microscopy
MDA-MB-231 and MDA-MB-468 cells were first seeded onto glass cover slips for 24 h, then treated with triptonide at the concentrations of 0–10 nM for 72 h. The cells were fixed with 4% paraformaldehyde and incubated with primary antibody against Twist1 at 4 °C overnight, followed by incubation with secondary antibodies, counterstained with 4, 6-diamidino-2-phenylindole (DAPI), and subjected to be imaged under confocal microscopy [36].
Immunohistochemical staining
The lung tumor tissues from xenografted mice were sectioned, and the slides were stained with anti-Twist1 primary antibody at 4 °C overnight, followed by incubation with HRP-labeled goat anti-rabbit secondary antibody for 1 h at room temperature, and stained with 3,3-diaminobenzidine (DAB) solution. The slides were imaged using Leica microscope.
Cell growth analysis
MDA-MB-231 and MDA-MB-468 cells were incubated with triptonide at the concentrations of 0–320 nM for 70 h; 10 μL MTT solution was added to each well and incubated at 37 °C for 2 h. The dual fluorescence wavelength absorbance at 560 and 590 nm was measured using SpectraMax M5 reader, respectively. The IC50, defined as the drug concentration at which cell growth was inhibited by 50%, was assessed by SPSS 16.0.
Colony formation assay
MDA-MB-231 and MDA-MB-468 cells were treated with triptonide at the concentrations of 0–10 nM for 72 h; the viable cells were harvested, and counted. The 5000 viable cells together with 0.5% low melting agarose were seeded into the 35 mm dishes and incubated at 37 °C in 5% CO2 for 10 days in the absence of triptonide; the number of colonies was counted under a Zoom-Stereo microscope SZX16 as we previously described [55].
Cell migration assay
MDA-MB-231 and MDA-MB-468 cells were seeded in six-well plates and incubated for 24 h, and then, the cell monolayer was wounded by a plastic tip. The wounded monolayer was then incubated with triptonide at the concentrations of 0–10 nM for 24 h. The migrated tumor cells were stained with Wright-Giemsa solution, imaged under a microscopy using five randomly chosen fields for each well, and statistically analyzed.
Cell invasion assay
The MDA-MB-231 cells were seeded into the upper chambers of trans-well plate pre-coated with 12.5% Matrigel at a density of 2.0 × 105 cells/mL and incubated without or with triptonide at the concentrations of 0–10 nM. After 24 h, the invaded cells in the lower chamber were fixed, stained with Wright-Giemsa solution, and photographed as we described before [56].
Tube forming assay
MDA-MB-231 cells were pre-treated with triptonide at the concentrations of 0–10 nM for 72 h and the viable 2 × 104 cells in 0.5 ml DMEM complete medium were transferred to each well of a 24-well plate containing 0.3 mL Matrigel matrix. After incubation at 37 °C, 5% CO2 for 8 h, the tubes were fixed and stained with Wright-Giemsa solution, and photographed by OLYMPUS FSX-100 microscope.
RT-PCR and real-time quantitative PCR
Total RNA was extracted from triptonide-treated cells, and reversely transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit. RT-PCR and real-time quantitative PCR (QT-PCR) were performed with SYBRGreen protocol as we previously described [57]. The primers of RT-PCR and QT-PCR are listed in the Additional file 1: Tables S1 and S2, respectively.
Western blotting
Proteins from MDA-MB-231 and MDA-MB-468 cells were extracted using the M-PER Mammalian Protein Extraction Kit. Equal amounts of protein were loaded onto each lane and resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis with tris–glycine running buffer, and the proteins were transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies at 4 °C overnight, followed by incubation with HRP-coupled secondary antibody for 1 h at room temperature. Blots were visualized using enhanced chemiluminescence detection reagents and exposed to X-ray film as we reported before [38].
Statistical analysis
All results represent the mean ± S.E. Differences between the groups were assessed by one-way ANOVA using GraphPad Prism 7.02. Statistical comparisons were performed using the Student's t test, and the significance of differences was indicated as *P < 0.05 and **P < 0.01.
Results
Triptonide potently inhibits TNBC cell proliferation and tumor growth
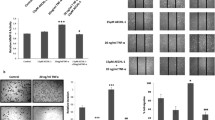
We first investigated the anti-TNBC effect of triptonide (Fig. 1A) in vitro. Triptonide inhibited the proliferation of all three TNBC cell lines, MDA-MB-231, MDA-MB-468, and BT-549, with IC50 values of 15.6, 14.4, and 12.1 nM, respectively (Fig. 1B). Triptonide also markedly reduced the colony numbers of highly metastatic MDA-MB-231 (Fig. 1C, D) and MDA-MB-468 cells (Fig. 1E, F) in a concentration-dependent manner, suggesting that triptonide is a potent inhibitor of TNBC cell tumorigenesis.
Triptonide potently inhibits TNBC cell proliferation and tumor growth in xenografted mice. The triple-negative breast cancer (TNBC) cell lines MDA-MB-231, MDA-MB-468, and BT-549 in the 96-well plate (n-6) were incubated with triptonide (TN, A) at the concentrations of 0–320 nM, respectively. Cell growth was detected by MTT assay (B). MDA-MB-231 and MDA-MB-468 cells were incubated with TN for 10 days at the concentrations of 0–10 nM; the colony numbers in each dish were imaged (C, E, 40 ×), counted, and statistically analyzed (D, F). The results represent three independent experiments. *P < 0.05, **P < 0.01. The five xenografted NOD/ SCID mice/group were treated with TN in saline at the dose of 3 mg/kg or saline as a control once a day. The tumor volume in the mice was recorded every other days (G). The tumors were first imaged (H), then weighed (I). The tumors were apparently bigger in control mice than TN-treated mice (J); notably, the tumors spread to the area out of breasts and were visible over the ribs in control xenografted mice, which was indicated by a red circle and an arrow (J, left panel), while the blue circle indicates the small tumor in the original transplantation site of the mouse breasts
We studied the anti-tumor and anti-metastatic effects of triptonide in xenografted mice. TNBC MDA-MB-231 cells together with Matrigel, which was used to mimic the extracellular matrix of the tumor microenvironment, were injected into the breast of NOD-SCID female mice. After 95 days, tumor volume reached 2400 mm3 in control mice, but only 100 mm3 in triptonide-treated mice (Fig. 1G). In the control group, all five mice had substantial tumor growth. Strikingly, in the triptonide group, three mice exhibited complete remission, while the two remaining mice had significantly reduced tumor growth (Fig. 1H). Similarly, tumor weights were reduced in triptonide-treated mice (reaching a tumor-inhibitory rate of 98%) compared to that of control mice (Fig. 1I). Of note, triptonide did not significantly affect the organ index (organ weight/mouse body weight) of the lung, heart, spleen, kidney, and liver, and no obvious complications were observed. Collectively, these data suggest that triptonide effectively inhibits TNBC tumor growth in xenografted mice without obvious side effects.
Triptonide effectively inhibits TNBC cell migration and lung metastasis
We investigated the effect of triptonide on TNBC metastasis in xenografted mice. In control mice, the tumors were large and visible over the ribs; however, no tumors were visible at this site in triptonide-treated mice (Fig. 1J), suggesting that triptonide effectively suppressed TNBC dissemination to nearby tissues. This was supported by H&E staining that revealed the presence of tumors in lung tissues of control mice, but not in lung tissues of triptonide-treated mice (Fig. 2A). These data suggest that triptonide effectively inhibits TNBC metastasis from the breast to the lung.
Triptonide notably inhibits TNBC cell invasion and lung metastasis. The lungs in triptonide (TN)-treated and control (Ctrl) mice were fixed, subjected to H&E staining, and imaged with a microscope (400 ×). Tumors were appeared in the lung tissues of control mice (A, up panels), but were absent in the lung tissues of TN-treated mice (A, low panels). The numbers of migrated MDA-MB-231 and MDA-MB-468 cells in the wounded area were first imaged and counted; then, the cell migration-inhibitory rate was calculated (B, C). Cell invasion was performed by a trans-well system. MDA-MB-231 and MDA-MB-468 cells in the bottom chamber that invaded from the up chamber were fixed, stained with Wright–Giemsa solution, imaged using OLYMPUS CKX31 microscope (D), and statistically analyzed (E). The tumor cell tube-forming assay showed that TN at the concentration of 10 nM almost completely inhibited MDA-MB-231 cell formation of capillary like structures (G). The results represent three independent experiments. *P < 0.05 and **P < 0.01
We further investigated the cellular mechanisms underlying triptonide-induced inhibition of TNBC dissemination. In a wound-healing assay, the migration of MDA-MB-231 and MDA-MB-468 cells was notably inhibited by triptonide in a concentration-dependent manner (Fig. 2B, C) with robust inhibition at 10 nM triptonide (Fig. 2D, E). Given that TNBC cells can directly form tumor blood vessels through vascular mimicry, we also examined the effect of triptonide on the ability of TNBC cells to form tube-like structures in vitro. Strikingly, 10 nM triptonide almost completely suppressed tube-like structure formation in MDA-MB-231 cells (Fig. 2F), suggesting that triptonide is a novel, potent inhibitor of TNBC cell vascular mimicry. Collectively, triptonide effectively inhibits TNBC cell migration, invasion, vascular mimicry, and metastasis.
Triptonide inhibits TNBC cell metastasis by triggering concurrent degradation of Twist1 and Notch1 oncoproteins
We studied the molecular mechanisms underlying triptonide-mediated anti-TNBC cell metastasis. Western blotting showed that triptonide notably decreased the levels of Twist1 in MDA-MB-231 (Fig. 3A, C) and MDA-MB-468 cells (Fig. 3B, C) in a concentration-dependent manner, but the levels of Twist1 mRNA in MDA-MB-231 and MDA-MB-468 cells were not significantly altered in response to triptonide, suggesting that triptonide may reduce Twist1 protein stability in TNBC cells. The protein levels of other EMT genes, such as Snail1, Snail2, Zeb1, and Zeb2, were not significantly affected by triptonide (Fig. 3A). Of note, triptonide did not significantly change the levels of Snail1 mRNA and protein in both MDA-MB-231 and MDA-MB-468 cells (Additional file 1: Figure S1),
Triptonide markedly reduces Twist1 protein levels in TNBC cells and in the tumor tissues of xenografted mice. MDA-MB-231 and MDA-MB-468 cells were treated with triptonide (TN) for 72 h at the concentrations of 0–10 nM. Twist1 protein levels were detected using Western blotting (A, B), and the bands were scanned and statistically analyzed (C). Additionally, the cells were treated with 10 nM TN for 0, 12, 24, 36, 48, and 72 h, respectively, Twist1 protein levels were measured (D, F) and statistically analyzed (E, G). Twsist1 protein levels in MDA-MB-231 and MDA-MB-468 cells were also detected using immunofluorescent staining and confocal microscopy (H, I, × 2400). Furthermore, Twist1 protein levels in the lung tissues of xenografted mice were detected by immunohistochemistry staining (J, × 1000) and statistically analyzed (K). The results represent three independent experiments. *P < 0 .05, **P < 0.01
We analyzed changes in Twist1 protein levels over time following triptonide treatment. Twist1 protein expression was almost completely abolished in MDA-MB-231 (Fig. 3D, E) and MDA-MB-468 cells (Fig. 3F, G) after 72 h of triptonide treatment. To corroborate our findings, immunofluorescent staining and confocal microscopy showed that triptonide markedly decreased the levels of Twist1 protein in MDA-MB-231 (Fig. 3H) and MDA-MB-468 cells (Fig. 3I). Furthermore, immunohistochemical analysis of lung sections from xenografted mice revealed that triptonide significantly reduced the levels of Twist1 protein in metastatic lung tissues compared to control mouse lung tissues (Fig. 3J, K). Therefore, triptonide decreases Twist1 protein levels in TNBC cells.
Interestingly, we observed that triptonide-induced Twist1 protein decay in TNBC cells was enhanced in the presence of the translational elongation inhibitor cycloheximide (CHX). Twist1 protein levels were reduced in a time-dependent manner after treating cells with 10 nM triptonide together with 20 μM CHX (Fig. 4A–D). Twist1 protein levels decreased faster in triptonide-treated MDA-MB-231 (Fig. 4E) and MDA-MB-468 cells (Fig. 4F) than in control cells without triptonide treatment, suggesting that triptonide promotes Twist1 degradation in TNBC cells.
Triptonide promotes Twist1 degradation in TNBC cells through the lysosomal system. MDA-MB-231 and MDA-MB-468 cells were first incubated with 10 nM triptonide (TN) for 24 h, followed by incubation with the translational elongation inhibitor cycloheximide (CHX) at the concentration of 20 μM. After CHX treatment for 0 h, 4 h, 8 h, and 12 h, respectively, Twist1 protein levels were measured using Western blotting (A, C), and the bands were scanned and statistically analyzed (B, D). Twist1 protein levels were sharply decreased in MDA-MB-231 (E) and MDA-MB-468 cells (F) in the presence of TN, suggesting that triptonide triggers Twist1 protein degradation in the cells. Additionally, 20 μM chloroquine significantly inhibited triptonide-induced Twist1 protein degradation in MDA-MB-231 (G, H) and MDA-MB-468 cells (I, J). The results represent three independent experiments. *P < 0.05, **P < 0.01
We further investigated whether triptonide-induced Twist1 degradation in TNBC cells occurs through the lysosomal or proteasomal systems. MDA-MB-231 and MDA-MB-468 cells were incubated for 12 h with 10 nM triptonide together with 20 μM of the lysosomal inhibitor chloroquine. Chloroquine significantly inhibited triptonide-triggered degradation of Twist1 in MDA-MB-231 (Fig. 4G, H) and MDA-MB-468 cells (Fig. 4I, J). However, when these cells were incubated for 12 h with 10 nM triptonide together with 20 μM of the proteasome inhibitor MG132, the levels of Twist1 protein were not significantly affected (Fig. 5A–D). Therefore, triptonide-triggered Twist1 protein degradation in TNBC cells occurs mainly through the lysosomal system.
Triptonide-mediated degradation of Twist1 in TNBC cells was not dependent on the ubiquitin–proteasome system. After MDA-MB-231 and MDA-MB-468 cells were treated with or without 10 nM triptonide (TN) and with or without 20 μM of the ubiquitin–proteasome inhibitor MG132, Twist1 protein levels were detected by Western blotting (A, C) and statistically analyzed (B, D). The results showed that TN-mediated degradation of Twist1 protein in these cells was not significantly changed, suggesting that TN-mediated degradation of Twist1 is not dependent on the ubiquitin–proteasome system. The results represent three independent experiments. *P < 0.05, **P < 0.01
We further investigated the effect of triptonide on the levels of other important signaling proteins in TNBC cells. Notably, Notch1 protein levels were markedly reduced in MDA-MB-231 (Fig. 6A, B) and MDA-MB-468 (Fig. 6C, D) cells with triptonide treatment in a concentration-dependent manner. We investigated the effect of triptonide on Notch1 downstream genes and signaling pathways. Triptonide significantly inhibited phosphorylation of NF-κB in MDA-MB-231(Fig. 6E, F) and MDA-MB-468 (Fig. 6G, H) cells in a dose-dependent manner. Additionally, triptonide significantly reduced the expression of tumor angiogenic genes VE-cadherin and VEGFR2 (Fig. 6 I, J) and diminished the levels of the EMT-inducing gene N-cadherin in MDA-MB-231 (Fig. 6I) and MDA-MB-468 cells (Fig. 6J). However, Notch1 mRNA levels in these cells were not significantly changed (Additional file 1: Figure S2A–D), suggesting that triptonide triggers Notch1 protein degradation. Triptonide did not significantly change the levels of several other signaling proteins, including p-ERK, p-AKT, p-SRC, p-LYN, p-CREB, p-JAK2, p-STAT3, p-MKK3, p-JNK, PARP, and PTEN (Additional file 1: Figure S2E).
Triptonide inhibits tumorigenic Notch1 and NF-κB signaling pathways in TNBC cells. MDA-MB-231 and MDA-MB-468 cells were treated with triptonide (TN) for 72 h at the concentrations of 0–10 nM. Notch1 protein levels were detected by Western blotting (A, C), and the bands were scanned and statistically analyzed (B, D). Similarly, TN-mediated reduction of Notch1 protein levels in MDA-MB-468 cells was also verified (C, D). Additionally, the levels of total NF-κB protein and phosphorylated NF-κB protein (p-NF-κB) were also measured by Western blotting (E, G), and the bands were scanned and statistically analyzed (F, H). The mRNA levels of VE-cadherin, VEGFR2, and N-cadherin in MDA-MB-231 (I) and MDA-MB-468 (J) cells were assessed by real time quantitative PCR (QT-PCR). The results represent three independent experiments. *P < 0.05, **P < 0.01
Together, these data indicate that triptonide suppresses TNBC cell tumorigenesis and metastasis mainly by triggering the degradation of Twist1 and Notch1 proteins, consequently inhibiting the NF-κB signaling pathway and diminishing the expression of VE-cadherin, VEGFR2, and N-cadherin (Fig. 7).
A schematic summary of triptonide-mediated inhibition of triple-negative breast cancer cell tumorigenesis and metastasis. Triptonide enters to TNBC cells and triggers the degradation of oncogenic Twist1 and Notch1 proteins, consequently inhibiting their downstream NF-κB signaling, and reducing the expression of angiogenic VE-cadherin and VEGFR2, and EMT-inducing N-cadherin. As a result, triptonide potently suppressed TNBC cell tumorigenesis, migration, invasion, and angiogenesis, tumor growth, and lung metastasis in xenografted mice
Discussion
The initiation and metastasis of TNBC are driven by the overexpression of numerous oncogenic genes and aberrant activation of multiple tumorigenic signaling pathways [6,7,8,9,10]. Despite decades of research, druggable targets for TNBC have been difficult to identify [3, 5, 12, 58], which has hindered the development of effective therapeutics for the disease. In the current study, we explored the ability of an active compound to inhibit TNBC cell tumorigenesis and dissemination in vitro and in vivo. Triptonide triggered the degradation of the Twist1 and Notch1 oncoproteins and inhibited NF-κB signaling and expression of VE-cadherin, VEGFR2, and N-cadherin in TNBC, resulting in potent suppression of TNBC cell tumorigenesis and metastasis. These data suggest that concurrent degradation of the oncoproteins Twist1 and Notch1 and subsequent inhibition of downstream metastatic genes and signaling pathways reverse the aggressive and metastatic phenotypes of TNBC. Our findings provide a new strategy for effectively inhibiting TNBC metastasis and offer a new drug candidate for TNBC treatment.
Twist1 functions as a transcription factor to drive the transcription of numerous oncogenic genes, and the overexpression of Twist1 triggers EMT and cancer metastasis [12, 13, 15, 59]. The reduction of Twist1 expression by thymoquinone [16] and tamoxifen [17] significantly inhibits TNBC tumor growth and metastasis and reverses drug resistance in an in vitro tumor cell model and in mice [16,17,18,19]. However, the efficacy of these anti-TNBC agents is generally moderate with no effective TNBC therapeutics in the clinic. In this investigation, we found that 10 nM triptonide nearly eliminates Twist1 protein expression in TNBC cells, which is more potent than other agents reported in the literature [16,17,18,19]. Our data indicate that triptonide is a novel, potent Twist1 protein inhibitor, warranting further development of Twist1-targeted drugs for the treatment of TNBC and other types of cancer with aberrant overexpression of Twist1.
Notch1 is a cell surface receptor that plays a pivotal role in supporting tumor cell stemness, EMT, and metastasis [10, 14]. Overexpression of Notch1 and the activation of the Notch1 signaling pathway are associated with poor prognosis in TNBC patients [24]. However, the efficacy of Notch1-based therapeutics against TNBC has been only moderate and Notch1-targeted drugs are absent in the clinic. In the current study, we found that 10 nM triptonide triggered significant Notch1 degradation in TNBC cells, suggesting that triptonide is a novel and potent Notch1 inhibitor. Whether triptonide directly binds to Twist1 and Notch1 proteins in TNBC and other cancer cells remains to be determined.
Notch1 lies upstream of the transcription factor NF-κB [60], which promotes the expression of numerous angiogenic and metastatic genes [61, 62]. In this study, triptonide significantly inhibited the phosphorylation of NF-κB in TNBC cells, thereby suppressing the expression of numerous oncogenic genes and inhibiting tumorigenesis and cancer metastasis.
Angiogenesis and vasculogenic mimicry play critical roles in tumor growth and cancer metastasis. Several anti-angiogenic drugs, such as Avastin and Sunitinib, have been used in cancer therapy; unfortunately, current anti-angiogenic drugs fail to cure TNBC patients and may actually promote TNBC invasion and metastasis by triggering tumor cell-mediated vasculogenic mimicry [63]. To date, effective drugs that suppress tumor vasculogenic mimicry are absent in the clinic. Triptonide potently suppressed the ability of TNBC cells to form tube-like structures, which is consistent with our recent report that triptonide strongly inhibits vasculogenic mimicry mediated by pancreatic cancer cells [34]. Our mechanistic studies revealed that triptonide suppresses expression of the angiogenic VE-cadherin and VEGFR2 genes, thereby reducing TNBC angiogenesis and vasculogenic mimicry. Our findings provide a new approach for targeting TNBC angiogenesis and vasculogenic mimicry.
As mentioned above, Gao et al. reported that triptonide diminishes TNBC tumor growth via inhibiting expression of several stemness genes, but promoting Snail1 overexpression and induces a Snail-associated feedback mechanism of triptonide-resistance in a single in vitro-produced triptonide-resistant HCC1806 cell line [39]; however, whether triptonide affects Snail1 expression in TNBC cell lines without in vitro artificial induction of triptonide-resistance has not been addressed in the paper [39]. In the current investigation, our data showed that the levels of Snail mRNA and protein in the TNBC cell lines MDA-MB-231 and MDA-MB-468 were not significantly affected by triptonide. These data suggest that the effect of triptonide on Snail1 expression may be limited only in the in vitro produced triptonide-resistant HCC1806 cell line, but is uncommon in TNBC cell lines. Of note, overexpression of the transcription factor Snail drives overexpression of various oncogenic and metastatic genes, activates multiple tumorigenic signaling pathways, and promotes EMT, tumorigenesis, cancer metastasis, and drug resistance [44,45,46,47,48,49,50,51,52,53]. Therefore, up-regulation of Snail1 should not be good idea for cancer therapy; instead, suppression of Snail1 should be a sensible strategy for treatment of malignant tumors.
Triptonide exerts anti-cancer effects through down-regulation of various oncogenic genes, including β-catenin, cMyc, Notch1, LXRα, SREBF1 [32,33,34,35,36,37,38, 64], particularly, triptonide directly binds to its receptor LXRα, reduces LXRα protein stability in pancreatic cancer cells, and exerts potent anti-cancer effect [32,33,34,35,36,37,38, 64]. In the current investigation, we found that triptonide triggers degradation of Twist1 and Notch1 oncoproteins in TNBC cells. Our study reveals a new mechanism of triptonide-mediated tumor suppression.
Triptonide exerts strong anti-cancer effects [32,33,34,35,36, 38, 64] with low toxicity in mice [31, 65], making it an attractive small molecule for TNBC drug development. Furthermore, the combination of triptonide with currently available chemotherapeutics, immunotherapies, and radiotherapy may help shape the future landscape of TNBC treatment.
Conclusion
Triptonide effectively suppressed the tumorigenesis and metastasis of TNBC via triggering the degradation of Twist1 and Notch1 oncoproteins, consequently downregulating expression of various metastatic and angiogenic genes, and inhibiting NF-κB signaling pathway in TNBC cells. Our findings provide a new strategy for treating highly lethal TNBC and offer a potential novel drug candidate for combatting this aggressive disease.
Abbreviations
- TNBC:
-
Triple-negative breast cancer
- EMT:
-
Epithelial–mesenchymal transition
- VEGFR2:
-
Vascular endothelial cell growth factor receptor 2
- DMEM:
-
Dulbecco's modified Eagle medium
- TN:
-
Triptonide
- CHX:
-
Cycloheximide
References
Chang-Qing Y, Jie L, Shi-Qi Z, Kun Z, Zi-Qian G, Ran X, Hui-Meng L, Ren-Bin Z, Gang Z, Da-Chuan Y, Chen-Yan Z. Recent treatment progress of triple negative breast cancer. Prog Biophys Mol Biol. 2020;151:40–53.
Blows FM, Driver KE, Schmidt MK, Broeks A, vanLeeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkilä P, Heikkinen T, Nevanlinna H, Akslen LA, Bégin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, García-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279.
Gupta GK, Collier AL, Lee D, et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers (Basel). 2020;12(9):2392.
Shen M, Pan H, Chen Y, Xu YH, Yang W, Wu Z. A review of current progress in triple-negative breast cancer therapy. Open Med (Wars). 2020;2020(15):1143–9.
Lee JS, Yost SE, Yuan Y. Neoadjuvant treatment for triple negative breast cancer: recent progresses and challenges. Cancers (Basel). 2020;12(6):1404.
Garrido-Castro AC, Spurr LF, Hughes ME, Li YY, Cherniack AD, Kumari P, Lloyd MR, Bychkovsky B, Barroso-Sousa R, Di Lascio S, Jain E, Files J, Mohammed-Abreu A, Krevalin M, MacKichan C, Barry WT, Guo H, Xia D, Cerami E, Rollins BJ, MacConaill LE, Lindeman NI, Krop IE, Johnson BE, Wagle N, Winer EP, Dillon DA, Lin NU. Genomic Characterization of de novo Metastatic Breast Cancer. Clin Cancer Res. 2020;2020(27):1105–18.
Rueda OM, Sammut SJ, Seoane JA, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567(7748):399–404.
Lee KL, Chen G, Chen TY, Kuo YC, Su YK. Effects of cancer stem cells in triple-negative breast cancer and brain metastasis: challenges and solutions. Cancers (Basel). 2020;12(8):2122.
Khaled N, Bidet Y. New insights into the implication of epigenetic alterations in the EMT of triple negative breast cancer. Cancers (Basel). 2019;11(4):559.
Li Y, Su P, Wang Y, Zhang H, Liang Y, Zhang N, Song X, Li X, Li J, Yang Q. Impact of histotypes on preferential organ-specific metastasis in triple-negative breast cancer. Cancer Med. 2020;2020(9):872–81.
Neelakantan D, Zhou H, Oliphant M, Zhang X, Simon LM, Henke DM, Shaw CA, Wu MF, Hilsenbeck SG, White LD, Lewis MT, Ford HL. EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat Commun. 2017;8:15773.
Deepak K, Vempati R, Nagaraju GP, et al. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153:104683.
Zhang D, Sun B, Zhao X, et al. Twist1 accelerates tumour vasculogenic mimicry by inhibiting Claudin15 expression in triple-negative breast cancer. J Cell Mol Med. 2020;24(13):7163–74.
Miao K, Lei JH, Valecha MV, et al. NOTCH1 activation compensates BRCA1 deficiency and promotes triple-negative breast cancer formation. Nat Commun. 2020;11(1):3256.
Devanand P, Sundaramoorthy S, Ryu MS, Jayabalan AK, Ohn T, Lim IK. Translational downregulation of Twist1 expression by antiproliferative gene, B-cell translocation gene 2, in the triple negative breast cancer cells. Cell Death Dis. 2019;10(6):410.
Khan MA, Tania M, Wei C, Mei Z, Fu S, Cheng J, Xu J, Fu J. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget. 2015;6(23):19580–91.
Ma G, He J, Yu Y, Xu Y, Yu X, Martinez J, Lonard DM, Xu J. Tamoxifen inhibits ER-negative breast cancer cell invasion and metastasis by accelerating Twist1 degradation. Int J Biol Sci. 2015;11(5):618–28.
Hata T, Rajabi H, Yamamoto M, et al. Targeting MUC1-C inhibits TWIST1 signaling in triple-negative breast cancer. Mol Cancer Ther. 2019;18(10):1744–54.
Wang SC, Sun HL, Hsu YH, et al. α-Linolenic acid inhibits the migration of human triple-negative breast cancer cells by attenuating Twist1 expression and suppressing Twist1-mediated epithelial-mesenchymal transition. Biochem Pharmacol. 2020;180:114152.
Gonzalez ME, Moore HM, Li X, et al. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc Natl Acad Sci USA. 2014;111(8):3098–103.
Xie X, Kaoud TS, Edupuganti R, et al. c-Jun N-terminal kinase promotes stem cell phenotype in triple-negative breast cancer through upregulation of Notch1 via activation of c-Jun. Oncogene. 2017;36(18):2599–608.
Giuli MV, Giuliani E, Screpanti I, Bellavia D, Checquolo S. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J Oncol. 2019;2019:8707053.
Gharaibeh L, Elmadany N, Alwosaibai K, Alshaer W. Notch1 in cancer therapy: possible clinical implications and challenges. Mol Pharmacol. 2020;98(5):559–76.
Zhong Y, Shen S, Zhou Y, et al. NOTCH1 is a poor prognostic factor for breast cancer and is associated with breast cancer stem cells. Onco Targets Ther. 2016;9:6865–71.
Mohammadi-Yeganeh S, Mansouri A, Paryan M. Targeting of miR9/NOTCH1 interaction reduces metastatic behavior in triple-negative breast cancer. Chem Biol Drug Des. 2015;86(5):1185–91.
Zhou YF, Sun Q, Zhang YJ, et al. Targeted inhibition of Notch1 gene enhances the killing effects of paclitaxel on triple negative breast cancer cells. Asian Pac J Trop Med. 2017;10(2):179–83.
Zeng LYK, Xiao YS, et al. (2020) Inhibition of Notch1 reverses EMT and chemoresistance to cisplatin via direct downregulation of MCAM in triple-negative breast cancer cells. Int J Cancer. 2020;147(2):490–504.
Paroni G, Zanetti A, Barzago MM, et al. Retinoic acid sensitivity of triple-negative breast cancer cells characterized by constitutive activation of the notch1 pathway: the role of Rarβ. Cancers (Basel). 2020;12(10):3027.
Valcourt DM, Dang MN, Scully MA, Day ES. Nanoparticle-mediated co-delivery of notch-1 antibodies and ABT-737 as a potent treatment strategy for triple-negative breast cancer. ACS Nano. 2020;14(3):3378–88.
Peng A, Li R, Hu J, Chen L, Zhao X, Luo H, et al. Flow rate gradient high-speed counter-current chromatography separation of five diterpenoids from Tripterygium wilfordii and scale-up. J Chromatogr A. 2008;1200(2):129–35.
Li XX, Du FY, Liu HX, Ji JB, Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J Ethnopharmacol. 2015;162:238–43.
Pan Y, Meng M, Zheng N, Cao Z, Yang P, Xi X, Zhou Q. Targeting of multiple senescence-promoting genes and signaling pathways by triptonide induces complete senescence of acute myeloid leukemia cells. Biochem Pharmacol. 2017;126:34–50.
Yang P, Dong F, Zhou Q. Triptonide acts as a novel potent anti-lymphoma agent with low toxicity mainly through inhibition of proto-oncogene Lyn transcription and suppression of Lyn signal pathway. Toxicol Lett. 2017;278:9–17.
Han H, Du L, Cao Z, Zhang B, Zhou Q. Triptonide potently suppresses pancreatic cancer cell-mediated vasculogenic mimicry by inhibiting expression of VE-cadherin and chemokine ligand 2 genes. Eur J Pharmacol. 2018;818:593–603.
Zhang M, Tan S, Yu D, Zhao Z, Zhang B, Zhang P, et al. Triptonide inhibits lung cancer cell tumorigenicity by selectively attenuating the Shh-Gli1 signaling pathway. Toxicol Appl Pharmacol. 2019;365:1–8.
Xiang S, Zhao Z, Zhang T, Zhang B, Meng M, Cao Z, et al. Triptonide effectively suppresses gastric tumor growth and metastasis through inhibition of the oncogenic Notch1 and NF-κB signaling pathways. Toxicol Appl Pharmacol. 2020;388:114870.
Zhang B, Meng M, Xiang S, Cao Z, Xu X, Zhao Z, et al. Selective activation of tumor-suppressive MAPKP signaling pathway by triptonide effectively inhibits pancreatic cancer cell tumorigenicity and tumor growth. Biochem Pharmacol. 2019;166:70–81.
Yang B, Zhang B, Cao Z, Xu X, Huo Z, Zhang P, et al. The lipogenic LXR-SREBF1 signaling pathway controls cancer cell DNA repair and apoptosis and is a vulnerable point of malignant tumors for cancer therapy. Cell Death Differ. 2020;27(8):2433–50.
Gao B, Chen J, Han B, Zhang X, Hao J, Giuliano AE, Cui Y, Cui X. Identification of triptonide as a therapeutic agent for triple negative breast cancer treatment. Sci Rep. 2021;2021(11):2408.
Maturi V, Morén A, Enroth S, Heldin CH, Moustakas A. Genomewide binding of transcription factor Snail1 in triple-negative breast cancer cells. Mol Oncol. 2018;2018(12):1153–74.
Baulida J, Díaz VM, Herreros AG. Snail1: a transcriptional factor controlled at multiple levels. J Clin Med. 2019;2019(8):757.
Xiao M, Hasmim M, Lequeux A, Moer KV, Tan TZ, Gilles C, Hollier BG, Thiery JP, Berchem G, Janji B, Noman MZ. Epithelial to mesenchymal transition regulates surface PD-L1 via CMTM6 and CMTM7 induction in breast cancer. Cancers (Basel). 2021;13(2021):1165.
Foubert E, De Craene B, Berx G. Key signalling nodes in mammary gland development and cancer: the Snail1-Twist1 conspiracy in malignant breast cancer progression. Breast Cancer Res. 2010;12:206.
Yamashita S, Miyagi C, Fukada T, Kagara N, Che YS, Hirano T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;2004(429):298–302.
Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, Basnet H, Zou Y, Shu W, Soni RK, Hendrickson RC, Hadjantonakis AK, Massagué J. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;2020(577):566–71.
Baulida J, García de Herreros A. Snail1-driven plasticity of epithelial and mesenchymal cells sustains cancer malignancy. Biochim Biophys Acta. 2015;2015(1856):55–61.
Zhang J, Lin X, Wu L, Huang JJ, Jiang WQ, Kipps TJ, Zhang S. Aurora B induces epithelial-mesenchymal transition by stabilizing Snail1 to promote basal-like breast cancer metastasis. Oncogene. 2020;2020(39):2550–67.
Herrera A, Herrera M, Peña C. The emerging role of Snail1 in the tumor stroma. Clin Transl Oncol. 2016;2016(18):872–7.
Subramaniyan B, Sridharan S, Howard CM, Tilley AMC, Basuroy T, DelaSerna I, Butt E, Raman D. Role of the CXCR4-LASP1 axis in the stabilization of Snail1 in triple-negative breast cancer. Cancers (Basel). 2020;12:2372.
López-Menéndez C, Vázquez-Naharro A, Santos V, Dubus P, Santamaría PG, Martínez-Ramírez Á, Portillo F, Moreno-Bueno G, Faraldo MM, Cano A. E2A modulates stemness, metastasis, and therapeutic resistance of breast cancer. Cancer Res. 2021;81:4529–44.
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;2015(527):525–30.
Kaufhold S, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Exp Clin Cancer Res. 2014;2014(33):62.
Wang X, Liu R, Zhu W, Chu H, Yu H, Wei P, Wu X, Zhu H, Gao H, Liang J, Li G, Yang W. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature. 2019;2019(571):127–31.
Lin LF, Li YT, Han H, Lin SG. MicroRNA-205-5p targets the HOXD9-Snail1 axis to inhibit triple negative breast cancer cell proliferation and chemoresistance. Aging (Albany NY). 2021;13(3):3945–56.
He J, Du L, Bao M, Zhang B, Qian H, Zhou Q, Cao Z. Oroxin A inhibits breast cancer cell growth by inducing robust endoplasmic reticulum stress and senescence. Anticancer Drugs. 2016;27(3):204–15.
Liu W, Lv C, Zhang B, Zhou Q, Cao Z. MicroRNA-27b functions as a new inhibitor of ovarian cancer-mediated vasculogenic mimicry through suppression of VE-cadherin expression. RNA. 2017;23(7):1019–27.
Zhang P, Zhang M, Yu D, Liu W, Hu L, Zhang B, Zhou Q, Cao Z. Lycorine inhibits melanoma cell migration and metastasis mainly through reducing intracellular levels of β-catenin and matrix metallopeptidase 9. J Cell Physiol. 2019;234(7):10566–75.
Garmpis N, Damaskos C, Garmpi A, et al. Molecular classification and future therapeutic challenges of triple-negative breast cancer. In Vivo. 2020;34(4):1715–27.
Temian DC, Pop LA, Irimie AI, Berindan-Neagoe I. The epigenetics of triple-negative and basal-like breast cancer: current knowledge. J Breast Cancer. 2018;21(3):233–43.
Zhu H, Bhaijee F, Ishaq N, Pepper DJ, Backus K, Brown AS, Zhou X, Miele L. Correlation of Notch1, pAKT and nuclear NF-κB expression in triple negative breast cancer. Am J Cancer Res. 2013;2013(3):230–9.
Fusella F, Seclì L, Busso E, Krepelova A, Moiso E, Rocca S, Conti L, Annaratone L, Rubinetto C, Mello-Grand M, Singh V, Chiorino G, Silengo L, Altruda F, Turco E, Morotti A, Oliviero S, Castellano I, Cavallo F, Provero P, Tarone G, Brancaccio M. The IKK/NF-κB signaling pathway requires Morgana to drive breast cancer metastasis. Nat Commun. 2017;2017(8):1636.
Wang M, Zhang Y, Xu Z, Qian P, Sun W, Wang X, Jian Z, Xia T, Xu Y, Tang J. RelB sustains endocrine resistant malignancy: an insight of noncanonical NF-κB pathway into breast Cancer progression. Cell Commun Signal. 2020;2020(18):128.
Sun H, Zhang D, Yao Z, et al. Anti-angiogenic treatment promotes triple-negative breast cancer invasion via vasculogenic mimicry. Cancer Biol Ther. 2017;18(4):205–13.
Chinison J, Aguilar JS, Avalos A, Huang Y, Wang Z, Cameron DJ, et al. Triptonide effectively inhibits Wnt/β-catenin signaling via C-terminal transactivation domain of β-catenin. Sci Rep. 2016;6:32779.
Xu L, Qiu Y, Xu H, Ao W, Lam W, Yang X. Acute and subacute toxicity studies on triptolide and triptolide-loaded polymeric micelles following intravenous administration in rodents. Food Chem Toxicol. 2013;57:371–9.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grants Nos. 81703595, 81772535, 81902647, and 82073225); a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); and Jiangsu Province’s Key Discipline of Medicine (XK201118).
Author information
Authors and Affiliations
Contributions
MZ, MM, YL, JQ, ZZ, YQ, YH, WL, ZZ, QZ, and PX conceived and performed experiments. QZ and PX wrote the manuscript and secured funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The tumor xenograft mice were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Soochow University as previously reported [35, 38].
Consent for publication
All of the authors have seen and approved this manuscript for publication in Breast Cancer Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Triptonide did not significantly change the levels of Snail1 mRNA and protein in the TNBC MDA-MB-231 and MDA-MB-468 cells. Figure S2. Triptonide did not significantly affect the levels of Notch1 mRNA and several signaling proteins in TNBC cells.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, M., Meng, M., Liu, Y. et al. Triptonide effectively inhibits triple-negative breast cancer metastasis through concurrent degradation of Twist1 and Notch1 oncoproteins. Breast Cancer Res 23, 116 (2021). https://doi.org/10.1186/s13058-021-01488-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-021-01488-7