Abstract
Background
Bacteria are the main pathogens that cause sepsis. The pathogenic mechanisms of sepsis caused by gram-negative and gram-positive bacteria are completely different, and their prognostic differences in sepsis remain unclear.
Methods
The PubMed, Web of Science, Cochrane Library, and Embase databases were searched for Chinese and English studies (January 2003 to September 2023). Observational studies involving gram-negative (G (−))/gram-positive (G (+)) bacterial infection and the prognosis of sepsis were included. The stability of the results was evaluated by sensitivity analysis. Funnel plots and Egger tests were used to check whether there was publication bias. A meta-regression analysis was conducted on the results with high heterogeneity to identify the source of heterogeneity. A total of 6949 articles were retrieved from the database, and 45 studies involving 5586 subjects were included after screening according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Twenty-seven high-quality studies and 18 moderate-quality studies were identified according to the Newcastle‒Ottawa Scale score. There was no significant difference in the survival rate of sepsis caused by G (−) bacteria and G (+) bacteria (OR 0.95, 95% CI 0.70–1.28). Subgroup analysis according to survival follow-up time showed no significant difference. The serum concentrations of C-reactive protein (CRP) (SMD = 0.39, 95% CI 0.02–0.76), procalcitonin (SMD = 1.95, 95% CI 1.32–2.59) and tumor necrosis factor-alpha (TNF-α) (MD = 0.31, 95% CI 0.25–0.38) in the G (−) bacterial infection group were significantly higher than those in the G (+) bacterial infection group, but there was no significant difference in IL-6 (SMD = 1.33, 95% CI − 0.18–2.84) and WBC count (MD = − 0.15, 95% CI − 0.96–00.66). There were no significant differences between G (−) and G (+) bacteria in D dimer level, activated partial thromboplastin time, thrombin time, international normalized ratio, platelet count, length of stay or length of ICU stay. Sensitivity analysis of the above results indicated that the results were stable.
Conclusion
The incidence of severe sepsis and the concentrations of inflammatory factors (CRP, PCT, TNF-α) in sepsis caused by G (−) bacteria were higher than those caused by G (+) bacteria. The two groups had no significant difference in survival rate, coagulation function, or hospital stay. The study was registered with PROSPERO (registration number: CRD42023465051).
Similar content being viewed by others
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. The host clears pathogens by activating the inflammatory response when pathogenic microorganisms invade the body. In sepsis, a systemic inflammatory response occurs due to the continuous activation of neutrophils and macrophages/monocytes, which leads to irreversible tissue damage and death [2]. The mechanism by which bacteria cause sepsis and septic shock involves bacterial components (cell wall, bacterial secretion products) and host responses (susceptibility, primary (immune) reaction, secondary (tissue) reaction, etc.) [3]. Initially, many studies suggested that the main microorganisms causing bacterial sepsis were gram-negative bacteria [4]. In the past 20 years, gram-positive bacteria, which are important pathogenic microorganisms that can also cause sepsis, have gradually attracted attention [5]. At present, the harmfulness of sepsis caused by G (−) bacteria and G (+) bacteria is still controversial. One study suggested that infection with G (+) bacteria caused a stronger host inflammatory response than infection with G (−) bacteria [6]. Another study suggested that there was no significant difference in the prognosis of sepsis caused by G (−) and G (+) bacteria [7]. To clarify the prognostic difference between sepsis caused by G (−) and G (+) bacteria, we conducted this systematic review and meta-analysis.
Methods
This review followed the PRISMA Statement [8].
Search strategy
The PubMed, Web of Science, Cochrane Library, and Embase databases were searched for Chinese and English studies in the past 20 years (January 2003 to September 2023). The complete search strategy is detailed in Additional file 1.
Study selection
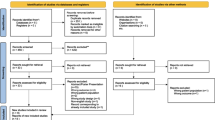
Two researchers performed the screening independently. The two researchers discussed with each other first if there were differences. A third investigator was consulted if disagreements could not be resolved. Screening was performed according to PRISMA guidelines.
Inclusion and exclusion criteria
-
(1)
The following inclusion criteria were used: (1) human subjects; (2) clinical research; (3) observational studies; (4) patients with sepsis; and (5) studies including prognostic outcomes associated with G (−) and G (+) bacteria.
-
(2)
The following exclusion criteria were used: (1) in vitro studies and animal studies; (2) only infants (age < 3 years) included in the study; (3) intervention studies; (4) conference abstracts, comments, letters, case reports, and expert opinions; (5) the language was not Chinese or English; (6) duplicate articles; (7) incomplete data provision; (8) measurement data not provided or unable to be converted to mean and standard deviation; and (9) research data obtained from the database.
Assessment of risk of bias
The risk of bias was assessed using the NOS by two researchers independently. The NOS consists of three parts: study population selection, comparability between groups, and outcome measures. The specific items and their scores are as follows: representativeness of the exposed cohort (1); selection of the nonexposed cohort (1); ascertainment of exposure (1); demonstration that the outcome of interest was not present at the start of the study (1); comparability of cohorts based on the design or analysis (2); assessment of outcome (1); sufficient follow-up length to allow outcomes to occur (1); and adequacy of follow-up of cohorts (1). Points are scored for each “yes” answer. According to the total score, studies were classified as high quality (7–9), moderate quality (4–6), and low quality (0–3).
Data extraction
Two researchers extracted information from the included studies, including (1) basic research information: author, year of publication, country, study type, sample size, source of sample, and whether to be included in the meta-analysis, site of infection, underlying host disease, whether patients with immunodeficiency and chemoradiotherapy for malignant tumors were excluded, and treatment measures, whether subjects were enrolled only from the ICU; (2) primary outcome data: survival; and (3) secondary outcome data: inflammatory factor concentrations, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, coagulation function, and length of hospital stay. It was preferred to obtain the relevant information directly from the publications. We obtained the data indirectly through the figures and datasets provided by the publication, if necessary.
Statistical analysis
Data synthesis was performed using RevMan software 5.3 and Stata 12. We performed pooled analyses of survival across time points. We chose data for 28-day survival if data for multiple survival times were presented in the same study. For continuous variables, the standardized mean difference (SMD)/mean difference (MD) and 95% confidence interval of the two groups were calculated. The odds ratio (OR) between the two groups and the 95% confidence interval were calculated for binary variables. To test heterogeneity, I2 statistics were computed, and a χ2 test was performed. Heterogeneity was considered high when I2 > 50%, and a random-effects model was used. Heterogeneity was considered insignificant when I2 ≤ 50%, and a fixed-effects model was used. Subgroup analysis was performed for some of the results. Meta-regression analysis was used to obtain the source of heterogeneity for results with high heterogeneity and more than 10 included articles. Sensitivity analyses were used to assess the robustness of the results. Funnel plots and Egger’s test were used to detect publication bias. The significance for all two-sided p values was set at less than 0.05.
Results
Study selection and characteristics
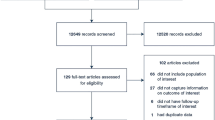
A total of 6949 articles were initially retrieved from the database. After screening, a total of 45 studies were ultimately included (Fig. 1). All studies were conducted at a secondary or tertiary care center. The basic information of the included studies is shown in Table 1 and Additional file 2. According to the NOS score, the studies were divided into 27 high-quality studies and 18 medium-quality studies, and no low-quality studies were found. The scores are detailed in Additional file 3.
Survival
A total of 20 studies had outcome measures associated with survival, including 28-day survival, hospital survival, ICU survival, and survival, without mention of follow-up time. A combined effect size analysis was performed for 20 studies (Fig. 2). We used a random-effects model due to the high heterogeneity of the results (I2 = 62%). The results showed that the survival rate of sepsis caused by G (+) bacteria (G (+) group) was not significantly different from that caused by G (−) bacteria (G (−) group) (OR 0.95, 95% CI 0.70–1.28, p = 0.74). No sources of heterogeneity were identified after a meta-regression analysis of 8 confounding factors (survival time points, sample size, whether subjects were enrolled only from the ICU, whether patients had only septic shock/severe sepsis, region, year of publication, whether only blood culture samples were collected, time of sampling, and the definition of sepsis) (Additional file 4). Subgroup analysis divided the studies into a 28-day survival group and an other survival group, and there was no difference between the two groups (p > 0.05). Egger’s test (p = 0.821) (Additional file 5) and funnel plot symmetry (Additional file 6) suggested that there was no significant publication bias (Fig. 2). Furthermore, we performed a subgroup analysis according to the definition of sepsis and found that the sepsis-1 group was less heterogeneous (I2 = 48%), suggesting that the definition of sepsis may be one of the sources of heterogeneity in this study (Additional file 7).
Severity of sepsis
Eleven studies reported the incidence of septic shock/severe sepsis. The random-effects model was used due to the high heterogeneity of the results (I2 = 63%). The incidence of septic shock/severe sepsis in the G (−) group was higher than that in the G (+) group (OR 1.73, 95% CI 1.09–2.76, p = 0.02). Meta-regression analysis suggested that whether patients were admitted only from the ICU might be the source of heterogeneity (p = 0.033) (Additional file 4). Egger’s test (p = 0.282) (Additional file 5) and funnel plot symmetry (Additional file 6) suggested that there was no significant publication bias (Fig. 3).
APACHE II score
A total of 10 studies reported APACHE II scores. The random-effects model was used due to the high heterogeneity of the results (I2 = 94%). The difference between the two groups was not significant (MD = 1.45, 95% CI − 0.41 ~ 3.31, p = 0.13). Meta-regression analysis revealed that the study region (p = 0.013 < 0.1), sample size (p = 0.041 < 0.1) and definition of sepsis (p = 0.093 < 0.1) may be sources of heterogeneity (Additional file 4). Egger’s test (p = 0.528) (Additional file 5) and funnel plot symmetry (Additional file 6) indicated that there was no significant publication bias (Fig. 4).
SOFA score
A total of five studies reported SOFA scores. There was no significant difference between the two groups (p = 0.06). After excluding a study [52] published 20 years ago, the SOFA score of the G (−) group was significantly higher than that of the G (+) group (MD = 1.66, 95% CI 0.69–2.64, p = 0.0008). Holub [52] was considered the source of heterogeneity (Fig. 5).
Length of stay
Four studies reported the length of hospital stay, and four studies reported the length of ICU stay. There was no significant difference between the G (−) group and the G (+) group (Fig. 6).
WBCs
A total of 12 studies mentioned white blood cells (WBCs). Subgroup analyses were performed according to the year of study publication. The combined effect sizes of studies published within ten years showed homogeneity (I2 = 20%). The random-effects model was used for analysis.The year of publication was considered a possible source of heterogeneity. The combined effect sizes of studies published within ten years showed that there was no significant difference between the G (−) and G (+) groups (MD = − 0.15, 95% CI − 0.96–00.66, p = 0.71) (Fig. 7).
Inflammatory factors
CRP
CRP concentrations were reported in 23 studies. Heterogeneity among the studies was high (I2 = 94%), and a random-effects model was used. The serum CRP concentration of the G (−) group was higher than that of the G (+) group (SMD = 0.39, 95% CI 0.02–0.76, p = 0.04). Meta-regression analysis revealed that admission to the ICU (p = 0.055 < 0.1) and study region (p = 0.05 < 0.1) might be the sources of heterogeneity (Additional file 4). Egger’s test (p = 0.77) (Additional file 5) and funnel plot symmetry (Additional file 6) indicated that there was no significant publication bias (Fig. 8).
PCT
Twenty studies reported serum PCT concentrations. A random-effects model was used due to the high interstudy heterogeneity (I2 = 97%). The serum PCT concentration of the G (−) group was significantly higher than that of the G (+) group (SMD = 1.95, 95% CI 1.32–2.59, p < 0.00001). Meta-regression analysis suggested that the study region (p = 0.061 < 0.1) might be the source of heterogeneity. Egger's test (p = 0.004) (Additional file 5) and funnel plot asymmetry (Additional file 6) showed publication bias (Fig. 9).
TNF-α and IL-6
Three studies reported serum concentrations of TNF-α, and five studies reported serum concentrations of interleukin-6 (IL-6). The serum TNF-α concentration in the G (−) group was significantly higher than that in the G (+) group (MD = 0.31, 95% CI 0.25–0.38, p < 0.00001). There was no significant difference in serum IL-6 concentration between the two groups (SMD = 1.33, 95% CI − 0.18–2.84, p = 0.08) (Additional file 7).
Coagulation function
Five studies reported D-D concentration, 2 studies reported APTT, 2 studies reported TT, 2 studies reported INR and 4 studies reported platelet (PLT) counts. After combining effect values, it was found that the G (−) and G (+) groups were not significantly different (Additional file 7).
Sensitivity analysis
Sensitivity analyses were performed separately for all results, which indicated that each result was stable.
Discussion
The meta-analysis revealed that sepsis caused by G (−) bacteria was more severe than that caused by G (+) bacteria. In addition, the concentrations of inflammatory factors in the G (−) group were significantly higher than those in the G (+) group. However, our study found that there was no significant difference in survival rate, coagulation function, length of stay, APACHE II score, or SOFA score between the G (−) and G (+) groups. We identified some sources of heterogeneity by meta-regression analysis, subgroup analysis, funnel plot, and Egger's test. Sensitivity analyses suggested that all results were stable.
Bacteria are one of the most common pathogens that cause sepsis, and there are significant differences in pathogenic mechanisms between G (−) bacteria and G (+) bacteria [4]. There are fundamental differences in the host response to infection with G (−) and G (+) bacteria, which are related to differences in their composition and structure [53].
Bacterial cell wall components include lipopolysaccharide (LPS), peptidoglycan (PGN), and lipoteichoic acid (LTA). LPS is the main component of the G (−) outer membrane. LPS and other cell wall components are released when bacteria multiply or die in the host. The toxic fraction lipid A causes the body's immune response [54]. The structure of the G (+) cell wall is different from that of G (−) bacteria, and its cell membrane is a single-cell membrane with PGN, LTA, etc., as the main components [55]. An experimental study found a significant increase in plasma concentrations of TNF-α, IFN-γ, and IL-10 one hour after intraperitoneal injection of LPS, whereas no significant increase was found after intraperitoneal injection of LTA [56]. In our meta-analysis, the serum concentrations of multiple proinflammatory factors were also elevated in patients with sepsis caused by G (−) bacteria. The results suggest that G (−) bacterial infection may cause a more severe systemic inflammatory response, which may be one of the important reasons for the increased severity of sepsis.
A total of 5259 patients had at least one positive microbiological culture in a study involving 15,202 subjects. Sixty-seven percent were gram-negative bacteria, 37% were gram-positive bacteria, and 16% were fungi [57]. The main site of infection was the lung (44.8%), followed by the abdomen (31.5%), urinary tract (6.2%), central venous catheter (4.6%), soft tissue (3.1%), and surgical wound (3.1%) [58]. Staphylococcus aureus and Pseudomonas species were the most common G (−) bacteria and G (+) bacteria. Different microorganisms and sites of infection interact in determining mortality [59]. As a reference, we recorded the information about the site of infection from each study. In general, many studies have suggested that sepsis caused by G (−) bacteria is more severe than that caused by G (+) bacteria [6]. A growing number of studies have different points of view. The pathogens causing sepsis used to be mainly G (−) bacteria, but they are being gradually replaced by G (+) bacteria [60]. The incidence and mortality of sepsis caused by gram-positive bacteria are increasing, which may be related to the resistance of G (+) bacteria [61]. The development of antibacterial drugs is underway, but the harmfulness of G (+) bacteria is not matched by the attention it receives [60]. In this study, G (−) bacteria caused more severe sepsis, but there were no differences in survival or length of hospital stay between the G (−) and G (+) groups. Bacteremia is thought to be associated with poor prognosis in sepsis. We performed a subgroup analysis of whether the patients were complicated with bacteremia and found that there was no difference in survival between the two groups. It is important to increase awareness of sepsis caused by G (+) bacteria.
Polymicrobial infection is a scenario that should be considered. Studies have shown that polymicrobial infection is a risk factor for severe sepsis [62]. The mortality of G (+) and G (−) infected patients was significantly increased when they were coinfected with COVID-19 [63]. The proportion of sepsis infections caused by fungi is increasing [64]. It is uncertain whether coinfection with other microorganisms is responsible for the difference in prognosis between the G (−) and G (+) groups. Some studies included only subjects with a single positive culture, while others included subjects with polymicrobial infection. This may have influenced our results.
Our study has some limitations. 1) Studies in languages other than Chinese or English were excluded, which may have resulted in an incomplete number of included studies. 2) Some results of this meta-analysis showed high heterogeneity. We identified some sources of heterogeneity through a series of methods, but some sources of heterogeneity are still unclear. 3) Because there are significant differences between children and adults in the prognosis and physiology of sepsis, we excluded studies involving only infants. However, we did not perform separate analyses for the other age groups. 4) Polymicrobial infection was not considered as a variable in this study.
Conclusion
In conclusion, sepsis caused by G (−) bacteria has higher serum inflammatory factor concentrations and greater disease severity than sepsis caused by G (+) bacteria. However, there was no significant difference in survival rate, length of stay, APACHE II score, SOFA score, or coagulation function between the two groups. This provides suggestions for the treatment of sepsis. The pathophysiological differences between G (−) and G (+) bacteria causing sepsis still need to be further studied.
Availability of data and materials
All data generated or analyzed during this study is included in this published article [and its supplementary information files.
Abbreviations
- G (−):
-
Gram-negative
- G (+):
-
Gram-positive
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NOS:
-
Newcastle–Ottawa scale
- APACHE II:
-
Acute physiology and chronic health evaluation
- SOFA:
-
Sequential organ failure assessment
- SMD:
-
Standardized mean difference
- MD:
-
Mean difference
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- CRP:
-
Serum C-reactive protein
- PCT:
-
Procalcitonin
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-6:
-
Interleukin-6
- WBC:
-
White blood cells
- D-D:
-
D dimer
- APTT:
-
Activated partial thromboplastin time
- TT:
-
Thrombin time
- INR:
-
International normalized ratio
- PLT:
-
Platelet
- LPS:
-
Lipopolysaccharides
- LTA:
-
Lipoteichoic acid
- PGN:
-
Peptidoglycan
- TLR4:
-
Toll-like receptor 4
- TRP:
-
Transient receptor potential
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Horn DL, Morrison DC, Opal SM, Silverstein R, Visvanathan K, Zabriskie JB. What are the microbial components implicated in the pathogenesis of sepsis? Report on a symposium. Clin Infect Dis. 2000;31(4):851–8.
Sriskandan S, Cohen J. Gram-positive sepsis. Mechanisms and differences from gram-negative sepsis. Infect Dis Clin N Am. 1999;13(2):397–412.
Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–42.
Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock. 2003;20(5):402–14.
Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than gram-positive bacteremia. Crit Care. 2010;14(2):27.
León C, Rodrigo MJ, Tomasa A, Gallart MT, Latorre FJ, Rius J, et al. Complement activation in septic shock due to gram-negative and gram-positive bacteria. Crit Care Med. 1982;10(5):308–10.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71.
Duan C, Wang Y, Wang Q, Li J, Xie J, Liu S, et al. Gram-negative bacterial infection causes aggravated innate immune response in sepsis: studies from clinical samples and cellular models. Biochem Biophys Res Commun. 2023;650:137–44.
Zhang ZB, Wang C, Han Y, Wang J, Lv JQ, Lin XR, et al. Diagnostic and prognostic value of serum LIF and RANTES in septic patients caused by bloodstream infection. Chin J Crit Care Med. 2023;43(4):296–300.
Bilgin M, Aci R, Keskin A, Yilmaz EM, Polat E. Evaluation of the relationship between procalcitonin level and the causative pathogen in intensive care patients with sepsis. Future Microbiol. 2023. https://doi.org/10.2217/fmb-2023-0010.
Chen Y, Jin Q, Chen Q, Lu GT, Zhang X. Differences in cytokine profiles and disease severity in elderly patients with sepsis. Chin J Clin Pharm Ther. 2022;38(18):2128–32.
Wu B, Zhang QF, Wang F, Ge YM, Liu Q, Xu AC. The relationship between serum levels of procalcitonin, endotoxin and C-reactive protein and pathogenic bacteria and prognosis in sepsis patients. Chin J Blood Purif. 2022;21(06):432–5.
Chen DF, Zheng F, Liu SF, Cao FS, Qiu GY. Study on inflammatory factors in sepsis patients caused by different bacterial infections. Chin J Nosocomiol. 2022;32(03):351–5.
Huang C, Xiong H, Li W, Peng L, Zheng Y, Liao W, et al. T cell activation profiles can distinguish gram negative/positive bacterial sepsis and are associated with ICU discharge. Front Immunol. 2022;13:1058606.
Liang P, Yu F. Predictive value of procalcitonin and neutrophil-to-lymphocyte ratio variations for bloodstream infection with septic shock. Med Sci Monit. 2022;28:935966.
Hu S, Yang J, Yu C, Feng Z, Zhang J, Yin W, et al. IL-6, IL-1β, and IL-10 levels in peripheral blood as indicators for early identification of gram-positive and gram-negative sepsis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37(6):532–7.
Yan S, Lian R, Sun L, Jin Z, Zhao C, Zhang G. Value of procalcitonin and critical illness score in etiological diagnosis and prognosis of sepsis caused by intra-abdominal infections. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(7):792–7.
Peng TT, Liu YH, Xuan K. Relationship between inflammatory factors, endotoxin changes and bacterial type and condition in sepsis patients. Chin J Nosocomiol. 2021;30(4):487–91.
Leijte GP, Rimmelé T, Kox M, Bruse N, Monard C, Gossez M, et al. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit Care. 2020;24(1):110.
Meng YX, Wang XZ, Kang FX, Yu H, Liu R. Procalcitonin, D-dimer and pathogens in patients with severe infection. Chin J Nosocomiol. 2019;29(16):2414–44.
Fernández-Grande E, Cabrera CM, González B, Varela C, Urra JM. Enhanced HLA-DR expression on T-lymphocytes from patients in early stages of non-surgical sepsis. Med Clin (Barc). 2019;152(9):346–9.
Gai L, Tong Y, Yan BQ. Research on the diagnostic effect of PCT level in serum on patients with sepsis due to different pathogenic causes. Eur Rev Med Pharmacol Sci. 2018;22(13):4238–42.
Zhang HW, Du H, Yu ZB, Xu F. Comparison of routine blood indices and inflammatory factors in patients with sepsis due to gram-negative or gram-positive bacteria. J Pathog Biol. 2018;13(11):1269–71.
Liu ZW, Miao FF, Zhang H, Mu N. Correlation analysis between serum levels of inflammatory markers, APACHE II score and the severity of bloodstream infections. Chin J Infect Chemother. 2018;18(05):477–81.
Lu B, Zhang Y, Li C, Liu C, Yao Y, Su M, et al. The utility of presepsin in diagnosis and risk stratification for the emergency patients with sepsis. Am J Emerg Med. 2018;36(8):1341–5.
Yunus I, Fasih A, Wang Y. The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS One. 2018;13(11):0206527.
Lang Y, Jiang Y, Gao M, Wang W, Wang N, Wang K, et al. Interleukin-1 receptor 2: a new biomarker for sepsis diagnosis and gram-negative/gram-positive bacterial differentiation. Shock. 2017;47(1):119–24.
Li W, Li ZG, Yi YF, Wei CF, Xue HW. Clinical study on patients with gastrointestinal related acute abdomen complicated with septic shock. Chin J Nosocomiol. 2017;27(19):4416–75.
Liu HH, Zhang MW, Guo JB, Li J, Su L. Procalcitonin and C-reactive protein in early diagnosis of sepsis caused by either gram-negative or gram-positive bacteria. Ir J Med Sci. 2017;186(1):207–12.
Gao L, Liu X, Zhang D, Xu F, Chen Q, Hong Y, et al. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp Ther Med. 2017;13(6):3479–83.
Liu YH, Wu M. Clinical significance of detection of blood coagulation - inflammation biomarkers in patients with sepsis caused by a bacterial bloodstream infection. J Pathog Biol. 2017;12(03):270–3.
Tunjungputri RN, van de Heijden W, Urbanus RT, de Groot PG, van der Ven A, de Mast Q. Higher platelet reactivity and platelet-monocyte complex formation in gram-positive sepsis compared to gram-negative sepsis. Platelets. 2017;28(6):595–601.
Zhuo HQ, Pei YH, Chen MQ, Dai LF, Wang X. Clinical value of combined detection of multiple indicators in bloodstream infection of gram-negative bacteria in patients with sepsis. Chin J Nosocomiol. 2016;26(24):5557–9.
Li S, Rong H, Guo Q, Chen Y, Zhang G, Yang J. Serum procalcitonin levels distinguish gram-negative bacterial sepsis from gram-positive bacterial and fungal sepsis. J Res Med Sci. 2016;21:39.
Surbatovic M, Popovic N, Vojvodic D, Milosevic I, Acimovic G, Stojicic M, et al. Cytokine profile in severe gram-positive and gram-negative abdominal sepsis. Sci Rep. 2015;5:11355.
Chen W, Niu SP, Zang XF, Zhao L, Sheng B. Early diagnostic value of combined inflammatory cytokines in bloodstream infection with different organisms. Chin J Emerg Med. 2015;24(4):369–73.
Zhao L, Zang X, Chen W, Sheng B, Gu X, Zhang J. Analysis of correlation between inflammatory parameters and severity of sepsis caused by bacterial bloodstream infection in septic patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27(6):448–53.
Guo SY, Zhou Y, Hu QF, Yao J, Wang H. Procalcitonin is a marker of gram-negative bacteremia in patients with sepsis. Am J Med Sci. 2015;349(6):499–504.
Aydemir H, Piskin N, Akduman D, Kokturk F, Aktas E. Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets. 2015;26(4):331–5.
Liu XQ, Sang L, Nong LB, Chen SB, He WQ, Li YM. Effect of bloodstream infections caused by different pathogens on serum procalcitonin. Chin J Pract Intern Med. 2014;34(07):702–5.
Gao HM, Lu L, Wang YQ. An investigation of the clinical characteristics and prognosis of 116 gram - positive and gram -negative sepsis. Chin J Crit Care Med. 2014;34(3):197–202.
Su MH, Shou ST. Prognostic value of presepsin for diagnosis and severity assessment of sepsis. Chin J Clin Lab Sci. 2014;32(02):106–11.
Chen W, Zhao L, Niu S, Wang S, Sheng B, Zhen J, et al. The diagnostic value of different pro-inflammatory factor in early diagnosis of sepsis in patients with bloodstream infection. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(3):165–70.
Janols H, Wullt M, Bergenfelz C, Björnsson S, Lickei H, Janciauskiene S, et al. Heterogeneity among septic shock patients in a set of immunoregulatory markers. Eur J Clin Microbiol Infect Dis. 2014;33(3):313–24.
Nakajima A, Yazawa J, Sugiki D, Mizuguchi M, Sagara H, Fujisiro M, et al. Clinical utility of procalcitonin as a marker of sepsis: a potential predictor of causative pathogens. Intern Med. 2014;53(14):1497–503.
Angeletti S, Battistoni F, Fioravanti M, Bernardini S, Dicuonzo G. Procalcitonin and mid-regional pro-adrenomedullin test combination in sepsis diagnosis. Clin Chem Lab Med. 2013;51(5):1059–67.
Labelle A, Juang P, Reichley R, Micek S, Hoffmann J, Hoban A, et al. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit Care Med. 2012;40(7):2016–21.
Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35(11):2538–46. https://doi.org/10.1097/01.CCM.0000284492.30800.00.
Feezor RJ, Oberholzer C, Baker HV, Novick D, Rubinstein M, Moldawer LL, et al. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect Immun. 2003;71(10):5803–13.
Blairon L, Wittebole X, Laterre PF. Lipopolysaccharide-binding protein serum levels in patients with severe sepsis due to gram-positive and fungal infections. J Infect Dis. 2003;187(2):287–91.
Holub M, Klucková Z, Helcl M, Príhodov J, Rokyta R, Beran O. Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clin Microbiol Infect. 2003;9(3):202–11.
Opal SM, Cohen J. Clinical gram-positive sepsis: does it fundamentally differ from gram-negative bacterial sepsis? Crit Care Med. 1999;27(8):1608–16.
Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16(3):379–414.
Dmitriev BA, Ehlers S, Rietschel ET. Layered murein revisited: a fundamentally new concept of bacterial cell wall structure, biogenesis and function. Med Microbiol Immunol. 1999;187(3):173–81.
Finney SJ, Leaver SK, Evans TW, Burke-Gaffney A. Differences in lipopolysaccharide- and lipoteichoic acid-induced cytokine/chemokine expression. Intensive Care Med. 2012;38(2):324–32.
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–87.
Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12(6):158.
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9.
Blaskovich MAT, Hansford KA, Butler MS, Ramu S, Kavanagh AM, Jarrad AM, et al. A lipoglycopeptide antibiotic for gram-positive biofilm-related infections. Sci Transl Med. 2022;14(662):2381.
Guo Q, Qu P, Cui W, Liu M, Zhu H, Chen W, et al. Organism type of infection is associated with prognosis in sepsis: an analysis from the MIMIC-IV database. BMC Infect Dis. 2023;23(1):431.
Montull B, Menéndez R, Torres A, Reyes S, Méndez R, Zalacaín R, et al. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. PLoS One. 2016;11(1):0145929.
Dar S, Erickson D, Manca C, Lozy T, Shashkina E, Kordalewska M, et al. The impact of COVID on bacterial sepsis. Eur J Clin Microbiol Infect Dis. 2023;42(10):1173–81.
Rodríguez-Créixems M, Alcalá L, Muñoz P, Cercenado E, Vicente T, Bouza E. Bloodstream infections: evolution and trends in the microbiology workload, incidence, and etiology, 1985–2006. Medicine (Baltimore). 2008;87(4):234–49.
Acknowledgements
Not applicable.
Funding
The fifth batch of "Longyi Scholar" Clinical Scientific and technological Innovation training Project of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine (PY2022011). Excellent Reserve talents of traditional Chinese Medicine in Shanghai University of traditional Chinese Medicine (2020).
Author information
Authors and Affiliations
Contributions
AT Propose study concepts and designs; drafting the manuscript; YS Propose study concepts and designs; drafting the manuscript; QD contributed to acquisition of the data; analysis and interpretation of the data; SW contributed to statistical expertise; YG contributed to drafting of the manuscript; CW contributed to statistical expertise; ZG contributed to analysis and interpretation of the data; WZ contributed to critical revision of the manuscript for important intellectual content; WC contributed to critical revision of the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Search strategy.
Additional file 2
. Characteristics of included studies.
Additional file 3
. NOS Score.
Additional file 4
. Meta-regression.
Additional file 5
. Egger's test.
Additional file 6
. Plot of funnel.
Additional file 7
. Forest plots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, A., Shi, Y., Dong, Q. et al. Prognostic differences in sepsis caused by gram-negative bacteria and gram-positive bacteria: a systematic review and meta-analysis. Crit Care 27, 467 (2023). https://doi.org/10.1186/s13054-023-04750-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04750-w