Abstract
Sepsis is life-threating organ dysfunction due to infection. Incidence of sepsis is increasing and the short-term mortality is improving, generating more sepsis survivors. These sepsis survivors suffer from additional morbidities such as higher risk of readmissions, cardiovascular disease, cognitive impairment and of death, for years following index sepsis episode. In the first year following index sepsis episode, approximately 60 % of sepsis survivors have at least one rehospitalisation episode, which is most often due to infection and one in six sepsis survivors die. Sepsis survivors also have a higher risk of cognitive impairment and cardiovascular disease contributing to the reduced life expectancy seen in this population, when assessed with life table comparisons. For optimal design of interventional trials to reduce these bad outcomes in sepsis survivors, in-depth understanding of major risk factors for these morbid events, their modifiability and a causal relationship to the pathobiology of sepsis is essential. This review highlights the recent advances, clinical and methodological challenges in our understanding of these morbid events in sepsis survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis has been redefined recently as life-threatening organ dysfunction caused by dysregulated host responses to infection and septic shock as a subset of sepsis in which particularly profound circulatory, cellular and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone [1, 2].
Globally, sepsis is common, with an estimated population incidence of 270 (95 % CI 176–412) cases per-100,000 person-years and acute mortality of 26.0 %[3]. A number of reasons suggest even this underestimates the magnitude of sepsis associated mortality and morbidity. First, as the authors’ of this paper [3] highlight, the incidence data is primarily critical care based, with limited data from low- and middle-income-countries. Second, at the bedside, sepsis cases represent either a new organ dysfunction or worsening of chronic organ dysfunction such as those seen in comorbid conditions [1], in the context of suspected or proven infection. The literature on prevalence of organ dysfunction outside the critical care environment is limited and when estimated appears frequently [4]. Alongside this underestimated incidence globally, the short-term mortality from sepsis is improving [5, 6]. This epidemiology pattern generates approximately 14 million sepsis survivors globally [3], increasing yearly, with ongoing health care needs [7].
In this background, after highlighting the conceptual approach and methodological challenges, this review focuses on the additional long-term risk of death, readmissions, cardiovascular disease, cognitive impairment and quality of life (QOL) alterations in sepsis survivors, followed by a brief overview of biological mechanisms contributing to these outcomes.
Conceptual Approach
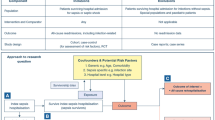
Sepsis and the outcomes following sepsis are best conceptualized as consequences of a complex interplay between baseline characteristics including pre-illness health status, risk factors for infection, dysregulated immune responses and those for developing acute organ dysfunction, health care setting, treatments provided and the response to treatments. The risk factors for infection include extremes of age, male sex, comorbidities, race, genetics, prior sepsis, surgery, any hospitalisation and frailty [8–16]. There is no accepted definition for dysregulated immune responses in sepsis [17], the risk factors for infection highlighted and potentially genetic variations [18] determine these immune responses. The mechanisms of organ dysfunction in sepsis are still debated [19, 20]; the risk factors for infection and immune responses are considered risk factors for organ dysfunction. In addition to short-term outcomes, all these characteristics also influence the long-term outcomes in sepsis survivors [21–24, 25••]. One of the most important lessons from the last decade of studying long-term outcomes of sepsis and other critical illnesses is that poor functional status is a risk factor for becoming critically ill as well as a frequent consequence. Similarly, many comorbidities, age and chronic diseases are risk factors both for sepsis and for impaired quality of life. Therefore, it is important in reviewing the literature to distinguish studies that have tried to separate out the potentially causal effects of sepsis from those that simply describe morbidity and mortality events [25••]. Understanding this fundamental concept helps to identify risk factors including those that are changeable, quantify this modifiable risk and target specific interventions at specific time-points in survivor trajectory to improve health in sepsis survivors (Fig. 1).
Conceptual framework for multiple interacting factors influencing sepsis associated long-term outcomes. The red triangle highlights the vicious cycle between pre-illness morbidity—sepsis—long-term outcomes pathway. The arrows represent direction of relationship between factors. Sepsis occurs in healthy and in subjects with co-morbidities, acutely altering pre-event health state. Thus, complex interactions between pre-sepsis health state and additional morbidity and frailty related to sepsis illness influence post-sepsis health state in sepsis-survivors
Challenges
There are a number of challenges to studying the potentially causal post-acute residual effects of sepsis. First, when studying risk factors and assessing modifiable risk, the acute effects of sepsis have to be separated by specifically looking for independent associations in the sepsis survivors population as opposed to the admission cohort with sepsis. Second, dose-response effect is important for biological plausibility arguments. An increase in sepsis severity may worsen post-acute sequelae, however, it will also increase the probability of short-term mortality. Therefore, the competing risk of death may hide the longer-term non-mortality effects of sepsis. Third, studies evaluating the long-term outcomes following sepsis often do not account for pre-illness trajectory. When this is accounted for, the independent effects of sepsis on long-term outcomes generate different results [9]. Fourth, studies without control arms will not provide an estimate of sepsis attributable risk [25••, 26••] and therefore are of little value in planning interventions. Fifth, if the long-term outcome studied cannot be defined for all individuals in a cohort either due to death or loss to follow-up and this missing data is differential, bias due to truncation-by-death occurs [27]. Typically, this occurs when evaluating long-term quality of life in survivors using follow up data from clinical trials and observational studies, when there is missing data from those sepsis survivors who die prior to obtaining a quality of life estimate. In the scenario where trial data is used, the probability of death in itself may be altered by the intervention and the baseline balance of randomisation is lost when assessing survivors. In observational studies, those survivors with missing data may be systematically different to those who have follow up data.
Methodological Approaches to Overcome Challenges
There are two ideal study designs to measure the causal effects of sepsis on long-term outcomes: a yet to be done large cohort study starting with a healthy population well before index sepsis episode to understand pre-illness trajectory, then these patients are followed through after sepsis with detailed measures of health status, processes of care, and biologic measures or an impossible controlled trial that randomly induces sepsis. The available observational studies use alternative methods to address this question with attendant limitations. To ascertain the pre-illness trajectory, a health care contact look back could be done and a look back period between one and 5 years is considered appropriate [28]. To account for bias secondary to lack of randomisation, there are numerous methods, the most well recognised being propensity score based matching. This approach compares the outcomes of patients who get septic with controls who were equally likely to get septic but did not [29]. The principal limitation being unobserved confounders may vary even after adjustment and matching. When studying long-term effects of sepsis, there are time-varying confounders and mediators such as age, comorbid conditions, chronic medications, ageing, frailty. Marginal structural models (MSMs) provide population-averaged causal effects, which go beyond the statistically independent associations generated by regression models and post estimation statistics [30]. Studies without control populations (patients without sepsis as exposure), sepsis attributable risk is imperceptible and is prone to inference bias if the control population chosen is not similar to sepsis cohort in all aspects except for the exposure (sepsis). This important concept is highlighted using cardiovascular events in sepsis survivors, and it is this attributable risk is what we hope to reduce using interventional studies.
Long-Term Outcomes
Mortality in Sepsis Survivors
Using fundamental principles of causality [31], we recently reported the limited strength of epidemiological evidence supporting the premise that sepsis causes an increase in all-cause mortality after hospital discharge (post-acute mortality) [25••]. Although the 1-year post-acute mortality was 16.1 % (95%CI =14.1–18.1 %; N = 43 studies), there was evidence of statistical and clinical heterogeneity, bias and residual confounding. Post-acute mortality was associated with age, male, co-morbidities, and deterioration in health prior to sepsis. Since publication of our systematic review, there has been two further publications [32•, 33•]. The well-conducted study by Prescott et al. focussed on patients older than 65 years and used double robust analysis [34] with a regression model for mortality and propensity score to account for covariates to show that sepsis is an independent risk factor for post-acute mortality [32•]. Using propensity score matching, Ou et al. also showed an increase in all cause mortality in sepsis survivors [33•]. Sepsis associated increased risk of death extends for up to ten years following index hospitalisation [25••, 32•, 33•, 35].
The magnitude of sepsis-post-acute mortality association varied dependent on the control population chosen for comparison. The additional hazard associated with sepsis was greatest when compared with general populations [25••]. The magnitude of additional risk of death in sepsis survivors decreased when the selected control population had more severe non-sepsis illnesses. The major implication being that the literature currently over estimates the risk of post-acute death caused by sepsis and therefore preventable fraction is likely to be lower, which needs to be accounted for during sample size estimation in future RCTs designed to reduce late mortality in sepsis survivors.
Rehospitalisation in Sepsis Survivors
Compared to non-sepsis admissions, sepsis survivors had a greater risk of rehospitalisation [10, 36–38], and rehospitalisation increases the risk of death. The average 30-day rehospitalisation rates in sepsis survivors are between 19.9 and 32 % [7, 36–43]. The cumulative rehospitalisation rates increases with follow-up time with the 90-day and 1-year rehospitalisation rates being 40 % [7, 36] and 63.0 % [7], respectively, implying persistent risk in sepsis survivors. Pneumonia survivors have lower 30-day rehospitalisation rates (16.5 % [44] and 18.2 % [45••]), implying potentially a dose-response effect.
The additional readmissions risk was significantly more common in sepsis survivors with matched hospitalisations with acute medical conditions comparisons and a proportion of these readmissions are potentially preventable [36]. In a retrospective cohort study, except for parenteral nutrition, the other risk factors for readmissions identified in univariate analyses such as transfusion, duration of antibiotics, disappeared after regression analyses to account for confounding [38]. In contrast, 30-day rehospitalisation after sepsis has been shown to be independently associated included age, malignancy diagnosis, hospitalizations in the year prior to sepsis admission and low haemoglobin concentrations at discharge [37].
The most common reason for rehospitalisation in sepsis survivors was infection [46] and infection-related rehospitalisation represented either unresolved/recurrent infection or new infections [38]. The risk factors and magnitude of infection-related readmissions differed based on definition used, implying clinical heterogeneity and confounding by indication in studies. Infection-related rehospitalisation was independently associated with index admission pathogens (multidrug resistant pathogens, E. Coli spp. and Bacteroides spp. infections), renal dysfunction and urinary tract source, with E Coli spp. and urinary tract being protective [43]. The most common site in infection-related rehospitalisation in sepsis survivors was pneumonia, whereas it was urosepsis in non-sepsis survivor rehospitalisation [46]. In this study, other independent risk factors for infection-related rehospitalisation were prolonged hospitalization, age and the presence of an indwelling catheter [46], implying admission case-mix is an important determinant for infected-related rehospitalisation in sepsis survivors.
As with post-acute mortality, further research is required to understand the true preventable fraction and modifiable risk factors for rehospitalisation, in particular infection-related rehospitalisation. Most studies censor death, which is a competing risk factor for readmissions. Independent associations generated by regression models represent strength of this relationship not a causal pathway, which is yet to be proven. The duration of additional risk of infection-related rehospitalisation and the underpinning mechanisms needs to be further characterised.
Cognitive Impairment in Sepsis Survivors
Hospitalization, regardless of aetiology, is associated with cognitive decline [47, 48]. The relationship between sepsis and cognitive decline is likely to be complex and bidirectional as pre-illness cognitive decline is a risk factor for pneumonia and sepsis [9, 49, 50••] and sepsis is also an independent risk factor for cognitive decline (OR 95 % CI 3.3 (1.5–7.3)) [49]. Thus, there is a threefold increase in risk of cognitive decline in sepsis survivors compared to control populations, but the rate of new cognitive decline in those sepsis survivors is similar to that seen with pre-illness deterioration [49]. Similarly, pneumonia is associated with cognitive decline and increases the hazard for dementia (OR 95 % CI 2.2 (1.6–3.6)) [50••]. This hazard ratio does not vary with sepsis severity, implying no dose response effect [50••]. Depression, which is common in sepsis survivors, is also a risk factor for pneumonia [51] and sepsis [52] and is significantly associated with post sepsis functional impairment [52].
Delirium is common during critical illness, prolongs hospitalisation and has minimal attributable mortality [53•]. Acute delirium is also associated with cognitive impairment including dementia and depression during longer-term follow-up of critical illness survivors [54, 55] and in hospitalised patients [56]. Sepsis is associated with delirium [57] and pre-sepsis episode cognitive decline. Thus, the post-sepsis cognitive decline could either have a causal relationship with sepsis or could be unrelated to sepsis but acting via the delirium pathway. A related concept that is difficult to test with new-onset cognitive decline or dementia is the extent of cognitive reserve, whether and how this reserve is modified by sepsis. This is important as incident cognitive decline is related to cognitive reserve and depends on follow-up duration [58].
Cardiovascular Complications in Sepsis Survivors
In the last 3 years, numerous studies have reported the long-term risk of cardiovascular events in sepsis survivors. Corrales-Medina et al. followed two community-based cohorts (>65 years of age and 45–64 years of age) and assessed the relationship between index pneumonia episode and subsequent cardiovascular disease (CVD) events over a 10-year period [59]. In this study, CVD events were myocardial infarction, stroke and fatal coronary heart disease. The older cohort had greater CVD events (34.9 % compared to 16.5 %). In both cohorts, sepsis was an independent risk factor for CVD events and the risk was greatest in the year following index pneumonia episode [59]. Of note, there were residual imbalances in covariates (e.g. hypertension, diabetes mellitus, smoking), despite incidence density sampling used to match cases to controls in this study [59]. Using propensity score based matching; Yende et al. assessed the independent risk of cardiovascular events after sepsis as an explanation for long term increased mortality risk in sepsis survivors [26••]. The primary outcome was the 1-year incidence rate of hospitalised cardiovascular events in sepsis survivors. Sepsis survivors had statistically significant higher rate of cardiovascular events when compared to propensity matched critically ill, hospitalised infected controls, and hospitalised non-infected controls. However, the magnitude of risk attributable to sepsis was minimal when cardiovascular event risks of acute care and baseline characteristics were accounted for [26••]. Jafarzadeh et al. assessed the cumulative 5-year risk of cardiovascular events in sepsis and used MSMs to generate a causal inference of additional risk. The odds ratio for sepsis associated any CVD was 2.39 (1.88–3.03), and there was a dose response with increase in severity (Bacteremia = 1.52 (1.21–1.90); Sepsis = 3.60 (2.59–5.00); and Septic shock = 4.55 (3.58–5.78)). In a community acquired bacteremia cohort from Denmark with matched general population controls and hospital controls, Dalager-Pedersen et al. show that the risk of myocardial infarction is greatest in the first 30-days following bacteremia. This independent association disappears by 6 months following index bacteremia [60]. Similar duration of additional risk for CVD events has been reported in other cohorts [33•, 61].
Quality of Life in Sepsis Survivors
Winters et al. performed a systematic review and identified 12 studies that used different validated tools to assess the QOL in sepsis survivors, the most common being Short Form 36 (SF-36) [62]. Overall, sepsis survivors have impaired QOL compared to population norms [62, 63] and this impaired QOL lasts as long as 5-years after index admission [64]. To account for pre-illness status and truncation-by-death issues highlighted earlier, Yende et al. used patients enrolled in two randomised controlled trials and who lived independently prior to the index sepsis episode to show impaired QOL in sepsis survivors [65]. However, the SF-36 scores in sepsis survivors were similar to patients with comorbid conditions such as chronic obstructive pulmonary disease, hypertension and congestive heart failure [66]. Sepsis survivors also have similar QOL to non-sepsis critically ill survivors [64, 66–68]. Prehospital QOL appears to be an important determinant of QOL after discharge following hospitalisation and the magnitude of additional risk due to critical illness is small compared to all cause hospitalisations [69]. These observations challenge a causal inference of sepsis effect on QOL.
Biological Explanations for Long-Term Effects in Sepsis Survivors
Despite the robustness of statistical methods and independent associations with some of the long-term outcomes following sepsis, the epidemiological causality and biological mechanisms are yet to be proven. The cause of death in sepsis survivors is seldom reported, and the mechanisms leading to accelerated death in sepsis survivors, if this observation is causal, are uncertain. Sepsis often occurs in patients with comorbid conditions [13, 70], frail and elderly [8]. Many of the long-term outcomes reported in sepsis survivors are also common in elderly and frail populations, implying accelerated ageing could be a potential mechanism. Aging is a multisystem process characterised by cell damage, responses to these cell damage events and the ensuing phenotype. The cell damage is considered secondary to genomic instability, telomere attrition and epigenetic alterations [71], which is a nascent literature in sepsis [72–74]. The cell damage events seen in aging results in abnormalities of nutrient sensing, mitochondrial function and cellular senesce [71], some of which are also seen in sepsis. Ageing results in impaired lymphopoiesis with relative preservation of myelopoiesis, with reduction in stem cells. The T cell memory pool is increased, with skewed expansion of CD8 T cells and CD4 differentiation into Th17 population with reduced B cell diversity. The lymphocytes from sepsis patients share a number of these features [75], implying accelerated aging of the immune system in sepsis.
In well-characterised caecal ligation and puncture murine models of sepsis, sepsis results in sustained central nervous system inflammation after recovery, which could explain the long-term cognitive impairments reported [76]. In similar murine models, elevated serum levels of high mobility group box 1 (HMGB1) was associated with cognitive dysfunction [77] and is hypothesised to act via the up regulated receptor for advanced glycation end products (RAGE) [78]. Thus, the hypothesis that neuronal inflammation in sepsis survivors explains cognitive impairment should be studied. Kaynar et al. did a RCT using CLP sepsis models in murines predisposed to atherosclerosis (ApoE-deficient) and with wild-type mice [79]. The authors showed that sepsis resulted in persistent inflammation that predisposes to accelerated atherosclerosis [79], which provides an explanation for CVS risk in sepsis survivors in addition to baseline demographic and other risk factors. In the future, understanding of mechanisms underpinning this accelerated atherosclerosis may identify potential intervention targets.
Readmissions due to infections in sepsis survivors could be either due to impaired immune system functions and/or imbalances in the gastrointestinal microbial flora (dysbiosis) in sepsis. The acute immune responses in sepsis characterised using pan leukocyte transcriptome profiles show activation of pro-inflammatory and anti-inflammatory pathways in the innate and adaptive immune systems and leaves residual immunosuppression in sepsis survivors [17, 80–82]. The mechanisms for post-acute immunosuppression in sepsis survivors are not well understood. To date, one pilot study involving just eight patients highlight abnormalities in T cells and impaired cytokines responses to extrinsic stimuli, long-after index sepsis episode [83]. Aside from the small sample size, the variable time interval from index sepsis episode to immune assessments (between 9 and 60 months) and high risk of bias preclude meaningful inferences. Similar changes were also observed in splenocytes and lung parenchyma in a landmark paper studying deaths from protracted sepsis [84]. A healthy microbiome consists of obligate anaerobes (e.g. Bacteroidetes, Firmicutes), and facultative anaerobes (e.g. Proteobacteria) with diverse with metabolic functions that maintain check on pathological bacterial density [85–87]. Acute illness, hospitalisation and antibiotics alter this balance towards a simpler and potentially pathogenic gut bacterial flora alongside increase in risk for Clostridium Difficile infections [87–89]. In a study that did not provide any microbiological evidence but presumed dysbiosis based on the microbiome literature, suggested dysbiosis as a risk factor for infection related rehospitalisation in sepsis survivors [10]. Thus, it is valid to hypothesise that both immunosuppression and dysbiosis in sepsis survivors potentially contribute to infection related rehospitalisation. Further studies are required to confirm these hypotheses.
Conclusions
Sepsis survivors have a different health trajectory prior to and following their acute illness, but the potentially causal role of sepsis on the observed long-term impairment and survival remains unclear. For some clinicians and health care administrators, the question of causality is irrelevant. It is a fact that survivors of sepsis, as well as many other acute illnesses requiring hospitalization, are profoundly functionally impaired and their health care needs as well as their caregivers’ needs must be addressed. However, for investigators designing interventions to prevent or treat these long-term sequelae, a fundamental understanding of the clinical and biological mechanisms causing these long-term morbid events in sepsis survivors is required.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10. doi:10.1001/jama.2016.0287.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock. JAMA. 2016;315(8):775. doi:10.1001/jama.2016.0289.
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis - Current Estimates and Limitations. Am J Respir Crit Care Med. 2015. doi:10.1164/rccm.201504-0781OC.
Churpek MM, Zadravecz FJ, Winslow C, Howell M, Edelson DP. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am J Respir Crit Care Med. 2015. doi:10.1164/rccm.201502-0275OC.
Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med. 2014;42(3):625–31. doi:10.1097/CCM.0000000000000026.
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308–16. doi:10.1001/jama.2014.2637.
Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–9. doi:10.1164/rccm.201403-0471OC.
Aalen OO, Valberg M, Grotmol T, Tretli S. Understanding variation in disease risk: the elusive concept of frailty. Int J Epidemiol. 2015;44(4):1408–21. doi:10.1093/ije/dyu192.
Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–41. doi:10.1164/rccm.201109-1660OC.
Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192(5):581–8. doi:10.1164/rccm.201503-0483OC.
Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–16.
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10.
Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–82. doi:10.1097/01.CCM.0000239114.50519.0E.
Chapman SJ, Hill AV. Human genetic susceptibility to infectious disease. Nat Rev Genet. 2012;13(3):175–88. doi:10.1038/nrg3114.
Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–63. doi:10.1001/archinte.167.15.1655.
Yende S, Iwashyna TJ, Angus DC. Interplay between sepsis and chronic health. Trends Mol Med. 2014;20(4):234–8. doi:10.1016/j.molmed.2014.02.005.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. doi:10.1038/nri3552.
Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med. 2016. doi:10.1016/s2213-2600(16)00046-1.
Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi:10.1097/SHK.0000000000000052.
Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187(5):509–17. doi:10.1164/rccm.201211-1983OC.
Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc. 2002;50(6):1108–12. doi:10.1046/j.1532-5415.2002.50268.x.
Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289(18):2387–92. doi:10.1001/jama.289.18.2387.
Lone NI, Walsh TS. Impact of intensive care unit organ failures on mortality during the five years after a critical illness. Am J Respir Crit Care Med. 2012;186(7):640–7. doi:10.1164/rccm.201201-0059OC.
Yende S, D’Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–7. doi:10.1164/rccm.200712-1777OC.
Shankar-Hari M, Ambler M, Mahalingasivam V, Jones A, Rowan K, Rubenfeld G. Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Critical Care. 2016;20(1):101. This systematic review challenges the causal relationship between sepsis and long-term mortality. Highlights methodological issues in the literatures.
Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189(9):1065–74. doi:10.1164/rccm.201307-1321OC. Landmark paper on cadiovascular events in sepsis survivors and value of control arms in determining attributable risk to sepsis.
McConnell S, Stuart EA, Devaney B. The truncation-by-death problem: what to do in an experimental evaluation when the outcome is not always defined. Eval Rev. 2008;32(2):157–86. doi:10.1177/0193841X07309115.
Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for charlson comorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128–39.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786.
Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60.
Hofler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol. 2005;2:11. doi:10.1186/1742-7622-2-11.
Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353. doi:10.1136/bmj.i2375. Highlights post-acute mortality in sepsis survivors using novel statistical methods.
Ou SM, Chu H, Chao PW, Lee YJ, Kuo SC, Chen TJ, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors. A nationwide population-based study. Am J Respir Crit Care Med. 2016;194(2):209–17. doi:10.1164/rccm.201510-2023OC. Highlights post acute mortality in sepsis survivors.
Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–7. doi:10.1093/aje/kwq439.
Linder A, Guh D, Boyd JH, Walley KR, Anis AH, Russell JA. Long-term (10-year) mortality of younger previously healthy patients with severe sepsis/septic shock is worse than that of patients with nonseptic critical illness and of the general population. Crit Care Med. 2014;42(10):2211–8. doi:10.1097/CCM.0000000000000503.
Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–7. doi:10.1001/jama.2015.1410.
Jones TK, Fuchs BD, Small DS, Halpern SD, Hanish A, Umscheid CA, et al. Post-acute care use and hospital readmission after sepsis. Ann Am Thorac Soc. 2015;12(6):904–13. doi:10.1513/AnnalsATS.201411-504OC.
Sun A, Netzer G, Small DS, Hanish A, Fuchs BD, Gaieski DF, et al. Association between index hospitalization and hospital readmission in sepsis survivors. Crit Care Med. 2016;44(3):478–87. doi:10.1097/CCM.0000000000001464.
Donnelly JP, Hohmann SF, Wang HE. Unplanned readmissions after hospitalization for severe sepsis at academic medical center-affiliated hospitals. Crit Care Med. 2015;43(9):1916–27. doi:10.1097/CCM.0000000000001147.
Chang DW, Tseng CH, Shapiro MF. Rehospitalizations following sepsis: common and costly. Crit Care Med. 2015;43(10):2085–93. doi:10.1097/CCM.0000000000001159.
Ortego A, Gaieski DF, Fuchs BD, Jones T, Halpern SD, Small DS, et al. Hospital-based acute care use in survivors of septic shock. Crit Care Med. 2015;43(4):729–37. doi:10.1097/ccm.0000000000000693.
Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43(4):738–46. doi:10.1097/CCM.0000000000000859.
Zilberberg MD, Shorr AF, Micek ST, Kollef MH. Risk factors for 30-day readmission among patients with culture-positive severe sepsis and septic shock: a retrospective cohort study. J Hosp Med. 2015;10(10):678–85. doi:10.1002/jhm.2420.
Borzecki AM, Chen Q, Restuccia J, Mull HJ, Shwartz M, Gupta K et al. Do pneumonia readmissions flagged as potentially preventable by the 3M PPR software have more process of care problems? A cross-sectional observational study. BMJ Qual Saf. 2015. doi:10.1136/bmjqs-2014-003911.
Krumholz HM, Lin Z, Keenan PS, Chen J, Ross JS, Drye EE, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–93. doi:10.1001/jama.2013.333. Landmark paper on readmissions literature.
Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29(2):87–95. doi:10.1177/0885066612467162.
Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–70. doi:10.1001/jama.2010.167.
Mathews SB, Arnold SE, Epperson CN. Hospitalization and cognitive decline: can the nature of the relationship be deciphered? Am J Geriatr Psychiatry. 2014;22(5):465–80. doi:10.1016/j.jagp.2012.08.012.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi:10.1001/jama.2010.1553.
Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188(5):586–92. doi:10.1164/rccm.201212-2154OC. Important paper that addresses challenges with pre-illness cognitive decline and pneumonia.
Davydow DS, Hough CL, Zivin K, Langa KM, Katon WJ. Depression and risk of hospitalization for pneumonia in a cohort study of older Americans. J Psychosom Res. 2014;77(6):528–34. doi:10.1016/j.jpsychores.2014.08.002.
Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry. 2013;21(9):887-97. doi:10.1016/j.jagp.2013.01.017. 10.1097/JGP.0b013e31825c0aed.
Klein Klouwenberg PM, Zaal IJ, Spitoni C, Ong DS, van der Kooi AW, Bonten MJ, et al. The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ. 2014;349:g6652. doi:10.1136/bmj.g6652. Conceptual paper exploring acute delerium and attributable mortality.
Wolters AE, van Dijk D, Pasma W, Cremer OL, Looije MF, de Lange DW, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care. 2014;18(3):R125. doi:10.1186/cc13929.
Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–20. doi:10.1097/CCM.0b013e3181e47be1.
Popp J. Delirium and cognitive decline: more than a coincidence. Curr Opin Neurol. 2013;26(6):634–9. doi:10.1097/wco.0000000000000030.
Tsuruta R, Oda Y. A clinical perspective of sepsis-associated delirium. J Intensive Care. 2016;4(1):1–7. doi:10.1186/s40560-016-0145-4.
Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17(4):593–601. doi:10.1017/s1355617710001748.
Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–74. doi:10.1001/jama.2014.18229.
Dalager-Pedersen M, Sogaard M, Schonheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014;129(13):1387–96. doi:10.1161/circulationaha.113.006699.
Mejer N, Gotland N, Uhre ML, Westh H, Schonheyder HC, Petersen A, et al. Increased risk of arterial thromboembolic events after Staphylococcus aureus bacteremia: a matched cohort study. J Infect. 2015;71(2):167–78. doi:10.1016/j.jinf.2015.03.010.
Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–83. doi:10.1097/CCM.0b013e3181d8cc1d.
Nesseler N, Defontaine A, Launey Y, Morcet J, Malledant Y, Seguin P. Long-term mortality and quality of life after septic shock: a follow-up observational study. Intensive Care Med. 2013;39(5):881–8. doi:10.1007/s00134-013-2815-1.
Uthbertson BH, Elders A, Hall S, Taylor J, MacLennan G, Mackirdy F, et al. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70. doi:10.1186/cc12616.
Yende S, Austin S, Rhodes A, Finfer S, Opal S, Thompson T et al. Long-Term Quality of Life Among Survivors of Severe Sepsis: Analyses of Two International Trials. Crit Care Med. 2016. doi:10.1097/CCM.0000000000001658.
Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28(11):3599–605.
Granja C, Dias C, Costa-Pereira A, Sarmento A. Quality of life of survivors from severe sepsis and septic shock may be similar to that of others who survive critical illness. Crit Care. 2004;8(2):R91–8. doi:10.1186/cc2818.
Orwelius L, Lobo C, Teixeira Pinto A, Carneiro A, Costa-Pereira A, Granja C. Sepsis patients do not differ in health-related quality of life compared with other ICU patients. Acta Anaesthesiol Scand. 2013;57(9):1201–5. doi:10.1111/aas.12164.
Feemster LC, Cooke CR, Rubenfeld GD, Hough CL, Ehlenbach WJ, Au DH et al. The Influence of Hospitalization or ICU Admission on Declines in Health-Related Quality of Life. Ann Am Thorac Soc. 2014. doi:10.1513/AnnalsATS.201404-172OC.
Esper AM, Martin GS. The impact of comorbid [corrected] conditions on critical illness. Crit Care Med. 2011;39(12):2728–35. doi:10.1097/CCM.0b013e318236f27e.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi:10.1016/j.cell.2013.05.039.
Weiterer S, Uhle F, Lichtenstern C, Siegler BH, Bhuju S, Jarek M, et al. Sepsis induces specific changes in histone modification patterns in human monocytes. PLoS One. 2015;10(3):e0121748. doi:10.1371/journal.pone.0121748.
Bomsztyk K, Mar D, An D, Sharifian R, Mikula M, Gharib SA, et al. Experimental acute lung injury induces multi-organ epigenetic modifications in key angiogenic genes implicated in sepsis-associated endothelial dysfunction. Crit Care. 2015;19:225. doi:10.1186/s13054-015-0943-4.
Bataille A, Galichon P, Ziliotis M-J, Sadia I, Hertig A. Epigenetic changes during sepsis: on your marks! Crit Care. 2015;19:358. doi:10.1186/s13054-015-1068-5.
Venet F, Lukaszewicz AC, Payen D, Hotchkiss R, Monneret G. Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Curr Opin Immunol. 2013;25(4):477–83. doi:10.1016/j.coi.2013.05.006.
Singer BH, Newstead MW, Zeng X, Cooke CL, Thompson RC, Singer K, et al. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One. 2016;11(2):e0149136. doi:10.1371/journal.pone.0149136.
Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–7. doi:10.2119/molmed.2012.00195.
Dal-Pizzol F, Pasquali M, Quevedo J, Gelain DP, Moreira JC. Is there a role for high mobility group box 1 and the receptor for advanced glycation end products in the genesis of long-term cognitive impairment in sepsis survivors? Mol Med. 2012;18:1357-8; author reply 9. doi:10.2119/molmed.2012.00317.
Kaynar A, Yende S, Zhu L, Frederick D, Chambers R, Burton C, et al. Effects of intra-abdominal sepsis on atherosclerosis in mice. Crit Care. 2014;18(5):469.
Sweeney TE, Shidham A, Wong HR, Khatri P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med. 2015;7(287):287ra71. doi:10.1126/scitranslmed.aaa5993.
Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi:10.1084/jem.20111354.
van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315(14):1469–79. doi:10.1001/jama.2016.2691.
Arens C, Bajwa SA, Koch C, Siegler BH, Schneck E, Hecker A, et al. Sepsis-induced long-term immune paralysis—results of a descriptive, explorative study. Crit Care. 2016;20(1):93. doi:10.1186/s13054-016-1233-5.
Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–605. doi:10.1001/jama.2011.1829.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi:10.1038/nature08821.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. doi:10.1126/science.1110591.
Pham TA, Lawley TD. Emerging insights on intestinal dysbiosis during bacterial infections. Curr Opin Microbiol. 2014;17:67–74. doi:10.1016/j.mib.2013.12.002.
Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2015;6:1543. doi:10.3389/fmicb.2015.01543.
Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M et al. Membership and Behavior of Ultra-Low-Diversity Pathogen Communities Present in the Gut of Humans during Prolonged Critical Illness. mBio. 2014;5(5). doi:10.1128/mBio.01361-14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs Shankar-Hari & Rubenfeld have no conflict of interests to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Sepsis and ICU
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shankar-Hari, M., Rubenfeld, G.D. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr Infect Dis Rep 18, 37 (2016). https://doi.org/10.1007/s11908-016-0544-7
Published:
DOI: https://doi.org/10.1007/s11908-016-0544-7