Abstract
Background
Sepsis survivors are at elevated risk for cardiovascular disease during long-term follow-up. Whether diabetes influences cardiovascular risk after sepsis survival remains unknown. We sought to describe the association of diabetes with long-term cardiovascular outcomes in adult sepsis survivors.
Methods
Population-based cohort study in the province of Ontario, Canada (2008–2017). Adult survivors of a first sepsis-associated hospitalization, without pre-existing cardiovascular disease, were included. Main exposure was pre-existing diabetes (any type). The primary outcome was the composite of myocardial infarction, stroke, and cardiovascular death. Patients were followed up to 5 years from discharge date until outcome occurrence or end of study period (March 2018). We used propensity score matching (i.e., 1:1 to patients with sepsis but no pre-existing diabetes) to adjust for measured confounding at baseline. Cause-specific Cox proportional hazards models with robust standard errors were used to estimate hazard ratios (HR) alongside 95% confidence intervals (CI). A main secondary analysis evaluated the modification of the association between sepsis and cardiovascular disease by pre-existing diabetes.
Results
78,638 patients with pre-existing diabetes who had a sepsis-associated hospitalization were matched to patients hospitalized for sepsis but without diabetes. Mean age of patients was 71 years, and 55% were female. Median duration from diabetes diagnosis was 9.8 years; mean HbA1c was 7.1%. Adult sepsis survivors with pre-existing diabetes experienced a higher hazard of major cardiovascular disease (HR 1.25; 95% CI 1.22–1.29)—including myocardial infarction (HR 1.40; 95% CI 1.34–1.47) and stroke (HR 1.24; 95% CI 1.18–1.29)—during long-term follow-up compared to sepsis survivors without diabetes. Pre-existing diabetes modified the association between sepsis and cardiovascular disease (risk difference: 2.3%; 95% CI 2.0–2.6 and risk difference: 1.8%; 95% CI 1.6–2.0 for the effect of sepsis—compared to no sepsis—among patients with and without diabetes, respectively).
Conclusions
Sepsis survivors with pre-existing diabetes experience a higher long-term hazard of major cardiovascular events when compared to sepsis survivors without diabetes. Compared to patients without sepsis, the absolute risk increase of cardiovascular events after sepsis is higher in patients with diabetes (i.e., diabetes intensified the higher cardiovascular risk induced by sepsis).
Similar content being viewed by others
Introduction
Sepsis is conceptualized as a life-threatening acute organ dysfunction in response to infection and represents a leading cause of morbidity and mortality worldwide [1,2,3,4,5]. Acute complications of sepsis may be related to both the infection itself and the host’s response and are characterized by distinct organ failures and high risk of mortality [3, 5, 6]. Moreover, there is an increasing awareness of the long-term health risks of sepsis, including—but not limited to—recurrent sepsis, clinical deconditioning and re-hospitalization, mental health problems, and cardiovascular events (which can be considered as part of the so-called post intensive care syndrome) [7,8,9,10,11,12,13,14,15,16,17].
Social determinants of health and baseline burden of disease such as nutrition, lifestyle choices, and comorbid conditions may all interact with the risk of both short- and long-term outcomes following sepsis [6, 18,19,20,21,22,23]. Diabetes mellitus may be a particularly important determinant of sepsis-related outcomes due to the associated cardiovascular changes and its high and ever-increasing prevalence [24,25,26]. Diabetes may affect post-sepsis cardiovascular disease either (1) directly, (2) through its association with other comorbidities such as hypertension, (3) through the varying severity of the sepsis episode, or (4) through the amplification of changes (e.g., inflammation cascade) that may occur after sepsis [23, 27]. However, once sepsis develops in patients with diabetes, the impact on organ failure, in-hospital events, and long-term clinical outcomes remains unclear [28, 29]. Overall, short-term mortality and risk of acute lung injury following sepsis may be reduced compared to patients without diabetes, while acute renal failure appears to be more common [23, 25, 30,31,32,33]. Diabetes is an established risk factor for cardiovascular disease, but the degree to which diabetes increases the risk of experiencing cardiovascular outcomes after sepsis (e.g., the potential additive risk in sepsis survivors) remains incompletely characterized [23, 27].
We sought to describe the association of pre-existing diabetes with long-term cardiovascular outcomes in adult sepsis survivors using population-based data from the province of Ontario. We hypothesized that pre-existing diabetes would be associated with a higher risk of cardiovascular disease in sepsis survivors.
Methods
Data sources and study population
We created the study cohort using population-based provincial health administrative databases contained at ICES, an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze healthcare and demographic data, without consent, for health system evaluation and improvement. These datasets were linked using unique encoded identifiers. Our study was developed in accordance with the amended Declaration of Helsinki, and this report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [34]. The use of data in this project was authorized under Sect. 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Our cohort included adults (age 18 years or older) in the province of Ontario, Canada, who survived a first sepsis-related hospitalization between April 2008 and April 2017. The study dates were chosen to optimize data completeness, and to allow a minimum follow-up of one year for all patients (to March 2018). Sepsis was identified using a previously validated algorithm [35, 36]. To control for baseline confounding, patients with pre-existing cardiovascular disease (identified during a 5-year lookback period) were excluded [14]. For all patients, the start of follow-up (i.e., index date) was defined as the date of hospital discharge. Patients were followed until outcome occurrence up to a maximum of five years or end of the study period.
Main exposure and outcomes of interest
Our exposure of interest was pre-existing diabetes at the time of sepsis hospitalization, defined using a previously validated algorithm [26]. This algorithm has high sensitivity and specificity but does not differentiate between type 1 or type 2 diabetes mellitus [26]. The composite primary outcome of interest was comprised of any of myocardial infarction, stroke, or cardiovascular death, defined using International Classification of Diseases 10-CA codes [37,38,39]. Secondary outcomes of interest included myocardial infarction, stroke, recurrent sepsis within 1 year, and the competing risk of non-cardiovascular death [14]. Table S1 in the supplement describes specific coding strategies used to define main variables of interest; details can be found elsewhere [14, 26, 35, 39].
Statistical analysis
Patients’ demographic, clinical, and hospital level characteristics were summarized using proportions for categorical variables and mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, as appropriate. Baseline characteristics of patients with or without diabetes were compared using standardized mean differences (SMD) [40]. SMD greater than 10% were considered relevant.
We used propensity score matching to control for measured confounding [40]. Specifically, we created a propensity score (i.e., disease risk score) for pre-existing diabetes using a logistic regression model including the following measured confounders identified using subject matter knowledge: age, sex, income quintile, long-term care residency, classic cardiovascular risk factors such as hypertension, dyslipidemia, and atrial fibrillation, and baseline comorbid conditions such as chronic kidney and pulmonary disease. To avoid overmatching on hospital characteristics that may lie within the causal pathway between pre-existing diabetes and cardiovascular disease, we did not match for severity of sepsis or intensity of organ support [14]. We then performed one to one greedy matching without replacement with a caliper width of 0.15 on the logit scale [40]. Outcome occurrence was summarized using cumulative incidence during long-term follow-up, alongside cumulative incidence functions [41]. To estimate the association between diabetes and time to (first) binary outcomes, we used cause-specific Cox proportional hazards model with robust standard errors based on the sandwich estimator to account for the matching procedure [42]. To estimate the association between diabetes and recurrent sepsis during the first year following hospital discharge (and to allow for multiple or recurring events), we used a Poisson regression model [43], also with robust standard errors. Effect estimates are reported as hazard ratios (HR) or incidence rate ratios (IRR) as appropriate, alongside 95% confidence intervals.
Secondary and sensitivity analyses
To further explore the impact of diabetes on long-term cardiovascular disease after sepsis, our main secondary analysis explored the modification of the association between sepsis and subsequent cardiovascular events by pre-existing diabetes. Specifically, we explored whether the previously identified effect of sepsis on cardiovascular disease during long-term follow-up varied across subgroups of patients with and without diabetes [14]. For this analysis, and in a similar way to previously reported [14], we included matched adult patients who survived a hospitalization (either related to sepsis or not). Details about cohort creation, matching, and the comparison between patients with and without sepsis can be found in the supplement and elsewhere [14]. We then fitted a multivariable Cox proportional hazards model (i.e., on the multiplicative scale) with sepsis, pre-existing diabetes, and their interaction as main covariates. To further assess effect measure modification on the additive and multiplicative scales, we also fitted generalized linear models with a binomial distribution and an identity or a log link to estimate absolute risk differences (i.e., additive scale) and risk ratios (i.e., multiplicative scale), respectively [44]. The presence of effect measure modification in all models was assessed based on a Wald test for the interaction term, and the effect of sepsis on long-term cardiovascular disease is reported separately for patients with and without pre-existing diabetes. Further details can be found in the supplement.
We also conducted several sensitivity analyses to assess the robustness of our main findings. First, we report the E-value for the point estimate and lower bound of the 95% confidence intervals for our main analysis on the primary outcome of interest [45]. Second, we utilized Fine and Gray models to take into account the competing risk of non-cardiovascular death, reporting sub-distribution HRs alongside 95% CI [46]. Third, since patients with chronic kidney disease may have a differential risk of sepsis and subsequent outcomes, we refitted our analysis restricting to patients without kidney failure at baseline (identified using International Classification of Diseases 10-CA codes during the lookback period) [47]. Fourth, we planned to further adjust, if needed, for baseline imbalances after matching (i.e., SMD greater than 10%) [48]. Fifth, since the intensity of the acute illness may modify the long-term cardiovascular risk following sepsis, we re-fitted our analysis while adjusting for in hospital characteristics (e.g., receipt of renal replacement therapy and mechanical ventilation) [23]. Further, since renal replacement therapy has been recently recognized as a potential risk factor of cardiovascular disease in sepsis survivors, we performed a causal mediation analysis considering the receipt of new dialysis as a potential mediator of the association between diabetes and the primary outcome (further details found in the supplement) [23, 49]. Sixth, we evaluated the impact of preadmission glycemic control of patients with diabetes on subsequent cardiovascular disease. To this end, we stratified patients by their measured HbA1c (i.e., less than 6.5%, 6.5% to 7.9%, more or equal than 8%). Seventh, we re-fitted our estimates considering those patients without previously identified diabetes but with a baseline HbA1c greater than or equal than 6.5% as having pre-existing diabetes. Eighth, we conducted a quantitative bias analysis to correct for potential misclassification of the exposure based on the expected accuracy of the coding algorithm for pre-existing diabetes [50]. Finally, we re-fitted our main analysis without the exclusion of patients with pre-existing cardiovascular disease since they may represent a population specifically at risk of subsequent cardiovascular outcomes after sepsis.
All analyses were performed using SAS Enterprise Guide version 7.1 (Cary, NC) and STATA v.14.2 (StataCorp, College Station, TX). A p-value of 0.05 was used as threshold for statistical significance and all tests were two-sided.
Results
Overall, 78,638 sepsis survivors with pre-existing diabetes were matched to sepsis survivors without diabetes during the study period (Fig. 1 and Table 1; Additional file 1: Table S2 shows the baseline characteristics of the complete unmatched sample). Mean age of patients was 71 years, and 55% were female. Median duration since diabetes diagnosis was 9.8 years (IQR 3.9–15.3); mean HbA1c on the last blood work prior to admission (available for 76% of sample) was 7.1% (SD: 1.6). Main comorbidities in patients with diabetes were hypertension (79%), active malignancy (28%), dementia (19%), and chronic kidney disease (10%). All demographics and comorbidities were balanced across both groups (i.e., SMD less than 10%).
On average, adult sepsis survivors with and without pre-existing diabetes had similar sources of infection and presence of septic shock during the initial hospitalization (Table 1). No relevant differences were observed in intensive care unit admission or renal replacement therapy (Table 1). Acute kidney injury was more common in patients with pre-existing diabetes (Table 1).
Major cardiovascular disease and secondary outcomes
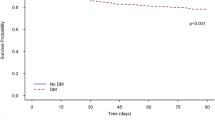
Participant follow-up information is presented in Additional file 1: Table S3. Median follow-up time for patients with and without pre-existing diabetes was 2.7 years (IQR 1.1–4.9) and 2.8 (IQR 1.2–5.0), respectively. Adult sepsis survivors with pre-existing diabetes experienced a higher hazard of major cardiovascular disease during long-term follow-up when compared to sepsis survivors without diabetes (HR 1.25; 95% CI 1.22–1.29; Table 2). Cumulative incidence functions are shown in Fig. 2. Adult sepsis survivors with pre-existing diabetes experienced a higher hazard of myocardial infarction (HR 1.40; 95% CI 1.34–1.47; Table 2) and stroke (HR: 1.24; 95% CI 1.18–1.29; Table 2). No significant difference was noted for the competing risk of non-cardiovascular death during long-term follow-up (HR 1.02; 95% CI 1.00–1.03; Table 2) or recurrent sepsis during the first year after hospital discharge (IRR 1.01; 95% CI 1.00–1.03; Table 2).
Cumulative incidence function1 for the primary composite outcome of myocardial infarction, stroke, or cardiovascular death among adult sepsis survivors in Ontario (2008–2017). Based on a sub-distribution proportional hazards Fine and Gray model including pre-existing diabetes as a binary indicator. Major cardiovascular disease defined as the composite of myocardial infarction, stroke, or cardiovascular death
Modification of the effect of sepsis on cardiovascular disease by diabetes
The effect measure modification analysis is summarized in Table 3, Additional file 1: Table S4 and Table S5. Cohort description, and overall association of sepsis (compared to no sepsis) on cardiovascular disease can be found elsewhere [14]. On the multiplicative scale (i.e., HR or risk ratios (RR)), pre-existing diabetes did not modify the previously estimated effect of sepsis on major cardiovascular disease during long-term follow-up (HR 1.27; 95% CI 1.23–1.31 and HR: 1.31; 95% CI 1.28–1.34 for the effect of sepsis among patients with and without diabetes, respectively; p value for interaction = 0.10; Table 3). Similar results were estimated when using a log-binomial model (RR 1.23; 95% CI 1.19–1.27 and RR: 1.25; 95% CI 1.22–1.28 for the effect of sepsis among patients with and without diabetes, respectively; p value for interaction = 0.42; Table 3).
The absolute risk (up to 5 years) of cardiovascular disease ranged from 7.2% in patients without sepsis and no pre-existing diabetes to 12.2% among patients who survived a sepsis episode and had prevalent diabetes (Table 3). On the additive scale, pre-existing diabetes modified the effect of sepsis on major cardiovascular disease during long-term follow-up (risk difference: 2.3%; 95% CI 2.0–2.6 and risk difference: 1.8; 95% CI 1.6–2.0 for the effect of sepsis among patients with and without diabetes, respectively; p value for interaction < 0.01; Table 3).
Sensitivity analyses
The main effect estimates were similar across several sensitivity analyses, namely while (1) using Fine and Gray models (sub-distribution HR 1.24; 95% CI 1.21–1.28; Additional file 1: Table S6), (2) restricting to patients without chronic kidney disease (HR 1.21; 95% CI 1.18–1.25; Additional file 1: Table S7), (3) adjusting for acute kidney injury upon admission (HR 1.25; 95% CI 1.21–1.28; Additional file 1: Table S7), (4) adjusting for renal replacement therapy and mechanical ventilation during the index hospitalization (HR 1.25; 95% CI 1.21–1.29; Additional file 1: Table S7), (5) keeping within the analytical sample patients with pre-existing cardiovascular disease (HR 1.21; 95% CI 1.19–1.23; Additional file 1: Table S7), (6) considering those patients without diabetes but with an HbA1c greater than or equal to 6.5% as being exposed (HR 1.24; 95% CI 1.20–1.27; Additional file 1: Table S7), (7) adjusting for potential misclassification of the exposure (RR 1.25; 95% CI 1.20–1.30; Figure S1). Increasing HbA1c at baseline was associated with an increased risk of cardiovascular disease during long-term follow-up (Additional file 1: Table S8). The E-value for the point estimate and lower bound of the main analysis was 1.61 and 1.56, respectively (Figure S2). There was no evidence of relevant mediation of the effect of pre-existing diabetes on subsequent cardiovascular events through the receipt of renal replacement therapy during the sepsis hospitalization (Additional file 1: Table S7).
Discussion
Our study shows that adult sepsis survivors with prevalent diabetes experience a higher hazard of long-term cardiovascular outcomes compared to similar patients without diabetes. We also observed that sepsis survivors with and without diabetes are at a similarly elevated relative risk for cardiovascular disease compared to patients without sepsis. The absolute risk increase of cardiovascular events after sepsis is higher in patients with diabetes (i.e., on the additive scale, diabetes intensified the higher cardiovascular risk induced by sepsis).
Pre-existing diabetes represents a classic cardiovascular risk factor in the general population, whereas mounting evidence from observational studies show the increased risk of cardiovascular disease in sepsis survivors [14,15,16, 27, 51,52,53]. Furthermore, the potential role for diabetes as an independent risk factor for cardiovascular disease in adult sepsis survivors has also been recently highlighted [21, 23, 54]. Prior studies have shown how, among other baseline comorbidities and characteristics of the sepsis episode, pre-existing diabetes may be associated with a higher risk of cardiovascular disease following sepsis; however, these studies were not designed specifically to quantify the impact of diabetes and were undertaken mostly within a prediction framework [21, 23].
A strength of our study is the matching of patients with and without diabetes and exploring several cardiovascular outcomes of interest, while also considering characteristics of the sepsis hospitalization. Our study highlights how diabetes appears to increase the risk of experiencing future cardiovascular outcomes after sepsis but does not increase the risk of other outcomes including recurrent sepsis or all-cause mortality. Furthermore, the presence of additive effect measure modification by diabetes of the association between sepsis and cardiovascular outcomes suggests that, in addition to being considered as independent risk factors, the combination of both carries potentially the highest risk during long-term follow-up. This is in alignment with previous findings showing a differential effect of sepsis on cardiovascular disease introduced by both age and sex [14]. Such groups of sepsis survivors at highest risk of subsequent cardiovascular outcomes could be the focus of future research evaluating potential mitigation strategies [23, 55].
Our study has several limitations. First, sepsis was identified using an administrative algorithm, and some degree of misclassification is expected [36]. This misclassification is more likely to lead to missed sepsis cases but could also lead to some patients being falsely classified as having sepsis with resulting bias toward the null of no association. Similarly, pre-existing cardiovascular disease was defined using administrative coding and some degree of false negative results are expected. Additionally, algorithms do not differentiate between type 1 or type 2 diabetes, and we could not assess differences (if any) between them. Second, our results are subject to residual and unmeasured confounding, where the association between diabetes and cardiovascular disease may be explained by a third characteristic such as baseline frailty (e.g., both associated with diabetes and cardiovascular events) [56]. However, our E-value showed that such a potential unmeasured confounder would need to have a moderate strength of association with both the exposure and outcome to explain our findings [45]. Third, our assessment of long-term outcomes is likely subject to the competing risk of non-cardiovascular mortality, especially in the face of its high occurrence among sepsis survivors [41, 46, 57]; we used formal methods to take this into account and our estimates were robust across different modeling techniques, including Fine and Gray sub-distribution hazards models [46]. Fourth, we did not include information on medications for diabetes (not available in ICES for those patients younger than 65 years of age) that may further modify the cardiovascular risk post-sepsis, either through metabolic control or other pathways yet to be elucidated. This may be of particular importance for the case of metformin given some observational data pointing toward improved outcomes in the critically ill population [58, 59]. In addition, we did not have information on newly diagnosed (e.g., post-sepsis) diabetes after hospitalization that may also influence the risk of long-term cardiovascular disease in sepsis survivors. Whether diabetes is a potential component of the post-intensive care syndrome (and the impact of this on subsequent cardiovascular events in sepsis survivors) remains unknown [60]. Fifth, our outcome assessment was also based on administrative algorithms and as such, some degree of misclassification is expected; however, this is likely non-differential and toward the null of no association [39]. Sixth, we did not capture information on pre-existing obesity, which could affect or modify the risk of cardiovascular disease in adult sepsis survivors with and without diabetes.
In conclusion, sepsis survivors with pre-existing diabetes face a higher long-term hazard of experiencing major cardiovascular outcomes when compared to sepsis survivors without diabetes. Pre-existing diabetes further intensifies the effect of sepsis on major cardiovascular events during long-term follow-up. Future studies should evaluate which subgroups (if any) of patients with diabetes remain at highest risk, and whether improvements in metabolic control and specific prescription patterns can mitigate the cardiovascular risk after sepsis in patients with diabetes.
Data and code availability
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
References
Rudd KE, Kissoon N, Di L, Bory S, Mutahunga B, Seymour CW, et al. The global burden of sepsis: barriers and potential solutions. Crit Care. 2018;22:123305059.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Critic Care Med. 2021;49(11):1974–82. https://doi.org/10.1007/s00134-021-06506-y.
Buchman TG, Simpson SQ, Sciarretta KL, Finne KP, Sowers N, Collier M, Chavan S, Oke I, Pennini ME, Santhosh A, Wax M. Sepsis among medicare beneficiaries: 1. The burdens of sepsis, 2012–2018. Critic Care Med. 2020;48(3):276.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395:200–11.
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75.
Shankar-Hari M, Rubenfeld GD. Understanding long-term outcomes following sepsis: implications and challenges. Curr Infect Dis Rep. 2016;18:37.
Prescott HC, Costa DK. Improving long-term outcomes after sepsis. Crit Care Clin. 2018;34:175–88.
Shankar-Hari M, Harrison DA, Ferrando-Vivas P, Rubenfeld GD, Rowan K. Risk factors at index hospitalization associated with longer-term mortality in adult sepsis survivors. JAMA Netw Open. 2019;2:e194900.
Mankowski RT, Yende S, Angus DC. Long-term impact of sepsis on cardiovascular health. Intensive Care Med. 2019;45:78–81.
Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–83.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787.
Shankar-Hari M, Ambler M, Mahalingasivam V, Jones A, Rowan K, Rubenfeld GD. Evidence for a causal link between sepsis and long-term mortality: A systematic review of epidemiologic studies. Crit Care. 2016;20:1–13. https://doi.org/10.1186/s13054-016-1276-7.
Angriman F, Rosella L, Lawler P, Ko D, Wunsch H, Scales D. Sepsis hospitalization and risk of subsequent cardiovascular events in adults: a population-based matched cohort study. Intensive Care Med. 2022;48:448–57.
Kosyakovsky LB, Angriman F, Katz E, Adhikari NK, Godoy LC, Marshall JC, et al. Association between sepsis survivorship and long-term cardiovascular outcomes in adults: a systematic review and meta-analysis. Intensive Care Med. 2021;47:931–42. https://doi.org/10.1007/s00134-021-06479-y.
Jentzer JC, Lawler PR, Van Houten HK, Yao X, Kashani KB, Dunlay SM. Cardiovascular events among survivors of sepsis hospitalization: a retrospective cohort analysis. J Am Heart Assoc. 2023;12:e027813.
Rousseau AF, Prescott HC, Brett SJ, Weiss B, Azoulay E, Creteur J, et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021;25:1–7.
Estenssoro E, Loudet CI, Ríos FG, Edul VS, Plotnikow G, Andrian M, Romero I, Piezny D, Bezzi M, Mandich V, Groer C. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9(9):989–98.
Estenssoro E, Loudet CI, Edul VS, Osatnik J, Ríos FG, Vásquez DN, Pozo MO, Lattanzio B, Pálizas F, Klein F, Piezny D. Health inequities in the diagnosis and outcome of sepsis in Argentina: a prospective cohort study. Crit Care. 2019;23(1):1.
Abu-Ashour W, Twells L, Valcour J, Randell A, Donnan J, Howse P, et al. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res Care. 2017;5:336.
Walkey AJ, Knox DB, Myers LC, Thai KK, Jacobs JR, Kipnis P, et al. Prognostic accuracy of presepsis and intrasepsis characteristics for prediction of cardiovascular events after a sepsis hospitalization. Crit Care Explor. 2022;4:e0674.
Shankar-Hari M, Saha R, Wilson J, Prescott HC, Harrison D, Rowan K, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. 2020;46:619–36. https://doi.org/10.1007/s00134-019-05908-3.
Angriman F, Rosella LC, Lawler PR, Ko DT, Martin CM, Wunsch H, Scales DC. Risk factors for major cardiovascular events in adult sepsis survivors: a population-based cohort study. Crit Care Med. 2023;51(4):471–83.
Yende S, van der Poll T. Diabetes and sepsis outcomes – it is not all bad news. Crit Care. 2009;13:117.
Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:1–6.
Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–6.
Ke C, Lipscombe LL, Weisman A, Zhou L, Austin PC, Shah BR, et al. Trends in the association between diabetes and cardiovascular events, 1994–2019. JAMA. 2022;328:1866–9.
Costantini E, Carlin M, Porta M, Brizzi MF. Type 2 diabetes mellitus and sepsis: state of the art, certainties and missing evidence. Acta Diabetol. 2021;58:1139–51. https://doi.org/10.1007/s00592-021-01728-4.
Wang Z, Ren J, Wang G, Liu Q, Guo K, Li J. Association between diabetes mellitus and outcomes of patients with sepsis: a meta-analysis. Med Sci Monit. 2017;23:3546.
Schuetz P, Castro P, Shapiro NI. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care. 2011;34:771–8. https://doi.org/10.2337/dc10-1185.
Akinosoglou K, Kapsokosta G, Mouktaroudi M, Rovina N, Kaldis V, Stefos A, et al. Diabetes on sepsis outcomes in non-ICU patients: a cohort study and review of the literature. J Diabetes Complicat. 2021;35:107765.
Shah BR, Victor JC, Chiu M, Tu JV, Anand SS, Austin PC, Manuel DG, Hux JE. Cardiovascular complications and mortality after diabetes diagnosis for South Asian and Chinese patients: a population-based cohort study. Diabetes Care. 2013;36(9):2670–6.
Jiang L, Cheng M. Impact of diabetes mellitus on outcomes of patients with sepsis: an updated systematic review and meta-analysis. Diabetol Metabol Syndr. 2022;14(1):1–7.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7.
Jolley RJ, Quan H, Jette N, Sawka KJ, Diep L, Goliath J, et al. Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open. 2015;5:1–10.
Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jetté N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19:139.
Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–6.
Porter J, Mondor L, Kapral MK, Fang J, Hall RE. How reliable are administrative data for capturing stroke patients and their care. Cerebrovasc Dis Extra. 2016;6:96–106.
Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, et al. The cardiovascular health in ambulatory care research team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:2014–2012.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–9.
Austin PC, Type I. Error rates, coverage of confidence intervals, and variance estimation in propensity-score matched analyses. Int J Biostat. 2009;5:13. https://doi.org/10.2202/1557-4679.1146/html.
Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6.
Pedroza C, Truong VT. Performance of models for estimating absolute risk difference in multicenter trials with binary outcome. BMC Med Res Methodol. 2016;16(1):1–2.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. https://doi.org/10.7326/M16-2607.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496.
Paik JM, Patorno E, Zhuo M, Bessette LG, York C, Gautam N, et al. Accuracy of identifying diagnosis of moderate to severe chronic kidney disease in administrative claims data. Pharmacoepidemiol Drug Saf. 2022;31:467–75.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. https://doi.org/10.1002/sim.3697.
VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32.
Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol. 2014;43:1969–85.
Ou S-MM, Chu H, Chao P-WW, Lee Y-JJ, Kuo S-CC, Chen T-JJ, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors a nationwide population-based study. Am J Respir Crit Care Med. 2016;194:209–17.
Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189:1065–74.
Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CCH, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–74.
Myers LC, Knox D, Thai KK, Kipnis P, Jacobs J, Lee C, et al. Predicting post-sepsis cardiovascular events with death as a competing risk. Ann Am Thorac Soc. 2022. https://doi.org/10.1513/AnnalsATS.202206-536RL.
Angriman F, Rosella LC, Lawler PR, Ko DT, Martin CM, Wunsch H, et al. Renin-angiotensin system inhibitors and major cardiovascular events after sepsis. Ann Am Thorac Soc. 2023;20:414.
Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. Ann Am Thorac Soc. 2019;16:22–8.
Mccaw ZR, Claggett BL, Tian L, Solomon SD, Berwanger O, Pfeffer MA, et al. Practical recommendations on quantifying and interpreting treatment effects in the presence of terminal competing risks: a review. JAMA Cardiol. 2021;7:450–6.
Jin BY, Song J, Kim J, Park JH, Kim SJ, Cho H, et al. Association between metformin and survival outcomes in in-hospital cardiac arrest patients with diabetes. J Crit Care. 2023;73:154171.
Gómez H, Del Rio-Pertuz G, Priyanka P, Manrique-Caballero CL, Chang CCH, Wang S, et al. Association of metformin use during hospitalization and mortality in critically Ill adults with type 2 diabetes mellitus and sepsis. Crit Care Med. 2022;50:935–44.
Preiser JC, de Longueville C. Could type 2 diabetes be a component of the post-intensive care syndrome? Crit Care. 2017;21:1–2. https://doi.org/10.1186/s13054-017-1607-3.
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from the Sepsis Canada Network (grant number 2021-1870). Parts of this material are based on data and information compiled and provided by Ontario Ministry of Health (MOH). The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. Parts of this report are based on Ontario Registrar General (ORG) information on deaths, the original source of which is ServiceOntario. The views expressed therein are those of the author and do not necessarily reflect those of ORG or the Ministry of Government and Consumer Services. Dr. Angriman is partially supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research and a Research Award from the Interdepartmental Division of Critical Care Medicine at the University of Toronto. Dr. Scales holds operating grants from the Canadian Institute for Health Research. Dr. Lawler is supported by a Heart and Stroke Foundation of Canada National New Investigator Award. Dr. Shah is supported by the University of Toronto as the Novo Nordisk Research Chair in Equitable Care of Diabetes and Related Conditions. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. The authors would like to acknowledge the support of the Acute & Intensive Care Outcomes Research Network (AICORN) during the conduct of the present research. The authors would also like to acknowledge the thoughtful feedback of Denis Boutin, Dr. Barna De, and Dr. Hertzel C. Gerstein on a previous version of this manuscript.
Funding
Sepsis Canada Network (grant number 2021-1870).
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation and data analysis were completed by FA and DCS. All authors contributed to data interpretation. The first draft of the manuscript was written by FA and DCS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The use of data in this project was authorized under Sect. 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Competing interests
The authors declare NO conflict of interest relevant to the contents of the present manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary appendix.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Angriman, F., Lawler, P.R., Shah, B.R. et al. Prevalent diabetes and long-term cardiovascular outcomes in adult sepsis survivors: a population-based cohort study. Crit Care 27, 302 (2023). https://doi.org/10.1186/s13054-023-04586-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04586-4